Effects of Immunization with Recombinant Schistosoma mansoni Enzymes AK and HGPRT: Murine Infection Control
Abstract
:1. Introduction
2. Material and Methods
2.1. Mice
2.2. Recombinant Enzymes AK and HGPRT
2.3. Immunization Protocol
2.4. Parasite and Challenge
2.5. Parasitological Parameters
2.6. Immunological Parameters
2.7. Eosinophils—Blood and Peritoneal Cavity
2.8. Histology
2.9. Statistical Analysis
3. Results
3.1. Immunization with a Combination of AK and HGPRT (MIX) Reduced the Number of Eggs in the Feces by 30.74% and Reduced the Number of Recovered Worms of the Animals by 29.00%
3.2. Immunization with AK, HGPRT and MIX Induce Production of IgG1 and IgE Antibodies
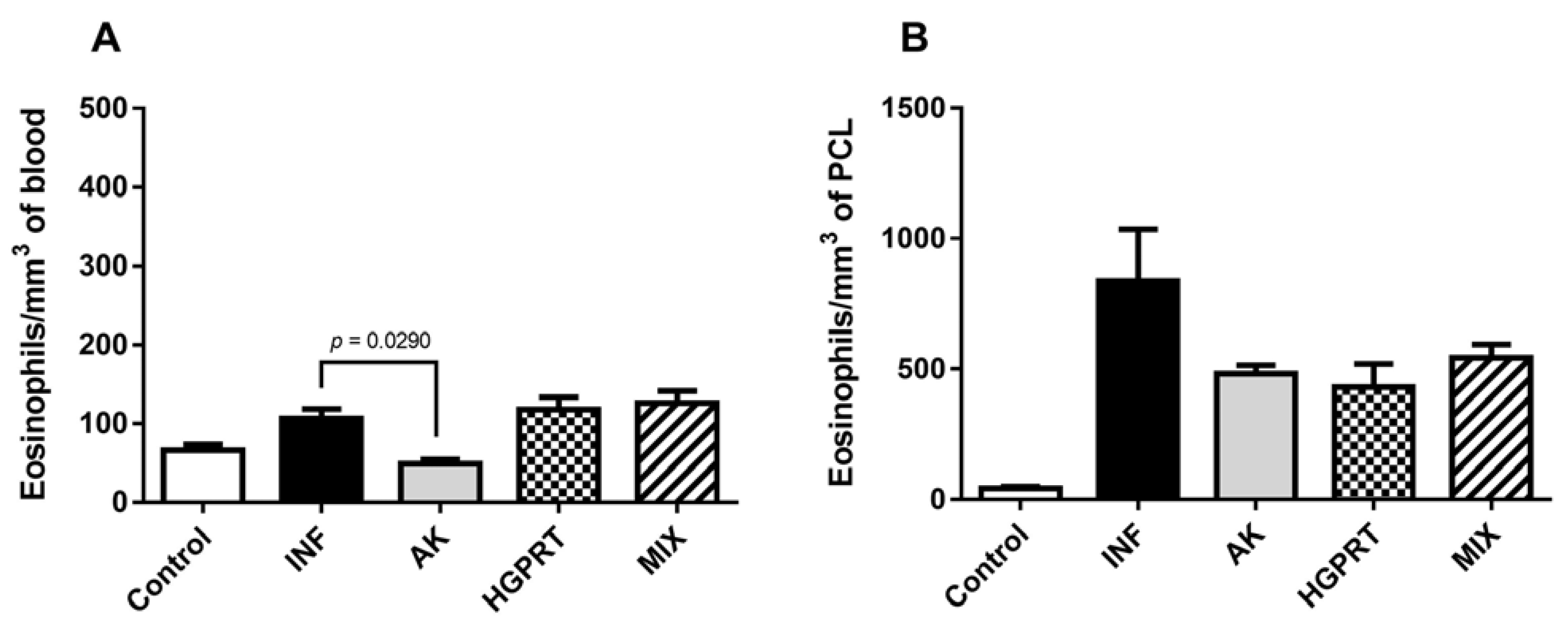
3.3. AK, HGPRT and MIX: Cytokines and Eosinophils
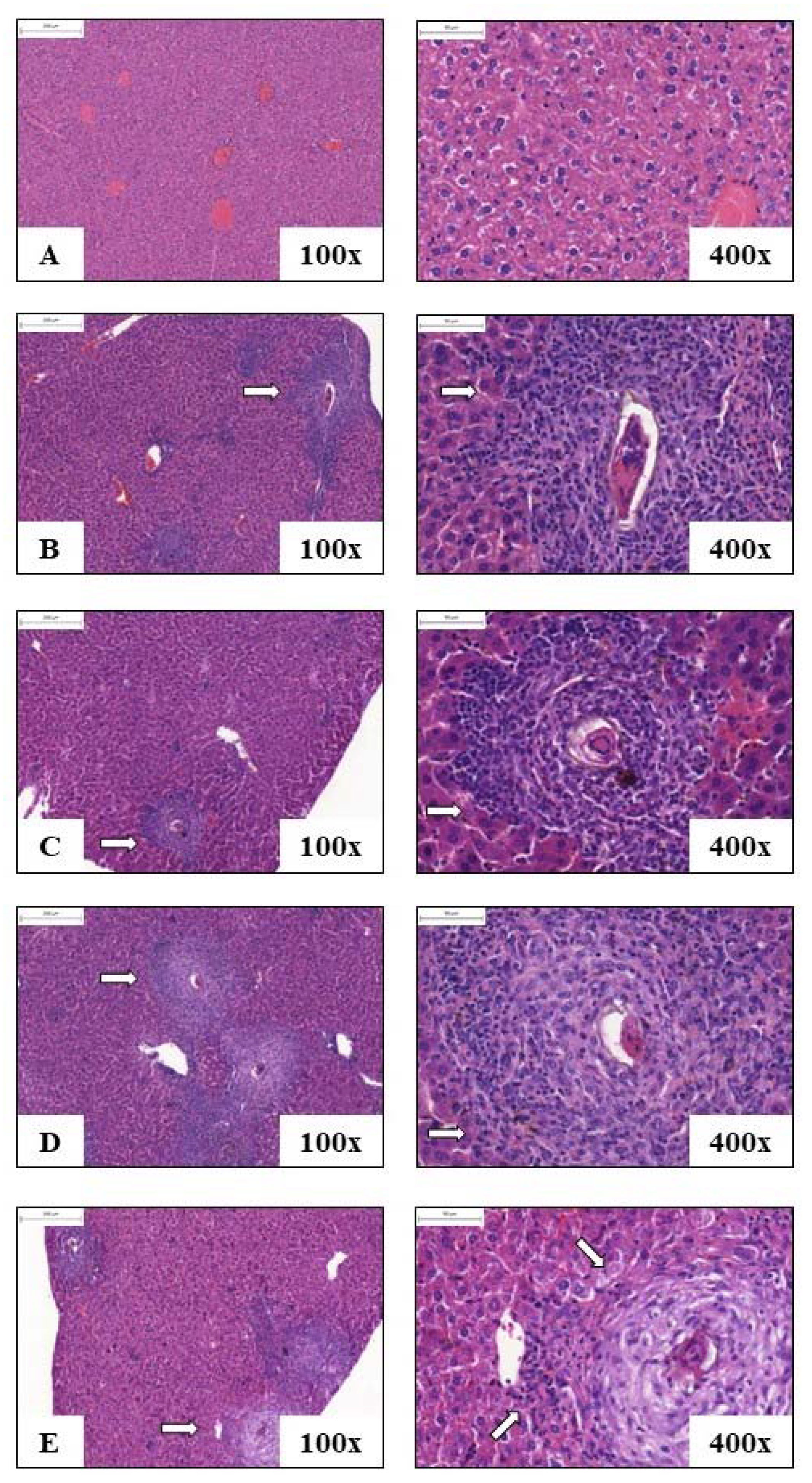
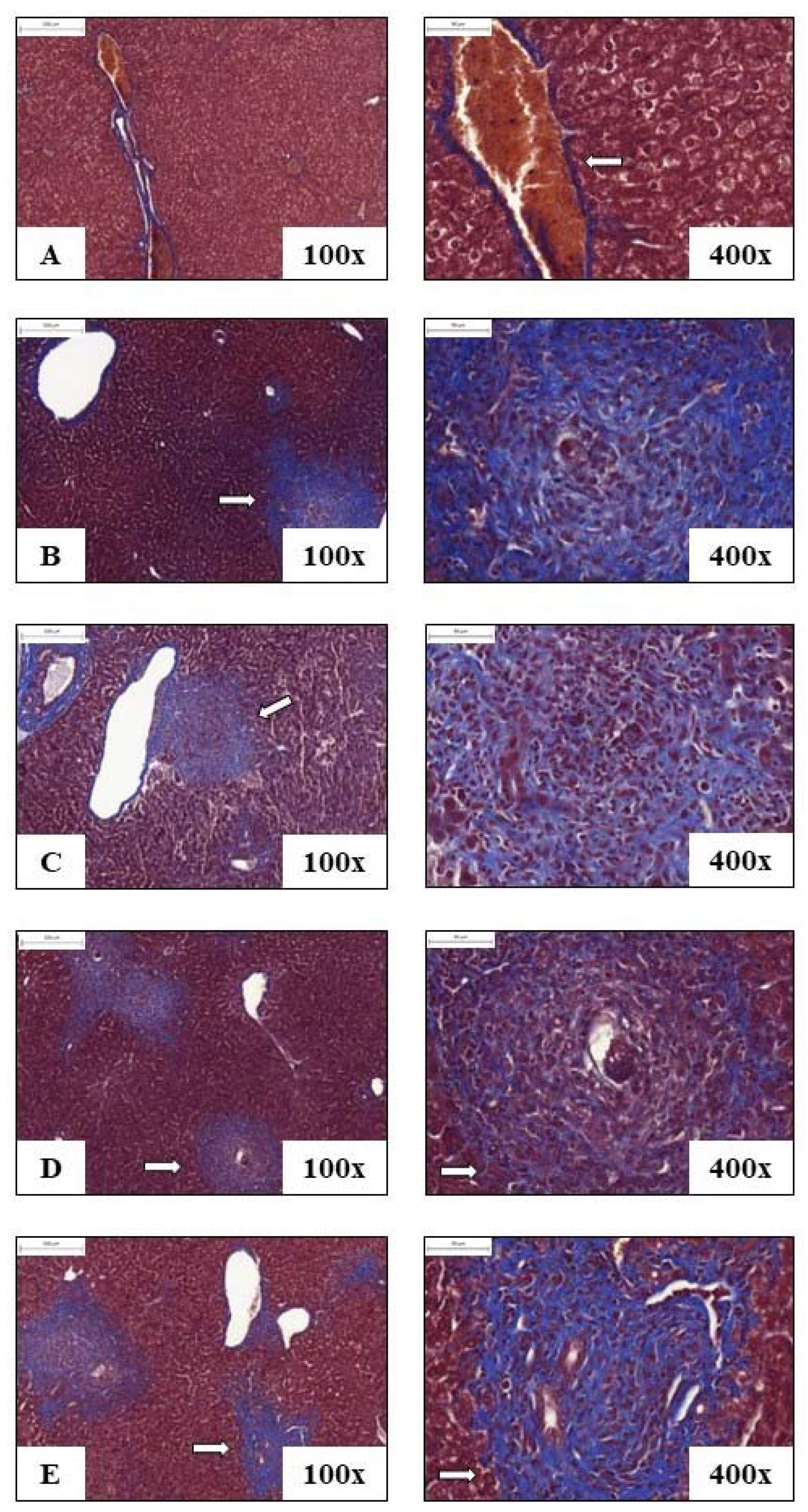
3.4. Liver Histology
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Schistosomiasis. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/s (accessed on 20 March 2022).
- Chitsulo, L.; Engels, D.; Montresor, A.; Savioli, L. The global status of schistosomiasis and its control. Acta Trop. 2000, 77, 41–51. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0001706X00001224 (accessed on 20 March 2022). [CrossRef] [PubMed] [Green Version]
- Modha, J.; Redman, C.; Thornhill, J.; Kusel, J. Schistosomes: Unanswered Questions on the Basic Biology of the Host–Parasite Relationship. Parasitol. Today 1998, 14, 396–401. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0169475898013210 (accessed on 20 March 2022). [CrossRef] [PubMed]
- Hotez, P.J.; Ferris, M.T. The antipoverty vaccines. Vaccine 2006, 24, 5787–5799. [Google Scholar] [CrossRef]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Latif, B.; Heo, C.C.; Razuin, R.; Shamalaa, D.V.; Tappe, D. Autochthonous Human Schistosomiasis, Malaysia. Emerg. Infect. Dis. 2013, 19, 1340–1341. Available online: http://wwwnc.cdc.gov/eid/article/19/8/12-1710_article.htm (accessed on 13 April 2022). [CrossRef] [PubMed]
- Liang, Y.-S.; Dai, J.-R.; Zhu, Y.-C.; Coles, G.C.; Doenhoff, M.J. Genetic analysis of praziquantel resistance in Schistosoma mansoni. Southeast Asian J. Trop. Med. Public Health 2003, 34, 274–280. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12971549 (accessed on 13 April 2022).
- Cioli, D.; Botros, S.S.; Wheatcroft-Francklow, K.; Mbaye, A.; Southgate, V.; Tchuem Tchuenté, L.-A.; Pica-Mattoccia, L.; Rita Troiani, A.; Seif el-Din, S.H.; Sabra, A.-N.A.; et al. Determination of ED50 values for praziquantel in praziquantel-resistant and -susceptible Schistosoma mansoni isolates. Int. J. Parasitol. 2004, 34, 979–987. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0020751904000980 (accessed on 13 April 2022). [CrossRef]
- Cioli, D.; Pica-Mattoccia, L.; Basso, A.; Guidi, A. Schistosomiasis control: Praziquantel forever? Mol. Biochem. Parasitol. 2014, 195, 23–29. [Google Scholar] [CrossRef]
- McManus, D.P.; Bergquist, R.; Cai, P.; Ranasinghe, S.; Tebeje, B.M.; You, H. Schistosomiasis—From immunopathology to vaccines. Semin. Immunopathol. 2020, 42, 355–371. [Google Scholar] [CrossRef]
- Wilson, R.A.; Coulson, P.S. Strategies for a schistosome vaccine: Can we manipulate the immune response effectively? Microbes Infect. 1999, 1, 535–543. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1286457999800938 (accessed on 17 April 2022). [CrossRef]
- Capron, A. Schistosomiasis: Forty Years’ War on the Worm. Parasitol. Today 1998, 14, 379–384. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0169475898013222 (accessed on 17 April 2022). [CrossRef] [PubMed]
- Molehin, A.J. Schistosomiasis vaccine development: Update on human clinical trials. J. Biomed. Sci. 2020, 27, 28. Available online: https://jbiomedsci.biomedcentral.com/articles/10.1186/s12929-020-0621-y (accessed on 30 April 2022). [CrossRef] [PubMed]
- Voet, D.; Voet, J.G. Biochesmitry, 4th ed.; John Wiley & Sons Inc: New York, NY, USA, 2010. [Google Scholar]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 7th ed.; W. H. Freeman: New York, NY, USA, 2017. [Google Scholar]
- Senft, A.W.; Miech, R.P.; Brown, P.R.; Senft, D.G. Purine metabolism in Schistosoma mansoni. Int. J. Parasitol. 1972, 2, 249–260. Available online: https://linkinghub.elsevier.com/retrieve/pii/0020751972900136 (accessed on 30 April 2022). [CrossRef] [PubMed]
- Dovey, H.F.; McKerrow, J.H.; Wang, C.C. Purine salvage in Schistosoma mansoni schistosomules. Mol. Biochem. Parasitol. 1984, 11, 157–167. Available online: https://linkinghub.elsevier.com/retrieve/pii/0166685184900628 (accessed on 30 April 2022). [CrossRef] [PubMed]
- Zhang, Y.; El Kouni, M.H.; Ealick, S.E. Structure of Toxoplasma gondii adenosine kinase in complex with an ATP analog at 1.1 Å resolution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2006, 62, 140–145. [Google Scholar] [CrossRef]
- Dovey, H.F.; McKerrow, J.H.; Aldritt, S.M.; Wang, C.C. Purification and characterization of hypoxanthine-guanine phosphoribosyltransferase from Schistosoma mansoni. A potential target for chemotherapy. J. Biol. Chem. 1986, 261, 944–948. [Google Scholar] [CrossRef]
- Keough, D.T.; Brereton, I.M.; De Jersey, J.; Guddat, L.W. The crystal structure of free human hypoxanthine-guanine phosphoribosyltransferase reveals extensive conformational plasticity throughout the catalytic cycle. J. Mol. Biol. 2005, 351, 170–181. [Google Scholar] [CrossRef]
- Bickle, Q.D.; Bogh, H.O.; Johansen, M.V.; Zhang, Y. Comparison of the vaccine efficacy of γ-irradiated Schistosoma japonicum cercariae with the defined antigen Sj62(IrV-5) in pigs. Vet. Parasitol. 2001, 100, 51–62. [Google Scholar] [CrossRef]
- Githui, E.K.; Damian, R.T.; Aman, R.A.; Ali, M.A.; Kamau, J.M. Schistosoma spp.: Isolation of microtubule associated proteins in the tegument and the definition of dynein light chains components. Exp. Parasitol. 2009, 121, 96–104. [Google Scholar] [CrossRef]
- Wilson, R.A. Proteomics at the schistosome-mammalian host interface: Any prospects for diagnostics or vaccines? Parasitology 2012, 139, 1178–1194. [Google Scholar] [CrossRef]
- Byram, J.E.; Goldberg, M.W.; Senft, A.W. Hemoglobinolytic Activity of Serum in Mice Infected with Schistosoma Mansoni. Am. J. Trop. Med. Hyg. 1981, 30, 96–101. [Google Scholar]
- Senft, A.W.; Crabtree, G.W. Purine metabolism in the schistosomes: Potential targets for chemotherapy. Pharmac. Ther. 1983, 20, 341–356. [Google Scholar] [CrossRef]
- Senft, A.W.; Crabtree, G.W.; Agarwal, K.C.; Scholar, E.M.; Agarwal, R.P.; Parks, R.E. Pathways of nucleotide metabolism in Schistosoma mansoni-III. Identification of enzymes in cell-free extracts. Biochem. Pharmacol. 1973, 22, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Serrao, V.H.B.; Pereira, H.D.; de Souza, J.R.T.; Romanello, L. Schistosoma mansoni Purine and Pyrimidine Biosynthesis: Structures and Kinetic Experiments in the Search for the Best Therapeutic Target. Curr. Pharm. Des. 2017, 23, 6967–6983. Available online: http://www.eurekaselect.com/156337/article (accessed on 30 April 2022). [CrossRef] [PubMed]
- Romanello, L.; Bachega, J.F.R.; Cassago, A.; Brandão-Neto, J.; Demarco, R.; Garratt, R.C.; Pereira, H.D. Adenosine kinase from Schistosoma mansoni: Structural basis for the differential incorporation of nucleoside analogues. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 126–136. [Google Scholar] [CrossRef]
- Romanello, L.; Zeraik, A.E.; de Freitas Fernandes, A.; Torini, J.R.; Bird, L.E.; Nettleship, J.E.; Rada, H.; Reddivari, Y.; Owens, R.J.; Serrão, V.H.B.; et al. In vitro and in vivo characterization of the multiple isoforms of Schistosoma mansoni hypoxanthine-guanine phosphoribosyltransferases. Mol. Biochem. Parasitol. 2019, 229, 24–34. [Google Scholar] [CrossRef]
- Olivier, L.; Stirewalt, M.A. An Efficient Method for Exposure of Mice to Cercariae of Schistosoma mansoni. J. Parasitol. 1952, 38, 19–23. [Google Scholar] [CrossRef]
- Katz, N.; Chaves, A.; Pellegrino, J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. 1972, 14, 397–400. [Google Scholar]
- Pellegrino, J.; Siqueira, A.F. Tecnica de perfusāo para colheita de Schistosoma mansoni em cobaias experimentalmente infestadas. Rev. Bras. Malariol. Doencas Trop. 1956, 8, 589–597. [Google Scholar]
- Delgado, V.S.; Suárez, D.P.; Cesari, I.M.; Incani, R.N. Experimental chemotherapy of Schistosoma mansoni with praziquantel and oxamniquine: Differential effect of single or combined formulations of drugs on various strains and on both sexes of the parasite. Parasitol. Res. 1992, 78, 648–654. [Google Scholar] [CrossRef]
- Merrifield, M.; Hotez, P.J.; Beaumier, C.M.; Gillespie, P.; Strych, U.; Hayward, T.; Bottazzi, M.E. Advancing a vaccine to prevent human schistosomiasis. Vaccine 2016, 34, 2988–2991. [Google Scholar] [CrossRef] [Green Version]
- Teixeira De Melo, T.; Michel De Araujo, J.; Do Valle Durães, F.; Caliari, M.V.; Oliveira, S.C.; Coelho, P.M.Z.; Fonseca, C.T. Immunization with newly transformed Schistosoma mansoni schistosomula tegument elicits tegument damage, reduction in egg and parasite burden. Parasite Immunol. 2010, 32, 749–759. [Google Scholar] [CrossRef]
- Nawaratna, S.S.K.; Gobert, G.N.; Willis, C.; Mulvenna, J.; Hofmann, A.; McManus, D.P.; Jones, M.K. Lysosome-associated membrane glycoprotein (LAMP)-preliminary study on a hidden antigen target for vaccination against schistosomiasis. Sci. Rep. 2015, 5, 15069. [Google Scholar] [CrossRef] [Green Version]
- Neris, D.M.; Pereira, H.D.M.; Correia, R.d.O.; de Oliveira, S.R.P.; Dejani, N.N.; Rodolpho, J.M.d.A.; de Souza, L.C.; Fattori, A.C.M.; Romanello, L.; de Souza, J.R.T.; et al. Evaluation of immunization with purine salvation pathway recombinant enzymes in Schistosoma mansoni worms and eggs in murine schistosomiasis. Int. J. Adv. Res. 2013, 1, 894–905. [Google Scholar]
- Ribeiro, F.; Dos Santos Vieira, C.; Fernandes, A.; Araújo, N.; Katz, N. The effects of immunization with recombinant Sm14 (rSm14) in reducing worm burden and mortality of mice infected with Schistosoma mansoni. Rev. Soc. Bras. Med. Trop. 2002, 35, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ahmad, G.; Torben, W.; Siddiqui, A.A. Sm-p80-based DNA vaccine made in a human use approved vector VR1020 protects against challenge infection with Schistosoma mansoni in mouse. Parasite Immunol. 2010, 32, 252–258. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, A.R.; Araújo, I.; Bacellar, O.; Magalhaes, A.; Pearce, E.; Harn, D.; Strand, M.; Carvalho, E.M. Human immune responses to Schistosoma mansoni vaccine candidate antigens. Infect. Immun. 2000, 68, 2797–2803. [Google Scholar] [CrossRef] [Green Version]
- El Ridi, R.; Shoemaker, C.B.; Farouk, F.; El Sherif, N.H.; Afifi, A. Human T- and B-cell responses to Schistosoma mansoni recombinant glyceraldehyde 3-phosphate dehydrogenase correlate with resistance to reinfection with S. mansoni or Schistosoma haematobium after chemotherapy. Infect. Immun. 2001, 69, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Capron, M.; Capron, A. Immunoglobulin E and effector cells in schistosomiasis. Science 1994, 264, 1876–1877. [Google Scholar] [CrossRef] [Green Version]
- Meyer, N.H.; Mayerhofer, H.; Tripsianes, K.; Blindow, S.; Barths, D.; Mewes, A.; Weimar, T.; Köhli, T.; Bade, S.; Madl, T.; et al. A crystallin fold in the interleukin-4-inducing principle of schistosoma mansoni eggs (IPSE/α-1) mediates IgE binding for antigen-independent basophil activation. J. Biol. Chem. 2015, 290, 22111–22126. [Google Scholar] [CrossRef] [Green Version]
- Pearce, E.J.; MacDonald, A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002, 2, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.H.S.; Britton, G.J.; Hill, E.V.; Verhagen, J.; Burton, B.R.; Wraith, D.C. Regulation of adaptive immunity; the role of interleukin. Front. Immunol. 2013, 4, 129. [Google Scholar] [CrossRef] [Green Version]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The Master Regulator of Immunity to Infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamai, K.; Ishikawa, H.; Mauviel, A.; Uitto, J. Interferon-γ coordinately upregulates matrix metalloprotease (MMP)-1 and MMP-3, but not tissue inhibitor of metalloproteases (TIMP), expression in cultured keratinocytes. J. Investig. Dermatol. 1995, 104, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, M.; Mwatha, J.K.; Joseph, S.; Jones, F.M.; Kadzo, H.; Ireri, E.; Kazibwe, F.; Kemijumbi, J.; Kariuki, C.; Kimani, G.; et al. Periportal Fibrosis in Human Schistosoma mansoni Infection Is Associated with Low IL-10, Low IFN-γ, High TNF-α, or Low RANTES, Depending on Age and Gender. J. Immunol. 2004, 172, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.M.; Dyer, K.D.; Cheever, A.W.; Ramalingam, T.; Pesnicak, L.; Domachowske, J.B.; Lee, J.J.; Lee, N.A.; Foster, P.S.; Wynn, T.A.; et al. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood 2006, 108, 2420–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lins, R.A.B.; Cavalcanti, C.B.D.L.; Araújo-Filho, J.L.S.; De Melo, M.R.; Chaves, M.E.C. A distribuição dos eosinófilos nas diferentes fases de evolução do granuloma hepático em camundongos infectados pelo Schistosoma mansoni. Rev. Soc. Bras. Med. Trop. 2008, 41, 173–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silveira-Lemos, D.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Alves Oliveira, L.F.; Costa-Silva, M.F.; Matoso, L.F.; de Souza, L.J.; Gazzinelli, A.; Corrêa-Oliveira, R. Eosinophil activation status, cytokines and liver fibrosis in Schistosoma mansoni infected patients. Acta Trop. 2008, 108, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, H.L.; Romanha, W.d.S.; Machado, M.P.; Mota, E.M.; Lenzi, J.A. Patologia Experimental com Enfoque no Granuloma Esquistossomótico. In Schistossoma Mansoni e Esquistossomose Uma Visão Multidisciplinar; Fiocruz: Rio de Janeiro, Brazil, 2008; pp. 575–627. ISBN 978-857541-150-6. [Google Scholar]
- Hams, E.; Aviello, G.; Fallon, P.G. The Schistosoma granuloma: Friend or foe? Front. Immunol. 2013, 4, 89. [Google Scholar] [CrossRef]




| Groups | Eggs/g of Feces | Reduction (%) | Adult Worms | Reduction (%) |
|---|---|---|---|---|
| INF | 106.6 ± 46.68 | - | 24.89 ± 11.7 | - |
| AK | 132.8 ± 52.79 | - | 23.64 ± 13.49 | 5.02 |
| HGPRT | 104.2 ± 40.16 | 2.25 | 24.55 ± 12.69 | 1.36 |
| MIX | 73.83 ± 29.78 | 30.74 | 17.67 ± 5.85 | 29.00 |
| Groups | Granulomas | Reduction (%) | Eggs in the Liver | Reduction (%) |
|---|---|---|---|---|
| INF | 13.75 ± 5.67 | - | 6.5 ± 1.29 | - |
| AK | 8 ± 0.00 | 41.81 | 3.75 ± 2.75 | 42.30 |
| HGPRT | 14.67 ± 1.52 | - | 9 ± 5.47 | - |
| MIX | 15.75 ± 4.5 | - | 9.75 ± 5.18 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattori, A.C.M.; Montija, E.d.A.; Fragelli, B.D.d.L.; Correia, R.d.O.; de Castro, C.A.; Romanello, L.; Nogueira, C.T.; Allegretti, S.M.; Soares, E.G.; Pereira, H.D.; et al. Effects of Immunization with Recombinant Schistosoma mansoni Enzymes AK and HGPRT: Murine Infection Control. Pathogens 2023, 12, 69. https://doi.org/10.3390/pathogens12010069
Fattori ACM, Montija EdA, Fragelli BDdL, Correia RdO, de Castro CA, Romanello L, Nogueira CT, Allegretti SM, Soares EG, Pereira HD, et al. Effects of Immunization with Recombinant Schistosoma mansoni Enzymes AK and HGPRT: Murine Infection Control. Pathogens. 2023; 12(1):69. https://doi.org/10.3390/pathogens12010069
Chicago/Turabian StyleFattori, Ana Carolina Maragno, Elisandra de A. Montija, Bruna D. de L. Fragelli, Ricardo de O. Correia, Cynthia Aparecida de Castro, Larissa Romanello, Camila T. Nogueira, Silmara M. Allegretti, Edson G. Soares, Humberto D. Pereira, and et al. 2023. "Effects of Immunization with Recombinant Schistosoma mansoni Enzymes AK and HGPRT: Murine Infection Control" Pathogens 12, no. 1: 69. https://doi.org/10.3390/pathogens12010069
APA StyleFattori, A. C. M., Montija, E. d. A., Fragelli, B. D. d. L., Correia, R. d. O., de Castro, C. A., Romanello, L., Nogueira, C. T., Allegretti, S. M., Soares, E. G., Pereira, H. D., & Anibal, F. d. F. (2023). Effects of Immunization with Recombinant Schistosoma mansoni Enzymes AK and HGPRT: Murine Infection Control. Pathogens, 12(1), 69. https://doi.org/10.3390/pathogens12010069






