Abstract
Leishmaniasis is a neglected disease caused by protozoa belonging to the Leishmania genus. Notably, the search for new, promising and potent anti-Leishmania compounds remains a major goal due to the inefficacy of the available drugs used nowadays. In the present work, we evaluated the effects of 1,10-phenanthroline-5,6-dione (phendione) coordinated to silver(I), [Ag(phendione)2]ClO4 (Ag-phendione), and copper(II), [Cu(phendione)3](ClO4)2·4H2O (Cu-phendione), as potential drugs to be used in the chemotherapy against Leishmania amazonensis and Leishmania chagasi. The results showed that promastigotes treated with Ag-phendione and Cu-phendione presented a significant reduction in the proliferation rate. The IC50 values calculated to Ag-phendione and Cu-phendione, respectively, were 7.8 nM and 7.5 nM for L. amazonensis and 24.5 nM and 20.0 nM for L. chagasi. Microscopical analyses revealed several relevant morphological changes in promastigotes, such as a rounding of the cell body and a shortening/loss of the single flagellum. Moreover, the treatment promoted alterations in the unique mitochondrion of these parasites, inducing significant reductions on both metabolic activity and membrane potential parameters. All these cellular perturbations induced the triggering of apoptosis-like death in these parasites, as judged by the (i) increased percentage of annexin-positive/propidium iodide negative cells, (ii) augmentation in the proportion of parasites in the sub-G0/G1 phase and (iii) DNA fragmentation. Finally, the test compounds showed potent effects against intracellular amastigotes; contrarily, these molecules were well tolerated by THP-1 macrophages, which resulted in excellent selective index values. Overall, the results highlight new selective and effective drugs against Leishmania species, which are important etiological agents of both cutaneous (L. amazonensis) and visceral (L. chagasi) leishmaniasis in a global perspective.
1. Introduction
Leishmaniasis is a tropical neglected disease caused by more than 20 species of protozoan parasites of the Leishmania genus that are transmitted by the bite of more than 90 species of sand flies from the Phlebotomus genus. Besides its endemic profile in 98 countries, leishmaniasis represents a serious public health concern in terms of control of transmission reservoirs [1]. Leishmaniasis presents a wide spectrum of clinical manifestations that appear to result from a combination of intrinsic properties of the parasite strain and species as well as various factors of the vertebrate host, such as age, genetic predisposition and immunological status [1,2]. In this regard, this disease can be traditionally classified into three main clinical forms: visceral, cutaneous (localized or diffuse) and mucocutaneous, which differ in their immunopathology, degree of morbidity and mortality [1,2].
Different chemotherapeutics with distinct mechanisms of action are available for the current treatment of leishmaniasis. Pentavalent antimonials, such as N-methyl glucamine antimoniate (glucantime®) and sodium stibogluconate (pentostan®) are the first-choice drugs. Amphotericin B, miltefosine and paromomycin are used as second options for treatment [1,3,4]. However, all antileishmanial therapies are very problematic, mainly due to the extensive toxicity, lack of efficacy, the necessity of parenteral administration, high costs, the emergence of drug resistance and a lack of access in regional areas [3,4]. Antimonials, which have been used in the treatment of leishmaniasis since the beginning of the last century, are responsible for important side effects in the host, and the resistance of the parasite to these drugs has been reported in several areas of the world. Miltefosine is an oral drug with few side effects, but it is extremely expensive, as are the lipid formulations of amphotericin B, which have many side effects and resistant parasites are usually reported [3,4].
In this scenario, coordination compounds have played an important role in the development of new chemotherapeutics with pharmacological applications [5,6]. Coordination compounds’ synthesis is relatively easy and the geometric possibilities resulting from the use of a metal center make this an attractive approach for the development of novel pharmacological agents [6]. The chemistry of metal complexes with heterocyclic linkers has attracted considerable interest in recent years, becoming a growing class of research due to the prospect of synthesis of a large number and a wide variety of synthetic binders (chelates), which behave like coordination agents for metal ions [6].
One of the oldest and best-studied N-heterocyclic chelating agents is 1,10-phenanthroline (phen), which acts as a platform for the synthesis of new reagents for biotechnological and medical purposes. Several published works have shown that phen-based compounds have antimicrobial properties against bacteria, fungi and protozoa, including different species of Leishmania [6,7,8]. 1,10-Phenanthroline-5,6-dione (phendione) was synthesized through the addition of two carbonyl groups attached at the 5,6-positions. Previous studies on the antibacterial properties of metal-free compounds, including heteroaromatic derivatives of phenanthrene, such as phen and phendione, have revealed that the inclusion of N-atoms in the phenanthrene ring has considerably increased its antimicrobial activity. In the search of new bioactive compounds derived from phen or phendione, a previous study described the synthesis of a series of new compounds, including [Cu(phendione)3](ClO4)2·4H2O(Cu-phendione) and [Ag(phendione)2]ClO4 (Ag-phendione), presenting a greater efficacy and a lower toxicity to multicellular organisms [9,10]. In this context, in vivo tests showed that Cu-phendione and Ag-phendione had a low toxicity for Swiss mice and Galleria mellonella larvae [6]. In addition, phendione-derived drugs, in both complexed or metal-free forms, were able to alter the functioning of a variety of microbial systems [6,8,11,12,13], such as bacteria (Acinetobacter baumannii [13], Escherichia coli [14], Klebsiella pneumoniae [15] and Pseudomonas aeruginosa [9]), yeasts (Candida albicans [6] and non-albicans Candida species [16]), filamentous fungi (Pseudallescheria boydii [6] and Phialophora verrucosa [11]) and parasites (Trichomonas vaginalis [12] and Leishmania braziliensis [17]).
Recently, our group showed that both Cu-phendione and Ag-phendione displayed a leishmanicidal effect against promastigote forms of L. braziliensis [17]. In this context, the present work aimed to amplify the studies with these potent compounds against other Leishmania species, since the extreme heterogeneous profile of leishmaniasis culminates in distinct responses to drugs. Based on this premise, L. amazonensis and L. chagasi, relevant etiologic agents of cutaneous and visceral forms of leishmaniasis, respectively, were selected to evaluate their susceptibility to the treatment with phendione and its silver and copper derivatives as well as to decipher the mechanisms of action and to elucidate the triggering death pathway. In addition, the effects of these test compounds on Leishmania-macrophage interaction were evaluated.
2. Materials and Methods
2.1. Parasites and Cultivation
Leishmania amazonensis (MHOM/BR/PH8) and Leishmania chagasi (MHOM/BR/1974/PP75) were obtained from Coleção de Leishmania from Fundação Oswaldo Cruz (FIOCRUZ; Leishmania Type Culture Collection-LTTC-WDCM 731), Rio de Janeiro, Brazil. Promastigotes were grown in Schneider’s insect medium (Sigma-Aldrich, St Louis, MO, USA), pH 7.2, containing 10% heat-inactivated fetal bovine serum (FBS) (Cultilab, São Paulo, SP, Brazil) at 28 °C.
2.2. Macrophages Cultivation
Human leukemia monocytic cell line (THP-1) was maintained in 25 cm2 tissue culture flasks with RPMI 1640 medium (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% FBS at 37 °C in an atmosphere containing 5% CO2. The culture medium was exchanged every three days. For interaction experiments, THP-1 cells in 24- or 96-well plates (2 × 105 cells/well) were differentiated into macrophages by treatment with phorbol-12-myristate-13-acetate (PMA; 40 ng/mL) (Sigma-Aldrich, St Louis, MO, USA) for 48 h. Then, the plates were washed twice with sterile phosphate-buffered saline (PBS; pH 7.2) to remove PMA and a new RPMI 1640 medium was added [18]. Differentiated cells, used in all experiments, showed similar morphological changes and the ability to adhere to the culture plates as macrophages.
2.3. Test Compounds
1,10-Phenanthroline-5,6-dione (phendione), [Cu(phendione)3](ClO4)2·4H2O (Cu-phendione) and [Ag(phendione)2]ClO4 (Ag-phendione) were prepared in accordance to published methods [19]. The simple salts AgNO3 and CuSO4 as well as 1,10-phenanthroline (phen) (Sigma-Aldrich, St Louis, MO, USA) were used as appropriated controls.
2.4. Effects of Coordination Compounds on Promastigotes’ Growth Rate
Promastigotes were counted using a Neubauer chamber and resuspended in fresh medium at a final concentration of 5 × 105 viable promastigotes per milliliter. The viability was assessed by mobility and a lack of staining after challenging with Trypan blue. Each metal-coordinated compound was added to the cultures at final concentrations ranging from 2.5 to 30 nM for L. amazonensis and from 5 to 50 nM for L. chagasi, starting from a stock solution in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St Louis, MO, USA). Phendione and phen were added to the cultures at final concentrations ranging from 5 to 50 nM and from 400 to 2000 nM, respectively, for both Leishmania species. After 72 h of incubation at 28 °C, the number of viable parasites was estimated. The 50% inhibitory concentration (IC50), i.e., the drug concentration that caused a 50% reduction in survival/viability, was determined by a linear regression analysis, by plotting the log number of promastigotes versus drug concentration using GraphPad Prism 5 computer software.
2.5. Protocol of Parasite Treatment: Looking for Potential Mechanisms of Action
For all the subsequent experiments (except when discriminated), promastigotes (5 × 105 cells/mL) were treated (or not) with each coordination compound for 72 h at concentrations corresponding to ½ × IC50, IC50 or 2 × IC50 values in order to detect the influence of these test compounds on the (i) morphology/morphometry, (ii) ultrastructure, (iii) general metabolism, (iv) mitochondrial dehydrogenase activity, (v) mitochondrial membrane potential, (vi) phosphatidylserine externalization, (vii) incorporation of propidium iodide, (viii) cell cycle, (ix) DNA fragmentation and (x) interaction with macrophages.
2.6. Morphology, Morphometry and Ultrastructure
Morphological alterations were evidenced by flow cytometry (FACSCalibur, BD Bioscience, Franklin Lakes, NJ, USA) using a two-parameter histogram of forward scatter (FSC) versus side scatter (SSC) to measure two morphological parameters, cell size and granularity, respectively. An ultrastructural analysis was performed by scanning electron microscopy (SEM). To do it, parasites were fixed for 40 min at 25 °C with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2. After fixation, the parasites were washed in a cacodylate buffer and postfixed with a solution of 1% OsO4, 0.8% potassium ferrocyanide and 5 mM CaCl2 in the same buffer for 20 min at 25 °C. The parasites were dehydrated in graded series of acetone (30–100%) and then dried by the critical point method, mounted on stubs, coated with gold (20–30 nm) and observed in a Jeol JSM 6490LV scanning electron microscope (JEOL Inc., Peabody, MA, USA).
2.7. General Metabolism
The general metabolism of the parasites was evaluated by a resazurin dye/Alamar blue (7-hydroxy-3H-phenoxazin-3-one-10-oxide) assay (Sigma-Aldrich, St Louis, MO, USA) [20]. L. amazonensis and L. chagasi promastigotes were incubated in sterile 96-well plates (in a total volume of 100 μL culture medium/well) and then resazurin was added to a final concentration of 0.0125% in PBS [21]. After a 4 h incubation at room temperature, parasites were analyzed in a microplate reader (SpectraMax spectrofluorometer, Molecular Devices, San Jose, CA, USA) using a pair of 590 and 544 nm as emission and excitation wavelengths, respectively. The viability was evaluated based on a comparison with untreated control cells. Parasites were also treated with sodium azide (40 µM) for 30 min in order to obtain nonviable cells to use as a positive control in the viability test.
2.8. Mitochondrial Dehydrogenases
The mitochondrial dehydrogenase activity was measured by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, St Louis, MO, USA). This reagent was added to a final concentration of 0.5 mg/mL in the culture medium and the parasites were incubated for 4 h in the dark at 28 °C. Then, the MTT solution was removed and 200 µL of DMSO was added to solubilize the formazan crystals. The mitochondrial metabolism was determined spectrophotometrically at 490 nm (SpectraMax Gemini 190, Molecular Devices, San Jose, CA, USA). Parasite cells incubated with sodium azide at 40 µM for 30 min were used as a positive control.
2.9. Mitochondrial Membrane Potential
In order to analyze the mitochondrial membrane potential (ΔΨm), parasites were incubated with 10 µg/mL Rhodamine 123 (R123) (Sigma-Aldrich, St Louis, MO, USA) for 30 min and washed three times with PBS, resuspended in the same buffer and analyzed by flow cytometry (FACSCalibur, BD Bioscience, Franklin Lakes, NJ, USA) equipped with a 15 mW argon laser emitting at 488 nm. As positive control of the depolarization of the mitochondrial membrane, parasites were incubated for 30 min with 2 μM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) (Sigma-Aldrich, St Louis, MO, USA), a mitochondrial protonophore. Data represented the analysis of 10,000 events and the results were expressed as the mean of the fluorescence intensity (MFI).
2.10. Phosphatidylserine Externalization and Incorporation of Propidium Iodide
Phosphatidylserine externalization and the passive incorporation of propidium iodide (PI) were evidenced by using the double labeling with annexin V conjugated to Alexa-Fluor and PI (Thermo Fisher Scientific, Waltham, MA, SUA). Parasite cells were washed twice with PBS and resuspended in an annexin-V binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4). Annexin V conjugated to Alexa-Fluor was added and the promastigotes were incubated for 20 min in the dark. After, parasite cells were washed with PBS and incubated with PI for 10 min. Finally, parasite cells were washed, resuspended in PBS and the intensity of the labeling of annexin V and PI was recorded in a FACSCalibur (BD Bioscience, Franklin Lakes, NJ, USA) flow cytometer and analyzed with Flowing software. Data represented the analysis of 10,000 events and the results were expressed as percentage of fluorescent cells (% FC).
2.11. Cell Cycle
To analyze the parasite cell cycle, promastigotes were fixed in 70% chilled methanol (diluted in PBS) overnight at −20 °C. Fixed promastigotes were washed thoroughly and then resuspended in 0.5 mL of PI (10 µg/mL in PBS) containing RNase A (100 µg/mL) (Thermo Fisher Scientific, Waltham, MA, SUA), and the mixture was incubated for 20 min in the dark at room temperature. The fluorescence intensity of PI was analyzed with a FACSCalibur (BD Bioscience, Franklin Lakes, NJ, USA) flow cytometer and CellQuest software. Data represented the analysis of 10,000 events and the results were expressed as % FC.
2.12. DNA Fragmentation
The detection of DNA fragmentation was performed by using a Terminal Deoxynucleotidyltranferase (TdT)-mediated dUTP Nick End Labeling (TUNEL) assay (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. Briefly, parasites were incubated in ice-cold 70% ethanol overnight at −20 °C. Then, the parasites were washed twice with PBS for ethanol removal and incubated with a DNA-labeling solution (TdT+ Brd UTP) for 60 min at 37 °C in a water bath. Finally, the parasites were resuspended in PBS and analyzed using the FACSCalibur (BD Bioscience, Franklin Lakes, NJ, USA) flow cytometer using Flowing software. Data represented the analysis of 10,000 events and the results were expressed as % FC.
2.13. Macrophage Toxicity
The effects of Cu-phendione and Ag-phendione on the viability of THP-1 cells were evaluated by MTT assay. THP-1 cells (2 × 105 cells/mL) were differentiated, as described previously, in 96-well culture plates in RPMI 1640 medium supplemented with 10% FBS. Then, each metal-coordinated compound was added to the cultures at final concentrations ranging from 0.1 to 3 µM and the macrophage cells were incubated for 24 h at 37 °C in a 5% CO2 atmosphere. Afterwards, an MTT reduction assay was carried out as described in Section 2.8. The 50% cytotoxic concentration (CC50) was determined by a linear regression analysis after 24 h of treatment with each test compound.
2.14. Leishmania-Macrophage Interaction
THP-1 cells (5 × 105 cells) were differentiated, as described in Section 2.2, in 24-well culture plates containing sterile cover glasses in each well. Then, L. amazonensis or L. chagasi stationary-phase promastigotes, obtained after 3 days of culture, were used to infect the mammalian cells (10 parasites per macrophage) for 24 h at 37 °C, 5% CO2, to allow parasite internalization. Afterwards, the cultures were washed with sterile PBS to remove noninternalized parasites and then fresh medium with 10% FBS was added. Infected THP-1 cells were treated for 24 h, under the same conditions, with concentrations that maintained 95% viability of the host cell: 0.025, 0.05 and 0.1 µM of Ag-phendione and 0.04, 0.08 and 0.16 µM of Cu-phendione. After this period, the cultures were fixed with methanol and stained with Giemsa. The percentage of infected macrophages was determined by randomly counting 200 cells on each of triplicate coverslips using a bright-field microscope. The association index was obtained by multiplying the percentage of infected macrophages by the number of amastigotes per macrophage. The 50% inhibitory concentration (IC50) for amastigotes was determined by a linear regression analysis. The selectivity index (SI) was calculated by dividing the CC50 value of THP-1 macrophage cells by the IC50 of amastigote forms.
2.15. Statistics
All experiments were performed in triplicate, in three independent experimental sets. The data were analyzed statistically by means of Student’s t test using GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA). p values of 0.05 or less were considered statistically significant.
3. Results and Discussion
3.1. Effects of Coordination Compounds on Leishmania’s Growth Rate
Initially, we evaluated the susceptibility of L. amazonensis and L. chagasi promastigotes to phen, its quinone derivative phendione, the phendione complexed with silver(I) (Ag-phendione) and copper(II) (Cu-phendione) as well as to the simple salts AgNO3 and CuSO4. To do it, promastigotes were incubated in the absence (control) or in the presence of different concentrations of each test compound and the cellular growth was compared with nontreated parasites for 72 h. For both Leishmania species, metal-free phendione was considerably less toxic than phen. When phendione was individually coordinated with silver or copper metals, a significant reduction in the IC50 values was verified (Table 1) (Supplementary Figure S1). In this sense, Ag-phendione and Cu-phendione had a similar action against L. amazonensis, displaying IC50 values of 7.8 nM and 7.5 nM, respectively, while the IC50 values calculated for phendione and phen were 19.1 nM and 870.0 nM, respectively (Table 1). Similar results were evidenced for L. chagasi, in which Cu-phendione was the most effective compound (IC50 = 20.0 nM) followed by Ag-phendione, phendione and phen (Table 1). Additionally, promastigotes cultured either in the presence of the simple salts or DMSO (the solvent of the test compounds) had no effect on the parasite proliferation when used in the same concentration of the coordination compounds (Figure S1).

Table 1.
Anti-Leishmania effects of Ag-phendione, Cu-phendione, phendione and phen.
The effects of these test compounds on other microorganisms’ growth rate were also evaluated. For instance, in a study conducted with the fungus P. verrucosa, an IC50 value of 7.0 μM was found for the phendione ligand. The addition of the transition metals silver and copper resulted in a potentiated effect of these compounds, with calculated IC50 values of 2.4 and 1.8 µM, respectively [11]. The effect of these metal complexes was also evaluated against the widespread and multidrug-resistant Gram-negative bacterium P. aeruginosa, for which the MIC50 values for Cu-phendione, Ag-phendione, phendione and phen were calculated as 7.43, 14.05, 31.15 and 579.28 µM, respectively [9]. Cu-phendione and Ag-phendione also affected the planktonic growth and biofilm formation of carbapenemase-producing Gram-negative bacterium A. baumannii [13]. These metal-phendione complexes also present a potent anti-T. vaginalis action when used alone or in combination with the reference drug metronidazole [12]. Recently, Lima and colleagues [17] showed that both metal-based compounds harmed the metallopeptidase gp63 activity of virulent strains of L. braziliensis, in addition to altering the parasite–macrophage interaction process.
3.2. Effects of Coordination Compounds on Leishmania’s Morphometry and Ultrastructure
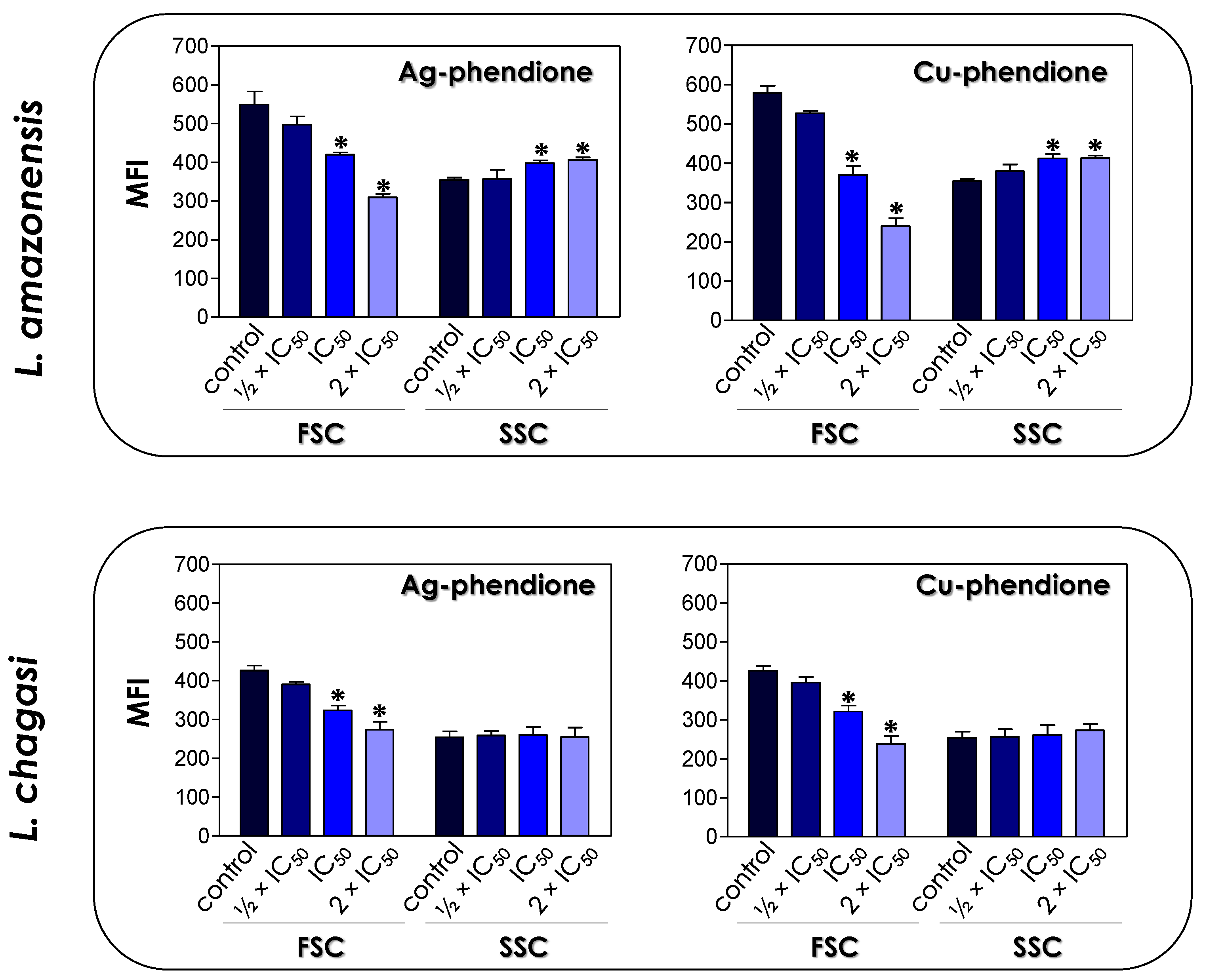
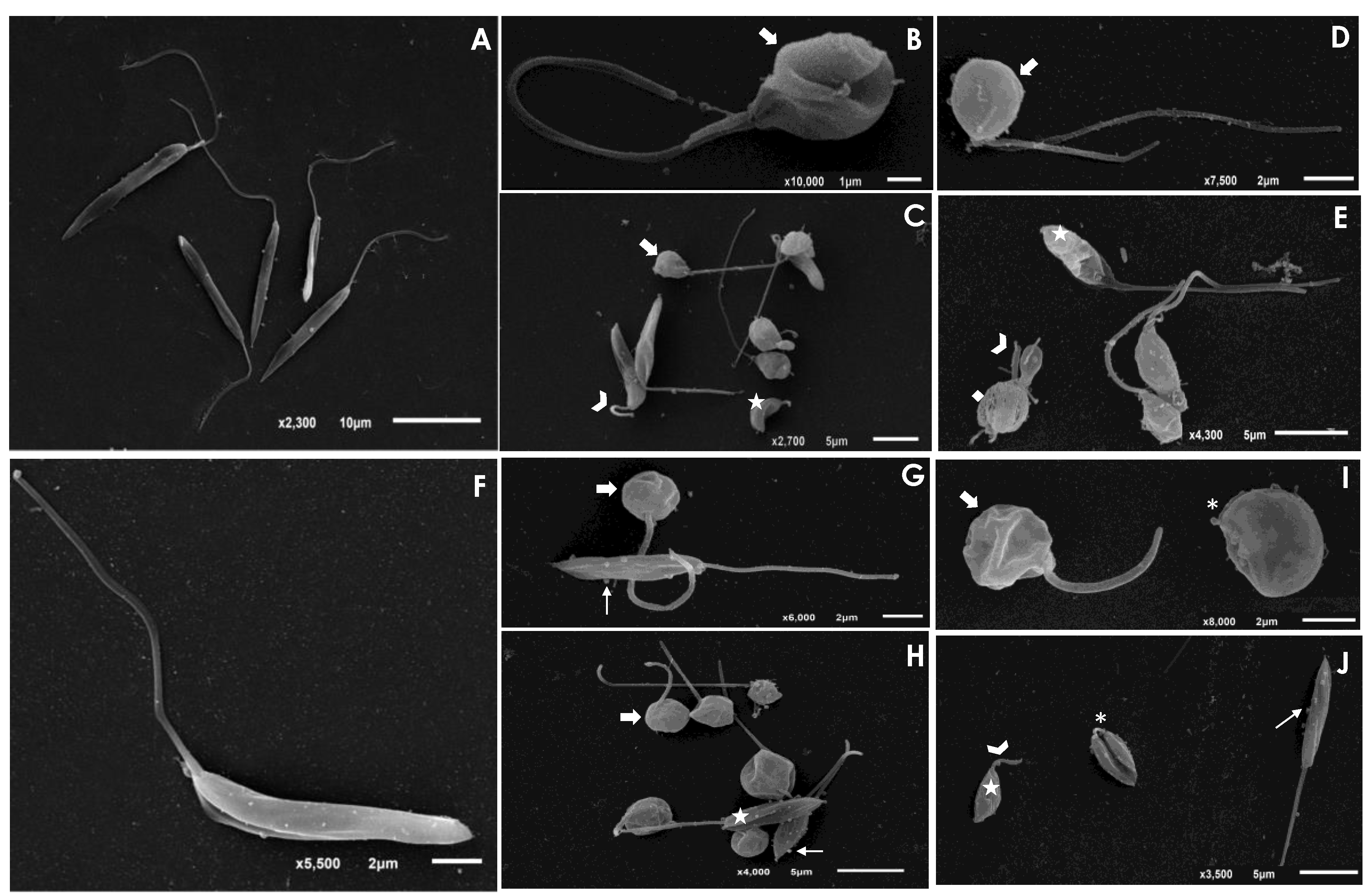
The treatment of L. amazonensis and L. chagasi promastigotes with Ag-phendione and Cu-phendione, at both IC50 and 2 × IC50 concentrations, induced a significant reduction in the parasite size in comparison to untreated cells as judged by a flow cytometry analysis, while the treatment with ½ × IC50 did not impact this morphometrical parameter (Figure 1). In parallel, an increase in the cell granularity of L. amazonensis was observed after the treatment with both test compounds, while no change in this morphometric parameter was detected in L. chagasi under the employed experimental conditions (Figure 1). In order to analyze the morphological changes caused by the test compounds in more detail, promastigotes were treated with the IC50 values of Ag-phendione and Cu-phendione and examined by SEM. The results showed that L. amazonensis (Figure 2A) and L. chagasi (Figure 2F) untreated parasites showed normal ultrastructural features, including regular cell surface, elongated cell body and a long and unique flagellum. The treatment of L. amazonensis promastigotes with Ag-phendione and Cu-phendione led to several ultrastructural alterations, such as a rounding of the cell body, cell shrinkage and a shortening of the flagellum (Figure 2B–E). Moreover, treatment with Cu-phendione also caused a discontinuity on the cell surface of the parasites (Figure 2E). Similarly, treatment of L. chagasi promastigotes with the IC50 value of both coordination compounds caused rounding in the cell body and cell shrinkage (Figure 2G–J). In addition, the parasites presented an increase in membrane protrusion resembling surface blebs compared to the control cells (Figure 2G–J). The treatment of L. chagasi with Cu-phendione also promoted the complete loss or shortening of the single flagellum (Figure 2I,J).
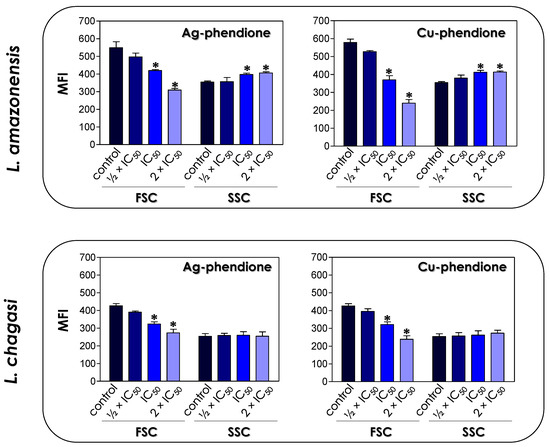
Figure 1.
Effects of coordination compounds on the cell size (FSC) and granularity (SSC) of L. amazonensis and L. chagasi promastigote forms. Parasites were treated or not (control) with the ½ × IC50, IC50 and 2 × IC50 values of Ag-phendione and Cu-phendione for 72 h and, subsequently, analyzed in a flow cytometer. FSC and SSC values were expressed as the mean of the fluorescence intensity (MFI). The data presented in the graph are representative of the analysis of 10,000 parasite cells in three experiments performed in triplicate and the results were considered significant when p < 0.05 (*).
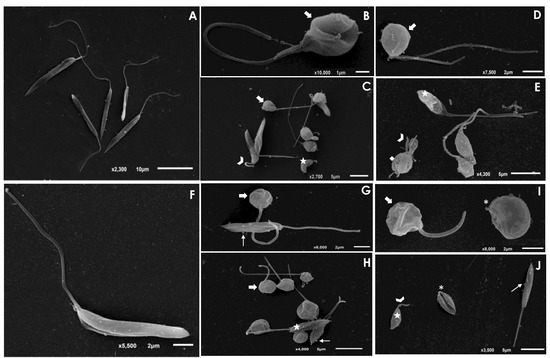
Figure 2.
Scanning electron microscopy (SEM) analysis of L. amazonensis and L. chagasi promastigotes after treatment with the IC50 value of Ag-phendione and Cu-phendione. Control cells of L. amazonensis (A) and L. chagasi (F) presented an elongated cell body and a long flagellum. The treatment of L. amazonensis with the IC50 value of Ag-phendione (B,C) and Cu-phendione (D,E) showed parasites displaying rounding in cell body (arrows), cell shrinkage (stars) and shortening of flagellum (arrowheads). In addition, treatment with Cu-phendione led to a discontinuity on the cell surface (diamond). L. chagasi treated with Ag-phendione (G,H) and Cu-phendione (I,J) also promoted rounding in cell body (thick arrows) and cell shrinkage (stars), besides the formation of blebs in the membrane (thin arrows). L. chagasi treated with Cu-phendione presented shortening (arrowhead) or loss (asterisks) of the flagellum.
In recent years, a series of compounds complexed to metals have been tested against Leishmania [22,23,24,25,26,27,28], and these new formulations may emerge as potential drugs in the treatment of leishmaniasis. A study using amino- and iminopyridyl compounds complexed with metals presented a potent effect against Trypanosoma cruzi and Leishmania [24]. Similar to the effects observed in the present work, the metal complexes induced morphological alterations such as a reduction or swelling of the cellular body and a shortening or loss of the flagellum [24]. The treatment of L. amazonensis with benzaldehyde thiosemicarbazone derived from limonene and complexed with copper, termed BenzCo, promoted morphological alterations in the parasite such as a reduction in the size of the parasites besides alterations in the ultrastructure [22]. Ternary nickel(II) complexes with a triazolopyrimidine derivative and different aliphatic or aromatic amines as auxiliary ligands have a potent antiproliferative effect against promastigotes and amastigotes of L. infantum and L. braziliensis, besides promoting ultrastructural alterations such as intense cytoplasmic vacuolization and alterations in the membranes forming the internal organelles [23]. The treatment of L. amazonensis promastigotes with biogenic silver nanoparticles (AgNp-bio), obtained by reducing silver nitrate using the nitrate reductase enzyme from Fusarium oxysporium, presented leishmanicidal activity at doses of 0.25 μg/mL in a time-dependent manner; furthermore, the treatment of infected peritoneal macrophages with the same concentration was also able to reduce both the number of infected macrophages and the number of intracellular amastigotes [25]. In another study, Alti and collaborators [26] showed a potent anti-L. donovani activity of gold–silver bimetallic nanoparticles (Au–Ag BNPs) synthesized through reduction with medicinal plant extracts and that presented IC50 values of 0.035 μg/mL. Similarly, gold and silver nanoparticles functionalized with 4′,7-dihydroxyflavone exhibited a promising activity against promastigote and amastigote forms of L. donovani [27]. Albalawi and collaborators [28] showed that copper nanoparticles (CuNPs) green-synthesized from Capparis spinosa’s fruit extract or combined with meglumine antimoniate (MA) suppressed the growth rate of L. major amastigotes in a typically dose-dependent mode. The IC50 values were 116.8 ± 3.05 and 21.3 ± 0.42 μg/mL for the CuNPs alone and CuNPs along with MA, respectively. Overall, these results indicate that coordination compounds can be considered potent and selective molecules against Leishmania.
3.3. Effects of Coordination Compounds on Leishmania’s General Metabolism and Mitochondrial Activity
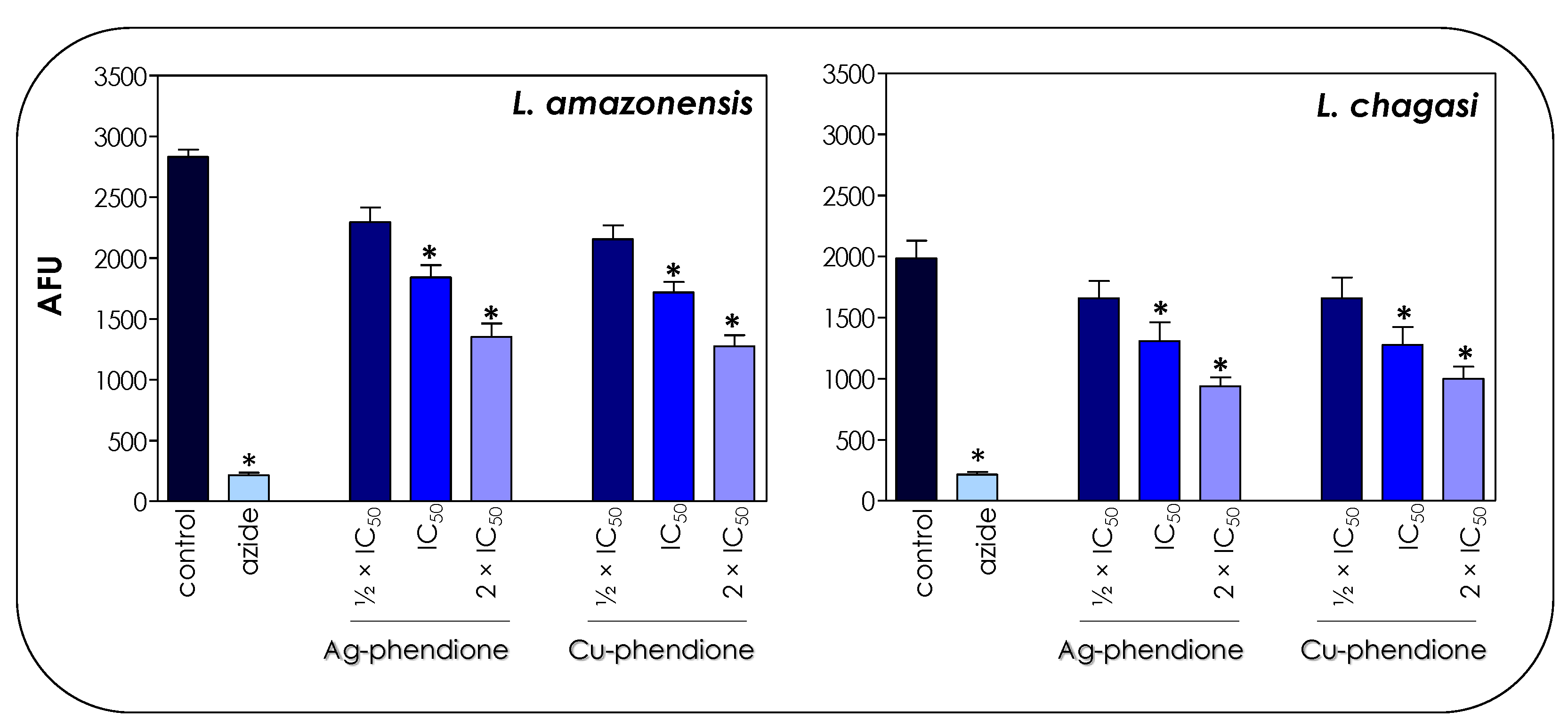
The toxicity of the coordination compounds on promastigote forms was tested by the colorimetric assay using resazurin dye/Alamar blue. Resazurin is a redox potential indicator dye, nontoxic for cells even during long incubation times and that has been used for the determination of cell viability [21]. In living cells, resazurin is reduced to the colorimetric resorufin dye, thereby changing its color from blue to red, while nonviable cells do not produce fluorescent signals [20]. These indicators have been used in different studies to assess the drug sensitivity of Leishmania promastigotes [20,29]. Herein, the results showed that both test compounds affected the global metabolism of both L. amazonensis and L. chagasi in a typically dose-dependent manner (Figure 3), displaying a statistically significant reduction when the treatment was performed at the concentrations corresponding to IC50 and 2 × IC50 values of both Ag-phendione and Cu-phendione. Parasites treated with sodium azide (40 μM) were used as a positive control of nonmetabolically active cells (Figure 3).
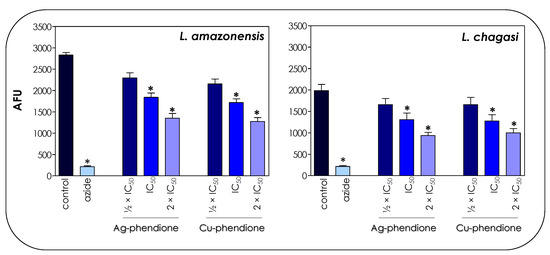
Figure 3.
Effects of coordination compounds on the general metabolism of L. amazonensis and L. chagasi promastigote forms. Parasites were incubated in the absence (control) and in the presence of Ag-phendione and Cu-phendione (½ × IC50, IC50 and 2 × IC50 values) for 72 h and, subsequently, incubated with resazurin. The reduction of resazurin was expressed as arbitrary fluorescence units (AFU). Parasites treated with sodium azide (40 µM) were used as a positive control of nonviable cells. The results were considered statistically significant when p < 0.05 (*).
In trypanosomatids, the mitochondrion corresponds to a single elongated organelle, which implies the necessity of its perfect functioning so that the protozoa can perform their vital functions [30,31]. On the other hand, the fact that mammalian cells present multiple mitochondria allows a mechanism of compensation in the presence of altered organelles. Thus, the mitochondrion of trypanosomatids acts as an attractive target for the development of new chemotherapeutics [30]. In this work, we verified the effects of the test compounds on both mitochondrial dehydrogenase activities (Figure 4) and mitochondrial membrane potential (Figure 5).
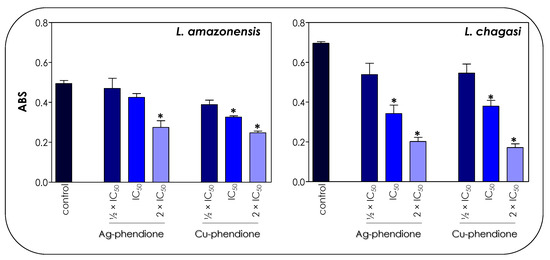
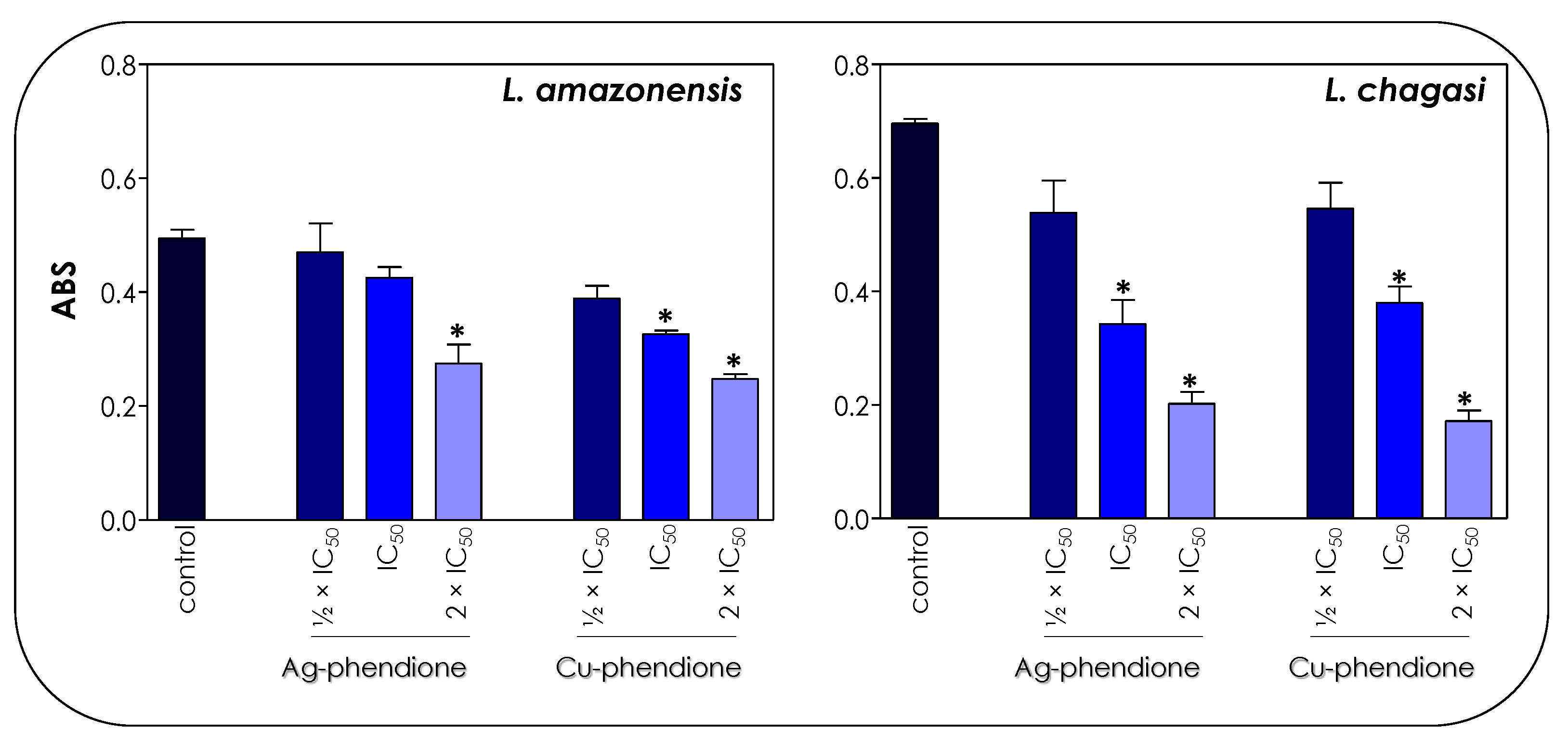
Figure 4.
Effects of coordination compounds on the mitochondrial metabolism of L. amazonensis and L. chagasi promastigotes. Parasites were incubated in the absence (control) and in the presence of the ½ × IC50, IC50 and 2 × IC50 values of Ag-phendione and Cu-phendione for 72 h and, subsequently, incubated with MTT for 4 h at 37 °C. Mitochondrial viability was determined by absorbance (ABS) quantification at 570 nm and the results were considered statistically significant when p < 0.05 (*).
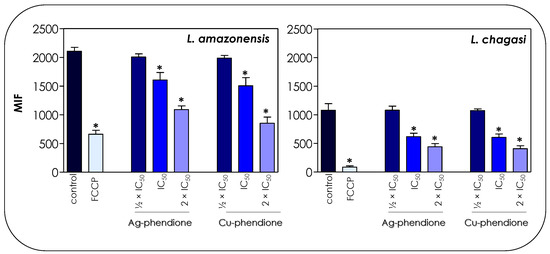
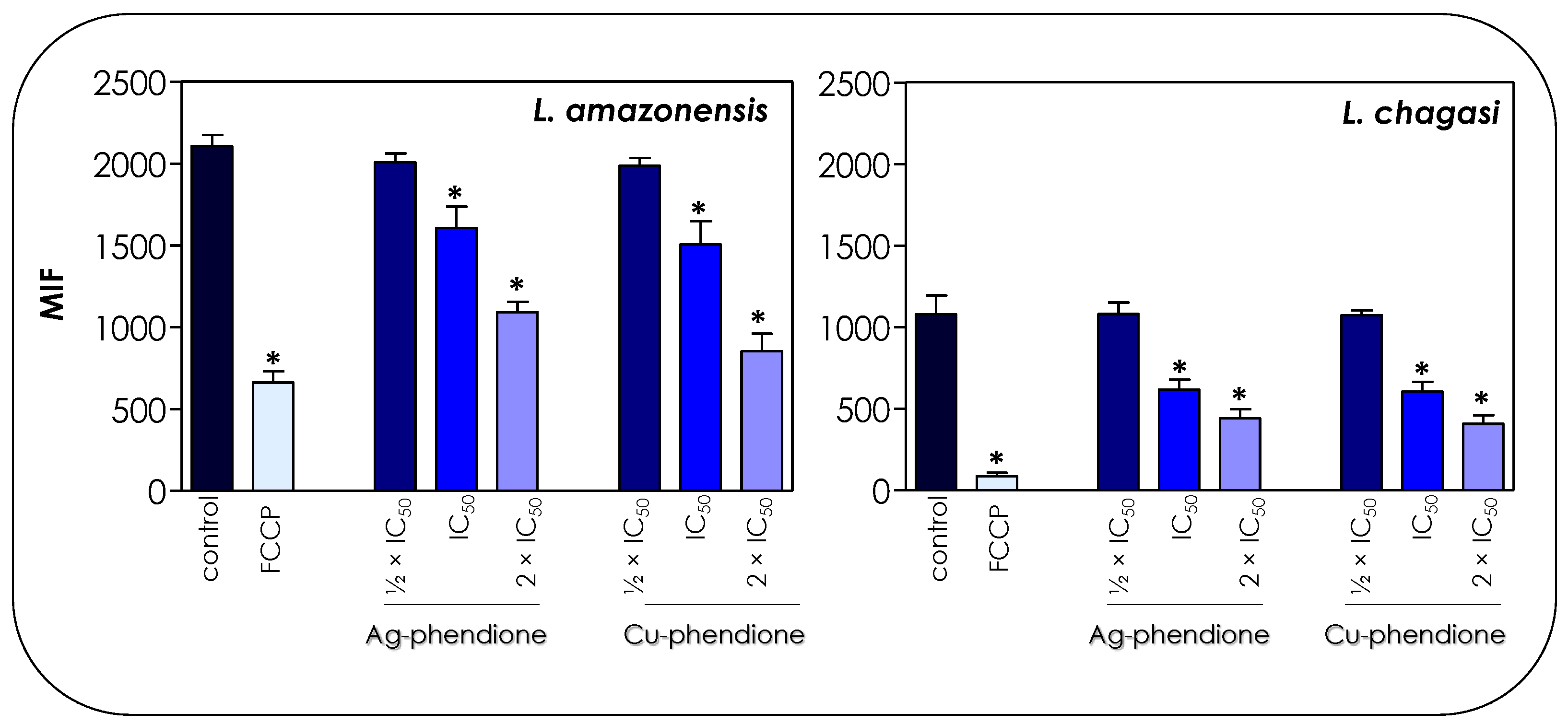
Figure 5.
Effects of coordination compounds on the mitochondrial membrane potential of L. amazonensis and L. chagasi promastigotes. Parasites were pretreated or not (control) for 72 h with the ½ × IC50, IC50 and 2 × IC50 values of Ag-phendione and Cu-phendione and incubated with Rhodamine 123 (10 μg/mL) for 20 min before analysis on a flow cytometer. FCCP was used as a membrane depolarization control. The data were expressed as the mean of the fluorescence intensity (MFI) and are representative of the analysis of 10,000 cells in experiments performed in triplicate. The results were considered statistically significant when p < 0.05 (*).
The treatment of parasite cells with Ag-phendione and Cu-phendione induced a reduction in mitochondrial dehydrogenase activities in a concentration-dependent manner (Figure 4). In this sense, IC50 and 2 × IC50 values of Cu-phendione caused a significant reduction in the enzymatic activity of mitochondrial dehydrogenases; the highest concentration led to approximately a 50 and 75% decay for L. amazonensis and L. chagasi, respectively, when compared to untreated promastigotes (Figure 4). The same range of inhibition was detected with Ag-phendione at 2 × IC50 values. The mitochondrial membrane potential is a key indicator of mitochondrial functionality and viability, because it reflects the process of electron transport and oxidative phosphorylation, the driving force behind ATP production [32]. In order to analyze the effect of the test coordination compounds on the mitochondrial membrane potential, parasite cells were labeled with Rhodamine 123 and analyzed in a flow cytometer. The results indicated that the treatment of L. amazonensis and L. chagasi with IC50 and 2 × IC50 values of either Ag-phendione or Cu-phendione significantly diminished the mitochondrial membrane potential when compared to the untreated control, indicating a mitochondrial membrane depolarization (Figure 5). Contrarily, no significant difference was observed when the parasites were treated with ½ × IC50 values of both test compounds. Parasites incubated with FCCP, a mitochondrial protonophore, were used as a positive control of mitochondrial membrane depolarization (Figure 5).
A similar result was found when promastigotes of L. amazonensis were treated with BenzCo and then labeled with Rhodamine 123 [22]. Similarly, the treatment of promastigotes of L. amazonensis with AgNp-bio also entailed the loss of the mitochondrial membrane potential of these parasites [25]. Gélvez and collaborators [33] verified through transmission electron microscopy that the treatment of L. amazonensis with nanocomposites containing silver nanoparticles (AgNPs), polyvinylpyrrolidone and MA promoted significant alterations in different organelles including myelin-like structure formation inside the mitochondrion.
The difference in susceptibility between L. amazonensis and L. chagasi to the test compounds may be a phenotype associated with the intrinsic characteristics of the Leishmania species [34]. Owing to the enormous genetic variability and plasticity of the genome in the Leishmania genus, alterations in different metabolic pathways are frequent and are related to varied responses to the same drug as well as therapeutic failures [35]. In this sense, the differences observed in cell metabolism and mitochondrial activity may be related to the particular characteristics of each Leishmania species, which will help in a greater or lesser response to different environmental changes.
Previous studies have shown that compounds complexed with copper have anti-trypanosomatid activity. Mixed-chelate copper(II) complexes Casiopeins® exhibited a potent anti-T. cruzi activity, similar to the reference drug Nifurtimox® [36]. Another study showed that the dinuclear copper(II) complexes using the triazolopyrimidine derivative 7-amino-1,2,4-triazolo [1,5-a]pyrimidine (7atp) showed a potent activity against Leishmania spp. and T. cruzi [37]. Copper nanoparticles exert different mechanisms on eukaryotic cells, such as oxidative stress, coordination effects and nonhomeostasis. Nanoparticles can cross a cell membrane and, inside the cell, can directly interact with oxidative organelles such as mitochondria, inducing reactive oxygen species (ROS) production, which can promote DNA strand breaks. Furthermore, Cu2+ ions have the ability to promote functional protein inactivation, due to their ability to move metal ions in specific metalloproteins or form chelates with biomolecules [38,39]. A series of mixed Cu(II)-phen complexes showed anticancer properties, inducing cell death mainly through a caspase-regulated apoptotic pathway leading to ROS production, the depolarization of the mitochondrial membrane, the overexpression of many apoptotic signalers and DNA cellular damage, triggering cell death [40]. Previous studies have reported that AgNPs can affect mitochondrial functioning as well as cell nucleus integrity [41]. The majority of the energy required for proper cellular function is provided by the mitochondrion, and damage to this organelle results in decreased or inefficient energy production, which may lead to cell death [41,42]. Furthermore, AgNPs can interact with membrane mitochondrial proteins and trigger a series of biological effects including mitochondrial dysfunction, oxidative stress, the interruption of ATP synthesis, DNA damage and consequent cell death by apoptosis or necrosis [41,42].
3.4. Effects of Coordination Compounds on Leishmania’s Cell Cycle
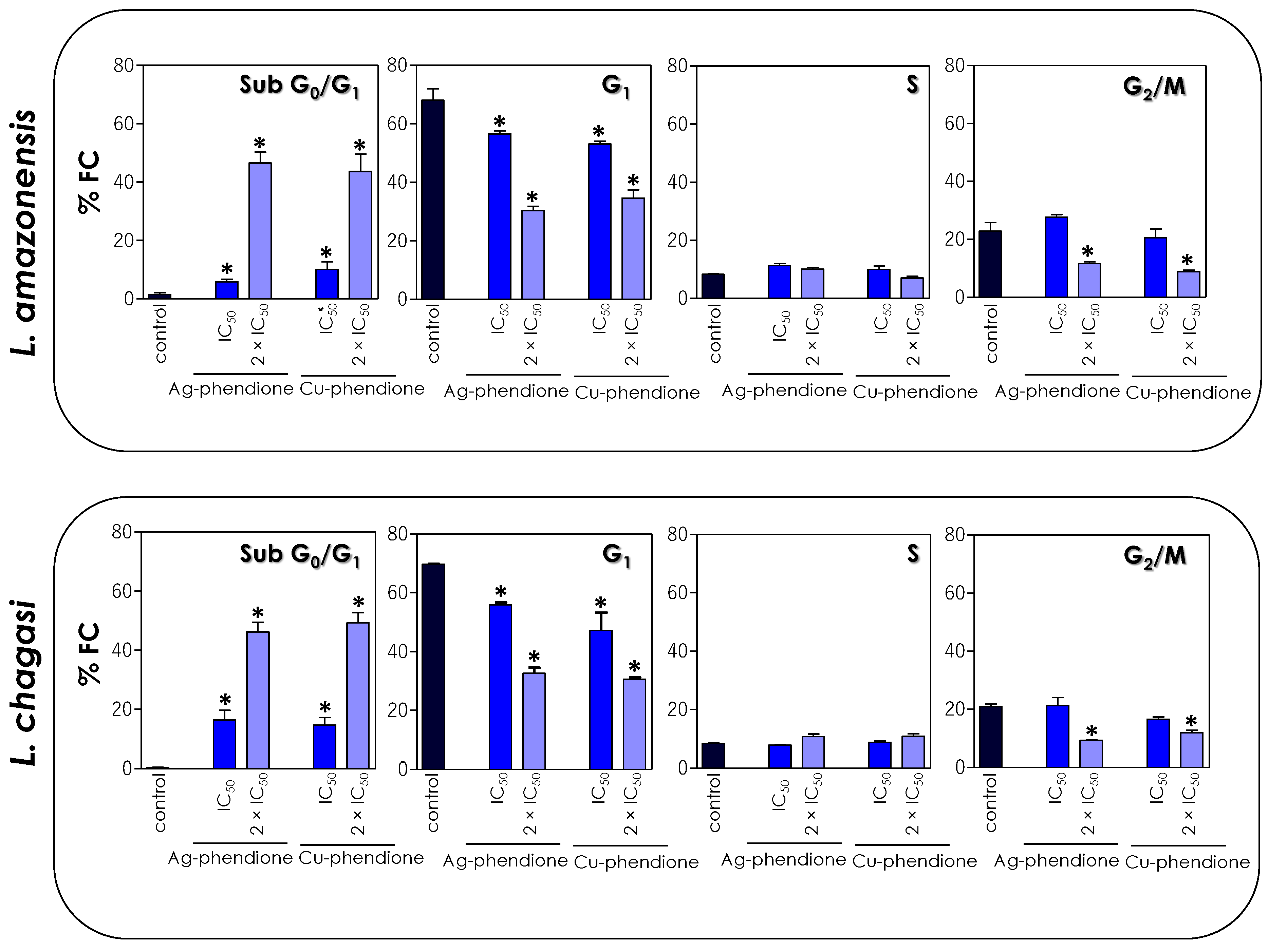
Since the test compounds drastically affected the mitochondrial functionality of the parasites, in this set of experiments, we decided to verify the effect of coordination compounds on the cell cycle progression of L. amazonensis and L. chagasi through flow cytometry. The results showed that the treatment with the coordination compounds induced an increase in the percentage of cells in the sub-G0/G1 phase of the cell cycle in a concentration-dependent manner, followed by a reduction in cells in the G0/G1 phase, when compared to untreated parasites (Figure 6). The S phase did not show any significant change in the percentage of cells, while a significant reduction in the number of cells was observed in the G2/M phase after treatment with the 2 × IC50 value (Figure 6).
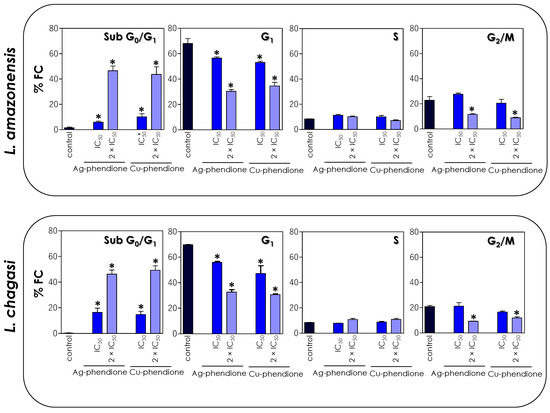
Figure 6.
Effects of coordination compounds on the cell cycle arrest of L. amazonensis and L. chagasi promastigotes. Parasites were incubated in the absence (control) and in the presence of the IC50 and 2 × IC50 values of Ag-phendione and Cu-phendione for 72 h and, subsequently, stained with PI and analyzed by flow cytometry. The graphs show the percentage of fluorescent cells (% FC) in the different phases of the cell cycle (sub-G0/G1, G1, S and G2/M). The data are representative of the analysis of 10,000 cells in experiments performed in triplicate, and the results were considered significant when p < 0.05 (*).
Compounds synthesized from transition metals have already been associated with cell cycle disruption in Leishmania. The treatment of L. donovani with copper salisylaldoxime (CuSAL) promoted an accumulation of parasite cells in the G1 phase of the cell cycle, with a consequent inhibition of the entry of parasites in the S phase [43]. In another study, the treatment of L. donovani with green-synthesized AgNPs also promoted cell cycle arrest in the sub-G0/G1 phase and a simultaneous decrease in both S and G2/M phases [44]. Similarly, in the present study, we verified that the treatment of L. amazonensis and L. chagasi promastigotes also promoted cell cycle arrest, inducing a significant increase in the proportion of cells in the sub-G0/G1 phase, followed by the interruption of the G1, S and G2/M phases, with a consequent inhibition of parasite proliferation. The accumulation of cells in the sub-G0/G1 phase of the cell cycle is a strong indication of the presence of cells with fragmented DNA, characteristic of a cell death process similar to apoptosis.
3.5. Effects of Coordination Compounds on Leishmania’s DNA Fragmentation
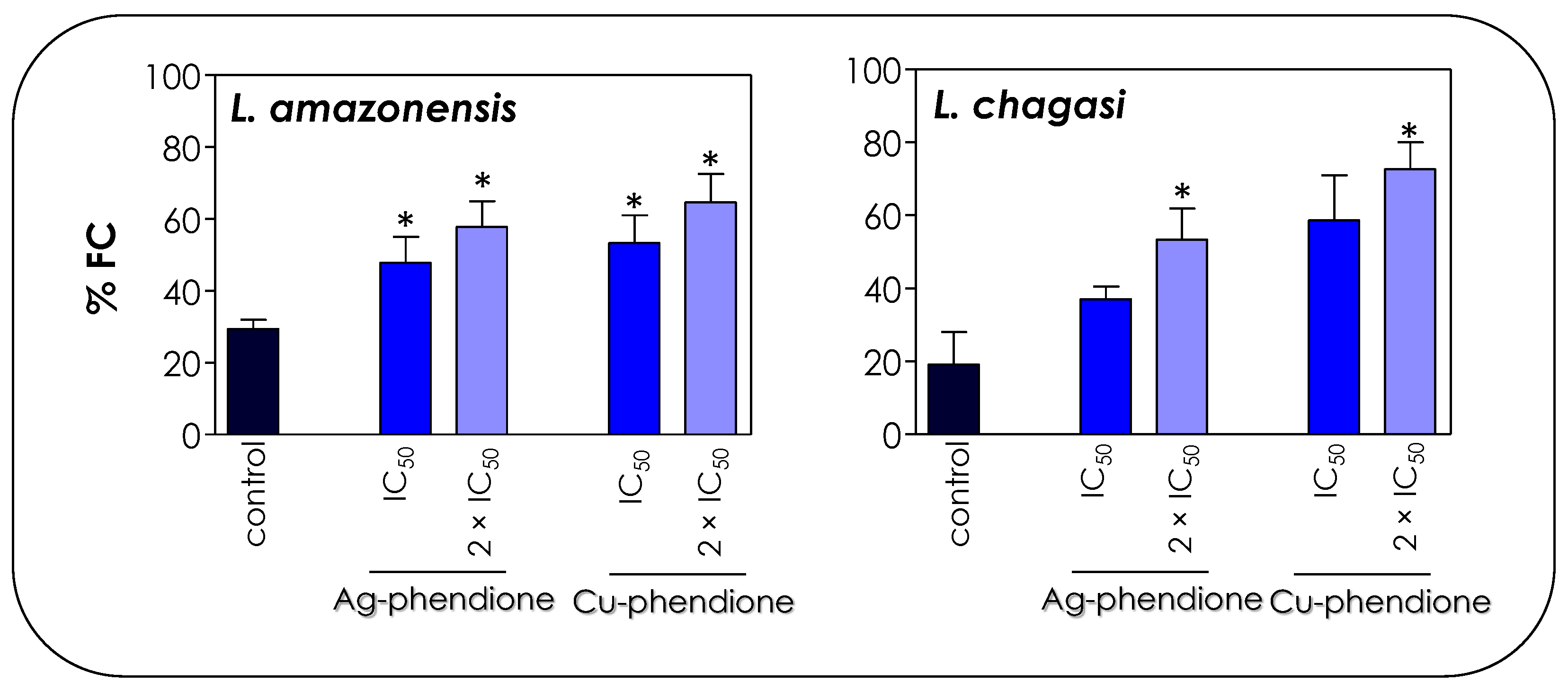
The cell cycle profile shown in Figure 6 is a strong marker for the apoptotic process, especially due to the accumulation of cells in the sub-G0/G1 phase that correspond to apoptotic bodies containing DNA fragments [45]. In dying cells, an endonuclease acts by cleaving the chromosomal DNA, causing the chromatin to fragment into multiple units [45]. In this sense, the TUNEL assay was performed in order to verify whether the compounds were able to induce DNA cleavage in Leishmania promastigotes. The treatment of promastigotes with IC50 and 2 × IC50 values of Ag-phendione and Cu-phendione during 72 h confirmed the DNA fragmentation through the increase in the incorporation of BrdU and a consequent increase in the % FC (Figure 7). Similarly, the treatment of L. donovani with copper salisylaldoxime (CuSAL) for 24 h was able to promote DNA fragmentation, indicating a process of cell death by apoptosis [43]. AgNPs also induced DNA fragmentation in L. donovani promastigotes, as verified by the TUNEL technique; however, gel electrophoresis showed that DNA breakage was not extensive, indicating the presence of high-molecular-weight DNA fragments of 700 bp [44]. Interestingly, the treatment of P. aeruginosa with bactericidal concentrations of Cu-phendione induced DNA fragmentation [46]. Ag-phendione and Cu-phendione were able to bind to double-stranded DNA via hydrogen bonding, hydrophobic and electrostatic interactions. In addition, Cu-phendione was able to induce topoisomerase I mediated DNA relaxation of supercoiled plasmid DNA, in addition to inducing oxidative DNA injuries [46].
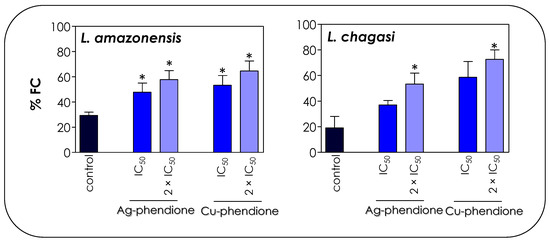
Figure 7.
Effects of coordination compounds on the DNA fragmentation of L. amazonensis and L. chagasi promastigotes. Parasites were incubated in the absence (control) and in the presence of the IC50 and 2 × IC50 values of Ag-phendione and Cu-phendione for 72 h and, subsequently, stained with dUTP-FITC in the presence of terminal deoxynucleotidyl transferase and RNase enzyme, followed by flow cytometry analysis. The graphs show the percentage of fluorescent cells (% FC). The data are representative of the analysis of 10,000 cells in experiments performed in triplicate, and the results were considered significant when p < 0.05 (*).
3.6. Effects of Coordination Compounds on Leishmania’s Phosphatidylserine Externalization
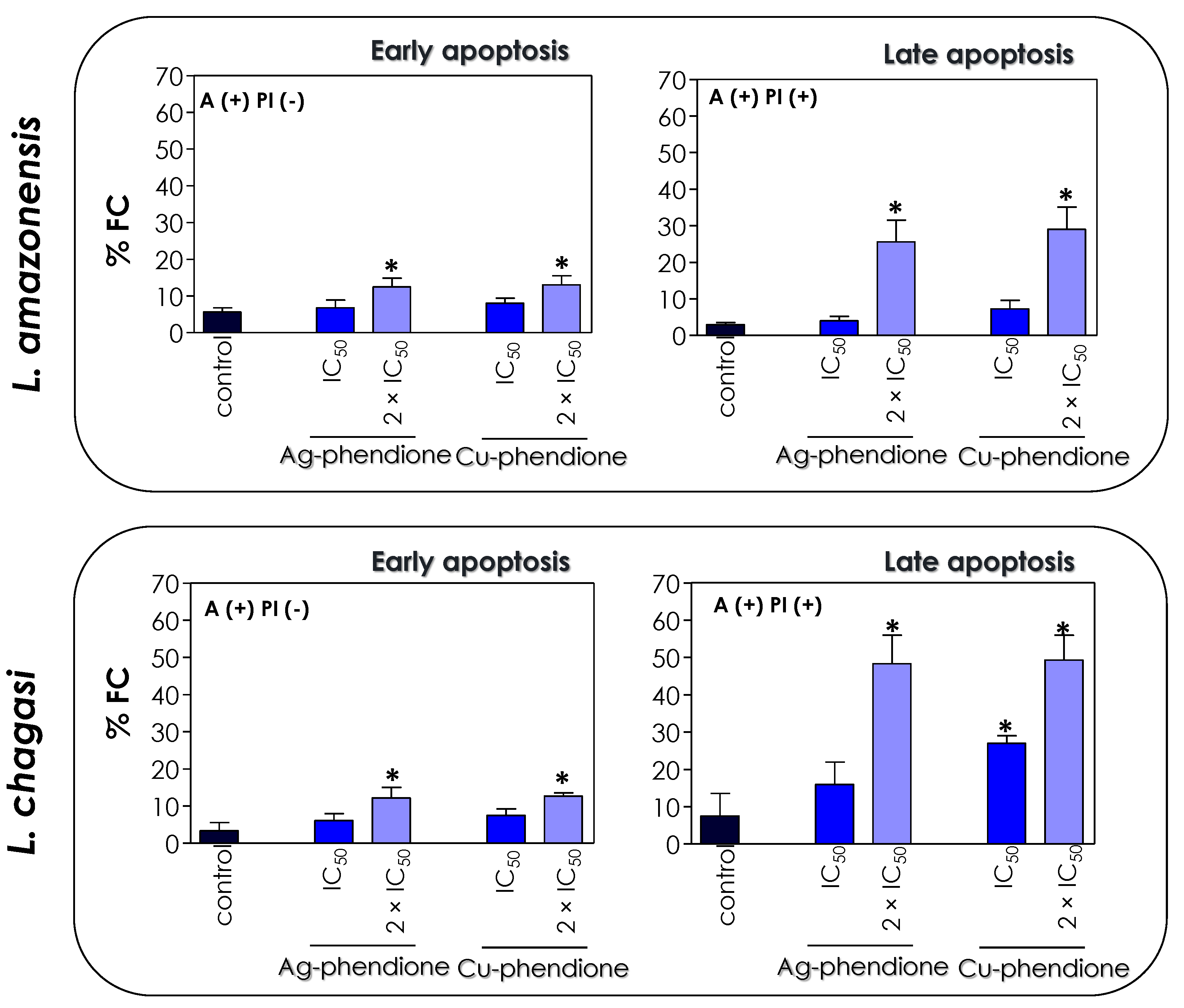
The induction of programmed cell death results in a series of biochemical events, such as an increase in the number of cells in the sub-G0/G1 phase of the cell cycle and the externalization of phosphatidylserine (PS) [41,42]. Since the treatment of L. amazonensis and L. chagasi with Ag-phendione and Cu-phendione induced the appearance of apoptotic characteristics, including a reduction in parasite size, a decrease in energy production, the arresting of cell cycle progression and DNA cleavage, we evaluated the externalization of PS in order to corroborate the hypothesis that the test coordination compounds act by inducing the apoptosis-like death pathway in Leishmania promastigotes. In eukaryotic cells, PS is present in the inner leaflet of the lipid bilayer and any change in this distribution causes a physiological event such as the clearance of apoptotic cells and, therefore, PS exposure has been implicated as an apoptotic marker [47]. Annexin V (AX-V) is a protein with affinity for PS, being used to evaluate cellular apoptotic processes. The translocation of PS from the inner to the outer side layer of the plasma membrane is a common phenomenon in eukaryotic cell apoptosis processes [47]. Although studies indicate that Leishmania does not have any detectable amounts of PS, other classes of phospholipids that bind to AX-V are present, such as phosphatidic acid, phosphatidylethanolamine; phosphatidylglycerol, phosphatidylinositol and cardiolipin [48]. Since AX-V can bind to both apoptotic and necrotic cells, due to the loss of cell membrane integrity, the simultaneous addition of PI, a nonpermeable dye that selectively binds to nucleic acids, allows one to differentiate between apoptotic cells [AX-V (+)/PI (−)] and late apoptotic/necrotic cells [AX-V (+)/PI (+)] [49].
In this work, we verified that the treatment of L. amazonensis and L. chagasi with the IC50 and 2 × IC50 values of Ag-phendione and Cu-phendione promoted an increase in the percentage of positive cells only for AX-V, as well as when doubly labeled with AX-V (+)/PI (+). In the presence of 2 × IC50 values of the coordination compounds, about 12.5% of the cells of both species of Leishmania were AX-V (+)/PI (−), indicating early stages of apoptotic events, and approximately 27% and 48% of L. amazonensis and L. chagasi promastigotes presented the AX-V (+)/PI (+) phenotype, respectively, characterizing events of late apoptosis and/or necrosis (Figure 8). Additionally, the treatment of L. chagasi with the IC50 value of Cu-phendione promoted a double labeling of 27% of the population. Previous studies showed that L. amazonensis treated with AgNp-bio also stimulated the PS exposure, as well as the loss of cell membrane integrity, as verified by double labeling with AX-V and PI [25]. Zahir and collaborators [44] showed that L. donovani promastigotes treated with Ag NPs for 24 h were positive for AX-V and PI or only for PI, suggesting that AgNPs induce cell death by necrosis. Similarly, an increase in the number of AX-V-positive cells was also observed after the treatment of L. major with green-synthesized AgNPs via ginger rhizome extract, indicating the induction of cell death via apoptosis [50].
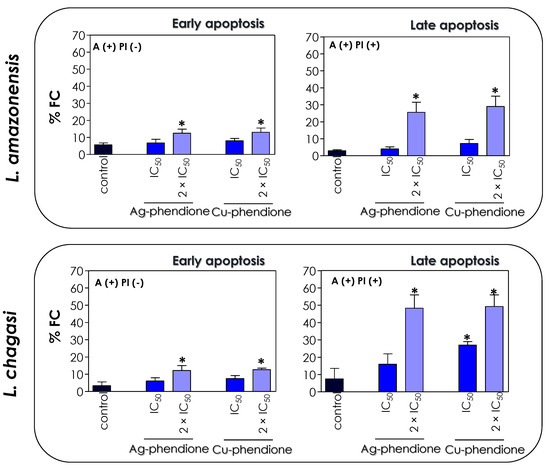
Figure 8.
Effects of coordination compounds on the expression of apoptosis-associated markers in L. amazonensis and L. chagasi promastigotes. Parasites were incubated in the absence (control) and in the presence of the IC50 and 2 × IC50 values of Ag-phendione and Cu-phendione for 72 h and subsequently co-stained with PI and annexin V–Alexa Fluor 488 and analyzed by flow cytometry. The graphs show the percentage of fluorescent cells (% FC) for annexin V [A(+) PI(−)] and for annexin V and PI [A(+) PI (+)]. The data are representative of the analysis of 10,000 cells in experiments performed in triplicate, and the results were considered significant when p < 0.05 (*).
The effect of metal-based compounds on the activation of caspase-like proteins as well as on the inhibition of antioxidant agents has also been studied as triggers of programmed cell death. The presence of caspase-like proteases, such as the CED-3/CPP32 group of proteases (caspase 3) and the ICE family of proteases (caspase 9), are well documented and play fundamental roles in the apoptotic cascade of Leishmania [51,52]. Compounds derived from silver and copper have already been reported in the activation of these proteases and their consequent action in triggering the process of cell death by apoptosis. The treatment of L. donovani with CuSAL activated the ICE family of proteases and the CED3/CPP32 group of proteases; in parallel, when in the presence of a specific inhibitor of these proteases, events derived from the caspase activation, such as DNA fragmentation, were prevented, suggesting that the activation of caspase-like proteases was involved in the CuSAL induced programmed cell death of Leishmania parasites [43]. Antioxidant molecules, such as trypanothione reductase (TR), participate in antioxidant reactions such as thiol–disulfide exchange, in addition to acting as electron donors in several metabolic pathways, being essential in maintaining the reduced environment and thus the survival of the parasite within the cell [44,53]. Silver ions are excellent inducers of ROS production in Leishmania as well as an effective TR inhibitor, leading to a process of cell death by apoptosis [44,53]. This analysis was corroborated by the treatment of L. donovani with green-synthesized AgNPs, which led to an increase in ROS production followed by a potent reduction in the total intracellular thiols, being accompanied by an increase in the G0/G1 phase of the cell cycle and DNA fragmentation, characteristic markers of the apoptotic death process [44].
The loss of mitochondrial membrane potential, DNA fragmentation, cell cycle arrest and PS externalization suggest that both tested coordinated compounds, Ag-phendione and Cu-phendione, induce cell death via an apoptosis-like mechanism [54]. However, new studies in order to evaluate other markers, such as cytochrome c release, endonuclease g, metacaspase and calpains [54], are needed in order to more accurately evaluate the mechanism of action of these promising compounds.
3.7. Effects of Coordination Compounds on THP-1 Macrophage Cells
Initially, the toxicity of Ag-phendione and Cu-phendione to THP-1 macrophages was assessed by MTT. The CC50 value calculated for Ag-phendione was 1870 nM and for Cu-phendione, it was 1470 nM after 24 h of treatment (Table 2). Dilutions of DMSO corresponding to the highest concentration of each compound had no effect on macrophage viability (data not shown). Based on the calculated CC50 values, coordination compounds showed an excellent selectivity index (SI) against the promastigote forms of both species of Leishmania. Ag-phendione and Cu-phendione presented an SI of 239.7 and 196.0, respectively, for L. amazonensis; in addition, for L. chagasi, the calculated values were 76.3 for Ag-phendione and 73.5 for Cu-phendione. The promising efficacy of the compounds against the promastigote forms of Leishmania stimulated the study of their effects on the amastigote forms of the parasites.

Table 2.
Cytotoxicity effects of Ag-phendione and Cu-phendione against THP-1 macrophages and anti-amastigote activity.
3.8. Effects of Coordination Compounds on Leishmania–Macrophage Interaction
The establishment and maintenance of the infection in the vertebrate host is a fundamental step for the development of the disease, and as such, the Leishmania–macrophage interaction is crucial in this process. In this work, we found that the coordination compounds were able to drastically reduce the survival of intracellular amastigotes without presenting a toxic effect to macrophages. THP-1 macrophages previously infected with L. amazonensis or L. chagasi and, subsequently, treated with the coordination compounds showed a significant reduction in the association index when compared to their respective untreated systems (Table 2). The results showed that both coordination compounds significantly suppressed the growth rate of intracellular amastigotes of L. amazonensis and L. chagasi. The obtained IC50 values for L. amazonensis were 43 nM for Ag-phendione and 35 nM for Cu-phendione, while L. chagasi showed IC50 values of 88 and 51 nM for Ag-phendione and Cu-phendione, respectively. Under these employed conditions, both Ag-phendione and Cu-phendione showed a higher toxicity to Leishmania amastigotes compared to THP-1 macrophage cells, resulting in excellent SI values (Table 2). Albalawi and collaborators [28] verified that green-synthesized CuNPs alone or combined with MA presented SI values of 11.34 and 18.60, respectively, against intramacrophage amastigote forms of L. major [28]. Green-synthesized AgNPs showed a low toxicity against J774A.1 macrophages with a CC50 of 115.5 µg/mL, while for intracellular amastigotes of L donovani, an IC50 value equivalent to 3.89 µg/mL was detected [44]. In another study, AgNp-bio did not present cytotoxicity on peritoneal macrophages at concentrations of 0.125, 0.25 and 0.50 μg/mL, indicating a good tolerability against macrophages infected with L. amazonensis [25]. Toxicity studies using RAW 264.7 macrophages revealed that a treatment with CuSAL at 30 μM for 8 h and 12 h reduced the number of intracellular amastigotes by 97% and 100%, respectively, without presenting any toxicity to the host cell [43].
Previously, our group found that the pretreatment of L. braziliensis promastigotes for 1 h with Ag-phendione and Cu-phendione induced a decrease in the association index with macrophages by 51.4% and 44.4%, respectively [17]. Vargas Rigo and collaborators [12] evaluated that Ag-phendione and Cu-phendione showed excellent selectivity for T. vaginalis compared to HMVII vaginal epithelial cells, erythrocytes and nontumor cell line 3T3-C1 and that Cu-phendione was the compound best tolerated in vitro by host cells, indicating the selectivity for the parasite and the safety of the complex. Previous studies indicated that Cu-phendione and Ag-phendione promoted a protective action on lung epithelial cells (A549) previously incubated with a P. aeruginosa supernatant, which is rich in elastase B (LasB), an important virulence factor of this bacterium. In addition, Cu-phendione was able to reduce the toxic effects of LasB in the G. mellonella model [55]. McCann and coworkers [6] had already verified that these compounds presented an excellent tolerability against different cell lineages. Studies conducted in vivo using the larvae of the insect G. mellonella indicated that treatment with up to 33.3 mg/kg of these metal-based compounds ensured 100% of survival of the larvae. In addition, studies using Swiss mice indicated that treatment with a concentration of up to 45 mg/kg/day ensured the survival of mice after 7 days of treatment. Moreover, blood samples taken from Swiss mice revealed that treatment with Ag-phendione and Cu-phendione did not affect the levels of two hepatic enzymes, aspartate aminotransferase and alanine aminotransferase, reinforcing the selective action of both coordination compounds against Leishmania parasites.
The mechanism of action induced by these test coordination compounds in trypanosomatids is still unknown. In this work, we proposed the mitochondrion as one of the major targets, as well as an arrest in the cell cycle, PS exposure and DNA fragmentation, followed by a cell death via apoptosis. Previous studies by our group [17] showed that these compounds also acted on the gp63 metalloprotease (an important virulence factor) of Leishmania promastigotes, an essential molecule in the parasite internalization process. The reduction in the expression of these molecules promoted by the treatment of promastigotes with Ag-phendione and Cu-phendione may be directly related to the reduction in the number of intramacrophagic amastigotes. Furthermore, the loss of mitochondrial viability as well as the expression of apoptotic/necrotic characteristics after treatment of promastigotes may be acting together to reduce the infection. Therefore, further studies are needed to determine the mechanisms responsible for the reduction of intracellular amastigotes.
4. Conclusions
Drugs currently available for the treatment of leishmaniasis have serious side effects and are often ineffective; in addition, an increasing number of cases of resistance has been reported [3,4]. The beneficial picture of phendione-based compounds [6,7,8] together with the need to develop new chemotherapeutic options for the prevention and/or treatment of infections caused by Leishmania led us to broaden our studies in an attempt to decipher the mechanisms of action of Ag-phendione and Cu-phendione against L. amazonensis and L. chagasi, which are etiological agents of cutaneous and visceral leishmaniasis, respectively.
In conclusion, Ag-phendione and Cu-phendione show a high potency in inhibiting the growth of promastigote forms of L. amazonensis and L. chagasi, in addition to altering the morphology of these parasites. The findings also indicated that the antileishmanial mechanisms of Ag-phendione and Cu-phendione point to an apoptosis-like cell death process due to the changes in mitochondrial viability/membrane potential, cell cycle arrest in sub-G0/G1 phase, DNA damage and annexin-V staining. Furthermore, the coordination compounds also showed a potent effect against the amastigote forms of both Leishmania species, with reduced toxicity to the host cell (presenting excellent selectivity indexes). Previous studies from our group have shown that these metal-based drugs have excellent tolerability to different cell lines as well as in in vivo animal models [6], confirming that they stand out as a new therapeutic option for the treatment of cutaneous and visceral leishmaniasis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens12010070/s1, Figure S1: Leishmania amazonensis (A) and Leishmania chagasi (B) proliferation profile.
Author Contributions
All authors conceived and designed the experiments. S.S.C.O. and V.S.S. performed the experiments. All authors analyzed the data. M.H.B., M.D., M.M. and A.L.S.S. contributed materials/reagents/analysis tools. All authors contributed to the research and approved the final version of the manuscript. All authors wrote and revised the paper. All authors agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; financial code—001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Denise Rocha de Souza, who is supported by a FAPERJ scholarship, for her technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodrigues, J.C.; Godinho, J.L.; de Souza, W. Biology of human pathogenic trypanosomatids: Epidemiology, lifecycle and ultrastructure. Subcell Biochem. 2014, 74, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Mohapatra, S. Drug resistance in leishmaniasis: Newer developments. Trop Parasitol. 2014, 4, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J. Leishmaniasis: An update of current pharmacotherapy. Expert. Opin. Pharmacother. 2013, 14, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.H.; Orvig, C. Boon and bane of metal ions in medicine. Science 2003, 300, 936–939. [Google Scholar] [CrossRef]
- McCann, M.; Santos, A.L.S.; Silva, B.A.; Romanos, M.T.V.; Pyrrho, A.S.; Devereux, M.; Kavanagh, K.; Fichtner, I.; Kellett, A. In vitro and in vivo studies into the biological activities of 1,10-phenanthroline, 1,10-phenanthroline-5,6-dione and its copper(II) and silver(I) complexes. Toxicol. Res. 2012, 1, 47–54. [Google Scholar] [CrossRef]
- Lima, A.K.; Elias, C.G.; Souza, J.E.; Santos, A.L.S.; Dutra, P.M. Dissimilar peptidase production by avirulent and virulent promastigotes of Leishmania braziliensis: Inference on the parasite proliferation and interaction with macrophages. Parasitology 2009, 136, 1179–1191. [Google Scholar] [CrossRef]
- Santos, A.L.S.; Sodre, C.L.; Valle, R.S.; Silva, B.A.; Abi-Chacra, E.A.; Silva, L.V.; Souza-Goncalves, A.L.; Sangenito, L.S.; Goncalves, D.S.; Souza, L.O.; et al. Antimicrobial action of chelating agents: Repercussions on the microorganism development, virulence and pathogenesis. Curr. Med. Chem. 2012, 19, 2715–2737. [Google Scholar] [CrossRef]
- Viganor, L.; Galdino, A.C.; Nunes, A.P.; Santos, K.R.; Branquinha, M.H.; Devereux, M.; Kellett, A.; McCann, M.; Santos, A.L.S. Anti-Pseudomonas aeruginosa activity of 1,10-phenanthroline-based drugs against both planktonic- and biofilm-growing cells. J. Antimicrob. Chemother. 2016, 71, 128–134. [Google Scholar] [CrossRef]
- McCann, M.; Kellett, A.; Kavanagh, K.; Devereux, M.; Santos, A.L.S. Deciphering the antimicrobial activity of phenanthroline chelators. Curr. Med. Chem. 2012, 19, 2703–2714. [Google Scholar] [CrossRef]
- Granato, M.Q.; Gonçalves, D.S.; Seabra, S.H.; McCann, M.; Devereux, M.; Santos, A.L.S.; Kneipp, L.F. 1,10-Phenanthroline-5,6-dione-based compounds are effective in disturbing crucial physiological events of Phialophora verrucosa. Front. Microbiol. 2017, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Vargas Rigo, G.; Petro-Silveira, B.; Devereux, M.; McCann, M.; Souza Dos Santos, A.L.S.; Tasca, T. Anti-Trichomonas vaginalis activity of 1,10-phenanthroline-5,6-dione-based metallodrugs and synergistic effect with metronidazole. Parasitology 2019, 146, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.F.; Galdino, A.C.; Viganor, L.; Schuenck, R.P.; Devereux, M.; McCann, M.; Santos, A.L.S.; Nunes, A. Antimicrobial action of 1,10-phenanthroline-based compounds on carbapenemase-producing Acinetobacter baumannii clinical strains: Efficacy against planktonic- and biofilm-growing cells. Braz. J. Microbiol. 2020, 51, 1703–1710. [Google Scholar] [CrossRef]
- Creaven, B.S.; Egan, D.A.; Karcz, D.; Kavanagh, K.; McCann, M.; Mahon, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterisation and antimicrobial activity of copper(II) and manganese(II) complexes of coumarin-6,7-dioxyacetic acid (cdoaH2) and 4-methylcoumarin-6,7-dioxyacetic acid (4-MecdoaH2): X-ray crystal structures of [Cu(cdoa)(phen)2].8.8H2O and [Cu(4-Mecdoa)(phen)2].13H2O (phen=1,10-phenanthroline). J. Inorg. Biochem. 2007, 101, 1108–1119. [Google Scholar] [CrossRef]
- Vianez Peregrino, I.; Ferreira Ventura, R.; Borghi, M.; Pinto Schuenck, R.; Devereux, M.; McCann, M.; Santos, A.L.S.; Ferreira Nunes, A.P. Antibacterial activity and carbapenem re-sensitizing ability of 1,10-phenanthroline-5,6-dione and its metal complexes against KPC-producing Klebsiella pneumoniae clinical strains. Lett. Appl. Microbiol. 2021, 73, 139–148. [Google Scholar] [CrossRef]
- Gandra, R.M.; Mc Carron, P.; Fernandes, M.F.; Ramos, L.S.; Mello, T.P.; Aor, A.C.; Branquinha, M.H.; McCann, M.; Devereux, M.; Santos, A.L.S. Antifungal potential of copper(II), manganese(II) and silver(I) 1,10-phenanthroline chelates against multidrug-resistant fungal species forming the Candida haemulonii complex: Impact on the planktonic and biofilm lifestyles. Front. Microbiol. 2017, 8, 1257. [Google Scholar] [CrossRef]
- Lima, A.; Elias, C.; Oliveira, S.; Santos-Mallet, J.R.; McCann, M.; Devereux, M.; Branquinha, M.H.; Dutra, P.; Santos, A.L.S. Anti-Leishmania braziliensis activity of 1,10-phenanthroline-5,6-dione and its Cu(II) and Ag(I) complexes. Parasitol. Res. 2021, 120, 3273–3285. [Google Scholar] [CrossRef]
- González, G.; Castillo, D.; Estevez, Y.; Grentzinger, T.; Deharo, E. Leishmania (Viannia) peruviana (MHOM/PE/LCA08): Comparison of THP-1 cell and murine macrophage susceptibility to axenic amastigotes for the screening of leishmanicidal compounds. Exp. Parasitol. 2009, 122, 353–356. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.; Coyle, B.; McKay, S.; McCormack, P.; Kavanagh, K.; Devereux, M.; McKee, V.; Kinsella, P.; O’Connor, R.; Clynes, M. Synthesis and X-ray crystal structure of [Ag(phendio)2]ClO4 (phendio = 1,10-phenanthroline-5,6-dione) and its effects on fungal and mammalian cells. Biometals 2004, 6, 635–645. [Google Scholar] [CrossRef]
- Mikus, J.; Steverding, D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol. Int. 2000, 48, 265–269. [Google Scholar] [CrossRef]
- Toté, K.; Vanden Berghe, D.; Levecque, S.; Bénéré, E.; Maes, L.; Cos, P. Evaluation of hydrogen peroxide-based disinfectants in a new resazurin microplate method for rapid efficacy testing of biocides. J. Appl. Microbiol. 2009, 107, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Britta, E.A.; Silva, A.P.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Silva, C.C.; Sernaglia, R.L.; Nakamura, C.V. Benzaldehyde thiosemicarbazone derived from limonene complexed with copper induced mitochondrial dysfunction in Leishmania amazonensis. PLoS ONE 2012, 7, e41440. [Google Scholar] [CrossRef][Green Version]
- Ramírez-Macías, I.; Maldonado, C.R.; Marín, C.; Olmo, F.; Gutiérrez-Sánchez, R.; Rosales, M.J.; Quirós, M.; Salas, J.M.; Sánchez-Moreno, M. In vitro anti-Leishmania evaluation of nickel complexes with a triazolopyrimidine derivative against Leishmania infantum and Leishmania braziliensis. J. Inorg. Biochem. 2012, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Sangenito, L.S.; Guedes, A.A.; Branquinha, M.H.; Kavanagh, K.; McGinley, J.; Santos, A.L.S.; Velasco-Torrijos, T. Glycosylated metal chelators as anti-parasitic agents with tunable selectivity. Dalton. Trans. 2017, 46, 5297–5307. [Google Scholar] [CrossRef] [PubMed]
- Fanti, J.R.; Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; Cataneo, A.; Andrade, C.; Panis, C.; Rodrigues, J.; Wowk, P.F.; Kuczera, D.; Costa, I.N.; et al. Biogenic silver nanoparticles inducing Leishmania amazonensis promastigote and amastigote death in vitro. Acta Trop. 2018, 178, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Alti, D.; Veeramohan Rao, M.; Rao, D.N.; Maurya, R.; Kalangi, S.K. Gold-silver bimetallic nanoparticles reduced with herbal leaf extracts induce ROS-mediated death in both promastigote and amastigote stages of Leishmania donovani. ACS Omega 2020, 5, 16238–16245. [Google Scholar] [CrossRef]
- Sasidharan, S.; Saudagar, P. Gold and silver nanoparticles functionalized with 4’,7-dihydroxyflavone exhibit activity against Leishmania donovani. Acta Trop. 2022, 231, 106448. [Google Scholar] [CrossRef]
- Albalawi, A.E.; Abdel-Shafy, S.; Khudair Khalaf, A.; Alanazi, A.D.; Baharvand, P.; Ebrahimi, K.; Mahmoudvand, H. Therapeutic potential of green synthesized copper nanoparticles alone or combined with meglumine antimoniate (Glucantime®) in cutaneous leishmaniasis. Nanomaterials 2021, 11, 891. [Google Scholar] [CrossRef]
- Zahid, M.; Johnson, M.M.; Tokarski, R.J.; Satoskar, A.R.; Fuchs, J.R.; Bachelder, E.M.; Ainslie, K.M. Evaluation of synergy between host and pathogen-directed therapies against intracellular Leishmania donovani. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 125–132. [Google Scholar] [CrossRef]
- Fidalgo, L.M.; Gille, L. Mitochondria and trypanosomatids: Targets and drugs. Pharm Res. 2011, 28, 2758–2770. [Google Scholar] [CrossRef]
- Menna-Barreto, R.F.; de Castro, S.L. The double-edged sword in pathogenic trypanosomatids: The pivotal role of mitochondria in oxidative stress and bioenergetics. Biomed. Res. Int. 2014, 2014, 614014. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Gélvez, A.; Diniz Junior, J.; Brígida, R.; Rodrigues, A. AgNP-PVP-meglumine antimoniate nanocomposite reduces Leishmania amazonensis infection in macrophages. BMC Microbiol. 2021, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Fernández, O.L.; Diaz-Toro, Y.; Ovalle, C.; Valderrama, L.; Muvdi, S.; Rodríguez, I.; Gomez, M.A.; Saravia, N.G. Miltefosine and antimonial drug susceptibility of Leishmania Viannia species and populations in regions of high transmission in Colombia. PLoS Negl. Trop. Dis. 2014, 8, e2871. [Google Scholar] [CrossRef] [PubMed]
- Santi, A.M.M.; Murta, S.M.F. Impact of genetic diversity and genome plasticity of Leishmania spp. in treatment and the search for novel chemotherapeutic targets. Front. Cell. Infect. Microbiol. 2022, 12, 826287. [Google Scholar] [CrossRef]
- Becco, L.; Rodríguez, A.; Bravo, M.E.; Prieto, M.J.; Ruiz-Azuara, L.; Garat, B.; Moreno, V.; Gambino, D. New achievements on biological aspects of copper complexes Casiopeínas®: Interaction with DNA and proteins and anti-Trypanosoma cruzi activity. J Inorg. Biochem. 2012, 109, 49–56. [Google Scholar] [CrossRef]
- Méndez-Arriaga, J.M.; Oyarzabal, I.; Escolano, G.; Rodríguez-Diéguez, A.; Sánchez-Moreno, M.; Salas, J.M. In vitro leishmanicidal and trypanocidal evaluation and magnetic properties of 7-amino-1,2,4-triazolo[1,5-a]pyrimidine Cu(II) complexes. J. Inorg. Biochem. 2018, 180, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
- Masuri, S.; Vaňhara, P.; Cabiddu, M.G.; Moráň, L.; Havel, J.; Cadoni, E.; Pivetta, T. Copper(II) phenanthroline-based complexes as potential anticancer drugs: A walkthrough on the mechanisms of action. Molecules 2021, 27, 49. [Google Scholar] [CrossRef]
- Riaz Ahmed, K.B.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. In Vitro. 2017, 38, 179–192. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Bhaumik, S.K.; Karmakar, S.; Paul, J.; Sawoo, S.; Majumder, H.K.; Roy, A. Copper salisylaldoxime (CuSAL) imparts protective efficacy against visceral leishmaniasis by targeting Leishmania donovani topoisomerase IB. Exp. Parasitol. 2017, 175, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Zahir, A.A.; Chauhan, I.S.; Bagavan, A.; Kamaraj, C.; Elango, G.; Shankar, J.; Arjaria, N.; Roopan, S.M.; Rahuman, A.A.; Singh, N. Green synthesis of silver and titanium dioxide nanoparticles using Euphorbia prostrata extract shows shift from apoptosis to G0/G1 arrest followed by necrotic cell death in Leishmania donovani. Antimicrob. Agents Chemother. 2015, 59, 4782–4799. [Google Scholar] [CrossRef] [PubMed]
- Matassov, D.; Kagan, T.; Leblanc, J.; Sikorska, M.; Zakeri, Z. Measurement of apoptosis by DNA fragmentation. Methods Mol. Biol. 2004, 282, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Galdino, A.; Viganor, L.; Pereira, M.M.; Devereux, M.; McCann, M.; Branquinha, M.H.; Molphy, Z.; O’Carroll, S.; Bain, C.; Menounou, G.; et al. Copper(II) and silver(I)-1,10-phenanthroline-5,6-dione complexes interact with double-stranded DNA: Further evidence of their apparent multi-modal activity towards Pseudomonas aeruginosa. J. Biol. Inorg. Chem. 2022, 27, 201–213. [Google Scholar] [CrossRef]
- Koonin, E.V.; Aravind, L. Origin and evolution of eukaryotic apoptosis: The bacterial connection. Cell Death Differ. 2002, 9, 394–404. [Google Scholar] [CrossRef]
- Weingärtner, A.; Kemmer, G.; Müller, F.D.; Zampieri, R.A.; Gonzaga dos Santos, M.; Schiller, J.; Pomorski, T.G. Leishmania promastigotes lack phosphatidylserine but bind annexin V upon permeabilization or miltefosine treatment. PLoS ONE 2012, 7, e42070. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Mohammadi, M.; Zaki, L.; KarimiPourSaryazdi, A.; Tavakoli, P.; Tavajjohi, A.; Poursalehi, R.; Delavari, H.; Ghaffarifar, F. Efficacy of green synthesized silver nanoparticles via ginger rhizome extract against Leishmania major in vitro. PLoS ONE 2021, 16, e0255571. [Google Scholar] [CrossRef]
- Das, M.; Mukherjee, S.B.; Shaha, C. Hydrogen peroxide induces apoptosis-like death in Leishmania donovani promastigotes. J. Cell Sci. 2001, 114, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Bertholet, S.; Debrabant, A.; Muller, J.; Duncan, R.; Nakhasi, H.L. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ. 2002, 9, 53–64. [Google Scholar] [CrossRef]
- Baiocco, P.; Ilari, A.; Ceci, P.; Orsini, S.; Gramiccia, M.; Di Muccio, T.; Colotti, G. Inhibitory effect of silver nanoparticles on trypanothione reductase activity and Leishmania infantum proliferation. ACS Med. Chem. Lett. 2010, 2, 230–233. [Google Scholar] [CrossRef]
- Basmaciyan, L.; Casanova, M. Cell death in Leishmania. Parasite 2019, 26, 71. [Google Scholar] [CrossRef] [PubMed]
- Galdino, A.; Viganor, L.; de Castro, A.A.; da Cunha, E.; Mello, T.P.; Mattos, L.M.; Pereira, M.D.; Hunt, M.C.; O’Shaughnessy, M.; Howe, O.; et al. Disarming Pseudomonas aeruginosa virulence by the inhibitory action of 1,10-phenanthroline-5,6-dione-based compounds: Elastase b (lasb) as a chemotherapeutic target. Front. Microbiol. 2019, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).