The Anti-Leishmania amazonensis and Anti-Leishmania chagasi Action of Copper(II) and Silver(I) 1,10-Phenanthroline-5,6-dione Coordination Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parasites and Cultivation
2.2. Macrophages Cultivation
2.3. Test Compounds
2.4. Effects of Coordination Compounds on Promastigotes’ Growth Rate
2.5. Protocol of Parasite Treatment: Looking for Potential Mechanisms of Action
2.6. Morphology, Morphometry and Ultrastructure
2.7. General Metabolism
2.8. Mitochondrial Dehydrogenases
2.9. Mitochondrial Membrane Potential
2.10. Phosphatidylserine Externalization and Incorporation of Propidium Iodide
2.11. Cell Cycle
2.12. DNA Fragmentation
2.13. Macrophage Toxicity
2.14. Leishmania-Macrophage Interaction
2.15. Statistics
3. Results and Discussion
3.1. Effects of Coordination Compounds on Leishmania’s Growth Rate
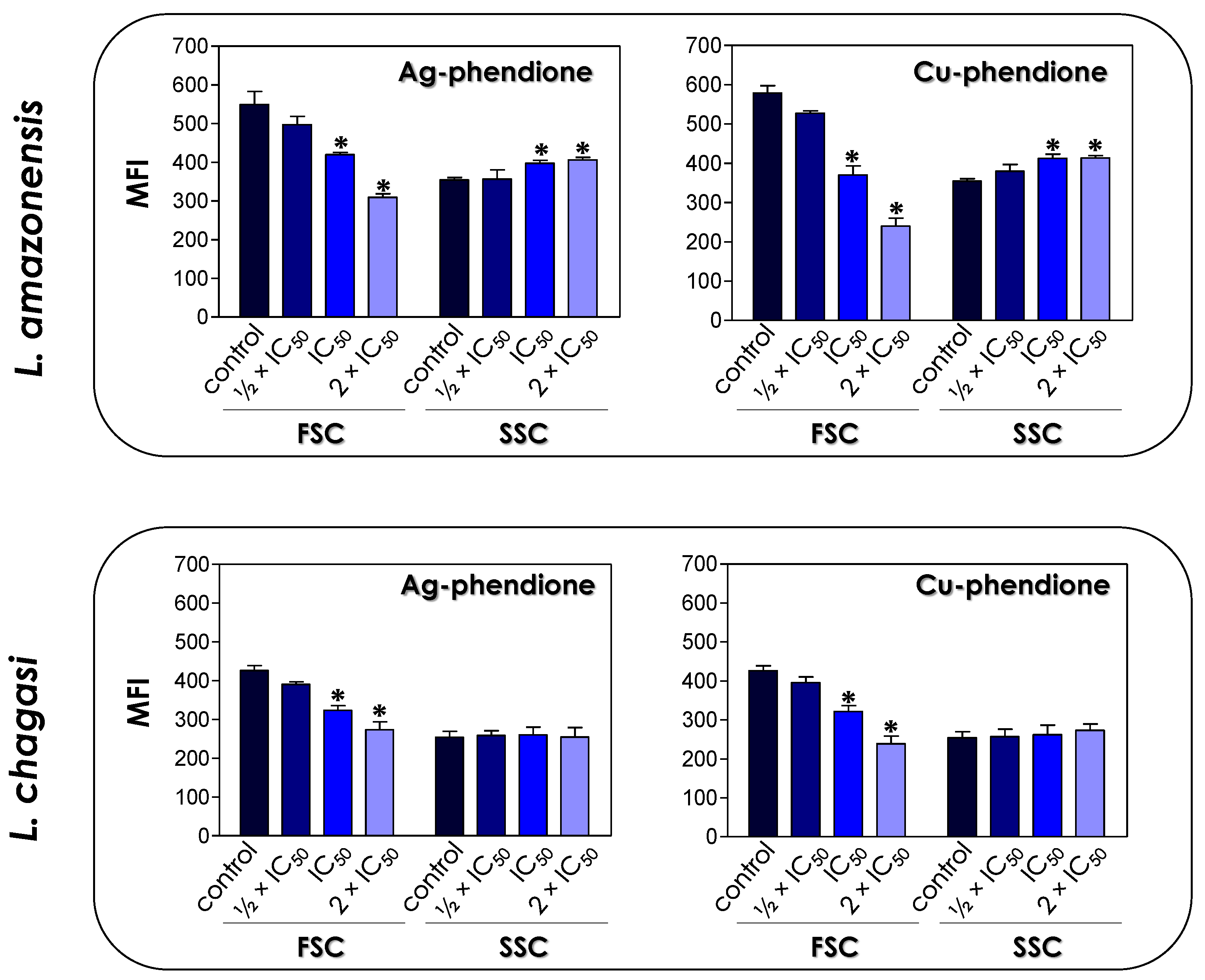
3.2. Effects of Coordination Compounds on Leishmania’s Morphometry and Ultrastructure
3.3. Effects of Coordination Compounds on Leishmania’s General Metabolism and Mitochondrial Activity
3.4. Effects of Coordination Compounds on Leishmania’s Cell Cycle
3.5. Effects of Coordination Compounds on Leishmania’s DNA Fragmentation
3.6. Effects of Coordination Compounds on Leishmania’s Phosphatidylserine Externalization
3.7. Effects of Coordination Compounds on THP-1 Macrophage Cells
3.8. Effects of Coordination Compounds on Leishmania–Macrophage Interaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, J.C.; Godinho, J.L.; de Souza, W. Biology of human pathogenic trypanosomatids: Epidemiology, lifecycle and ultrastructure. Subcell Biochem. 2014, 74, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Mohapatra, S. Drug resistance in leishmaniasis: Newer developments. Trop Parasitol. 2014, 4, 4–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundar, S.; Chakravarty, J. Leishmaniasis: An update of current pharmacotherapy. Expert. Opin. Pharmacother. 2013, 14, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.H.; Orvig, C. Boon and bane of metal ions in medicine. Science 2003, 300, 936–939. [Google Scholar] [CrossRef]
- McCann, M.; Santos, A.L.S.; Silva, B.A.; Romanos, M.T.V.; Pyrrho, A.S.; Devereux, M.; Kavanagh, K.; Fichtner, I.; Kellett, A. In vitro and in vivo studies into the biological activities of 1,10-phenanthroline, 1,10-phenanthroline-5,6-dione and its copper(II) and silver(I) complexes. Toxicol. Res. 2012, 1, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.K.; Elias, C.G.; Souza, J.E.; Santos, A.L.S.; Dutra, P.M. Dissimilar peptidase production by avirulent and virulent promastigotes of Leishmania braziliensis: Inference on the parasite proliferation and interaction with macrophages. Parasitology 2009, 136, 1179–1191. [Google Scholar] [CrossRef]
- Santos, A.L.S.; Sodre, C.L.; Valle, R.S.; Silva, B.A.; Abi-Chacra, E.A.; Silva, L.V.; Souza-Goncalves, A.L.; Sangenito, L.S.; Goncalves, D.S.; Souza, L.O.; et al. Antimicrobial action of chelating agents: Repercussions on the microorganism development, virulence and pathogenesis. Curr. Med. Chem. 2012, 19, 2715–2737. [Google Scholar] [CrossRef]
- Viganor, L.; Galdino, A.C.; Nunes, A.P.; Santos, K.R.; Branquinha, M.H.; Devereux, M.; Kellett, A.; McCann, M.; Santos, A.L.S. Anti-Pseudomonas aeruginosa activity of 1,10-phenanthroline-based drugs against both planktonic- and biofilm-growing cells. J. Antimicrob. Chemother. 2016, 71, 128–134. [Google Scholar] [CrossRef] [Green Version]
- McCann, M.; Kellett, A.; Kavanagh, K.; Devereux, M.; Santos, A.L.S. Deciphering the antimicrobial activity of phenanthroline chelators. Curr. Med. Chem. 2012, 19, 2703–2714. [Google Scholar] [CrossRef]
- Granato, M.Q.; Gonçalves, D.S.; Seabra, S.H.; McCann, M.; Devereux, M.; Santos, A.L.S.; Kneipp, L.F. 1,10-Phenanthroline-5,6-dione-based compounds are effective in disturbing crucial physiological events of Phialophora verrucosa. Front. Microbiol. 2017, 8, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas Rigo, G.; Petro-Silveira, B.; Devereux, M.; McCann, M.; Souza Dos Santos, A.L.S.; Tasca, T. Anti-Trichomonas vaginalis activity of 1,10-phenanthroline-5,6-dione-based metallodrugs and synergistic effect with metronidazole. Parasitology 2019, 146, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.F.; Galdino, A.C.; Viganor, L.; Schuenck, R.P.; Devereux, M.; McCann, M.; Santos, A.L.S.; Nunes, A. Antimicrobial action of 1,10-phenanthroline-based compounds on carbapenemase-producing Acinetobacter baumannii clinical strains: Efficacy against planktonic- and biofilm-growing cells. Braz. J. Microbiol. 2020, 51, 1703–1710. [Google Scholar] [CrossRef]
- Creaven, B.S.; Egan, D.A.; Karcz, D.; Kavanagh, K.; McCann, M.; Mahon, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterisation and antimicrobial activity of copper(II) and manganese(II) complexes of coumarin-6,7-dioxyacetic acid (cdoaH2) and 4-methylcoumarin-6,7-dioxyacetic acid (4-MecdoaH2): X-ray crystal structures of [Cu(cdoa)(phen)2].8.8H2O and [Cu(4-Mecdoa)(phen)2].13H2O (phen=1,10-phenanthroline). J. Inorg. Biochem. 2007, 101, 1108–1119. [Google Scholar] [CrossRef] [Green Version]
- Vianez Peregrino, I.; Ferreira Ventura, R.; Borghi, M.; Pinto Schuenck, R.; Devereux, M.; McCann, M.; Santos, A.L.S.; Ferreira Nunes, A.P. Antibacterial activity and carbapenem re-sensitizing ability of 1,10-phenanthroline-5,6-dione and its metal complexes against KPC-producing Klebsiella pneumoniae clinical strains. Lett. Appl. Microbiol. 2021, 73, 139–148. [Google Scholar] [CrossRef]
- Gandra, R.M.; Mc Carron, P.; Fernandes, M.F.; Ramos, L.S.; Mello, T.P.; Aor, A.C.; Branquinha, M.H.; McCann, M.; Devereux, M.; Santos, A.L.S. Antifungal potential of copper(II), manganese(II) and silver(I) 1,10-phenanthroline chelates against multidrug-resistant fungal species forming the Candida haemulonii complex: Impact on the planktonic and biofilm lifestyles. Front. Microbiol. 2017, 8, 1257. [Google Scholar] [CrossRef]
- Lima, A.; Elias, C.; Oliveira, S.; Santos-Mallet, J.R.; McCann, M.; Devereux, M.; Branquinha, M.H.; Dutra, P.; Santos, A.L.S. Anti-Leishmania braziliensis activity of 1,10-phenanthroline-5,6-dione and its Cu(II) and Ag(I) complexes. Parasitol. Res. 2021, 120, 3273–3285. [Google Scholar] [CrossRef]
- González, G.; Castillo, D.; Estevez, Y.; Grentzinger, T.; Deharo, E. Leishmania (Viannia) peruviana (MHOM/PE/LCA08): Comparison of THP-1 cell and murine macrophage susceptibility to axenic amastigotes for the screening of leishmanicidal compounds. Exp. Parasitol. 2009, 122, 353–356. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.; Coyle, B.; McKay, S.; McCormack, P.; Kavanagh, K.; Devereux, M.; McKee, V.; Kinsella, P.; O’Connor, R.; Clynes, M. Synthesis and X-ray crystal structure of [Ag(phendio)2]ClO4 (phendio = 1,10-phenanthroline-5,6-dione) and its effects on fungal and mammalian cells. Biometals 2004, 6, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Mikus, J.; Steverding, D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol. Int. 2000, 48, 265–269. [Google Scholar] [CrossRef]
- Toté, K.; Vanden Berghe, D.; Levecque, S.; Bénéré, E.; Maes, L.; Cos, P. Evaluation of hydrogen peroxide-based disinfectants in a new resazurin microplate method for rapid efficacy testing of biocides. J. Appl. Microbiol. 2009, 107, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Britta, E.A.; Silva, A.P.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Silva, C.C.; Sernaglia, R.L.; Nakamura, C.V. Benzaldehyde thiosemicarbazone derived from limonene complexed with copper induced mitochondrial dysfunction in Leishmania amazonensis. PLoS ONE 2012, 7, e41440. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Macías, I.; Maldonado, C.R.; Marín, C.; Olmo, F.; Gutiérrez-Sánchez, R.; Rosales, M.J.; Quirós, M.; Salas, J.M.; Sánchez-Moreno, M. In vitro anti-Leishmania evaluation of nickel complexes with a triazolopyrimidine derivative against Leishmania infantum and Leishmania braziliensis. J. Inorg. Biochem. 2012, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Sangenito, L.S.; Guedes, A.A.; Branquinha, M.H.; Kavanagh, K.; McGinley, J.; Santos, A.L.S.; Velasco-Torrijos, T. Glycosylated metal chelators as anti-parasitic agents with tunable selectivity. Dalton. Trans. 2017, 46, 5297–5307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanti, J.R.; Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; Cataneo, A.; Andrade, C.; Panis, C.; Rodrigues, J.; Wowk, P.F.; Kuczera, D.; Costa, I.N.; et al. Biogenic silver nanoparticles inducing Leishmania amazonensis promastigote and amastigote death in vitro. Acta Trop. 2018, 178, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Alti, D.; Veeramohan Rao, M.; Rao, D.N.; Maurya, R.; Kalangi, S.K. Gold-silver bimetallic nanoparticles reduced with herbal leaf extracts induce ROS-mediated death in both promastigote and amastigote stages of Leishmania donovani. ACS Omega 2020, 5, 16238–16245. [Google Scholar] [CrossRef]
- Sasidharan, S.; Saudagar, P. Gold and silver nanoparticles functionalized with 4’,7-dihydroxyflavone exhibit activity against Leishmania donovani. Acta Trop. 2022, 231, 106448. [Google Scholar] [CrossRef]
- Albalawi, A.E.; Abdel-Shafy, S.; Khudair Khalaf, A.; Alanazi, A.D.; Baharvand, P.; Ebrahimi, K.; Mahmoudvand, H. Therapeutic potential of green synthesized copper nanoparticles alone or combined with meglumine antimoniate (Glucantime®) in cutaneous leishmaniasis. Nanomaterials 2021, 11, 891. [Google Scholar] [CrossRef]
- Zahid, M.; Johnson, M.M.; Tokarski, R.J.; Satoskar, A.R.; Fuchs, J.R.; Bachelder, E.M.; Ainslie, K.M. Evaluation of synergy between host and pathogen-directed therapies against intracellular Leishmania donovani. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 125–132. [Google Scholar] [CrossRef]
- Fidalgo, L.M.; Gille, L. Mitochondria and trypanosomatids: Targets and drugs. Pharm Res. 2011, 28, 2758–2770. [Google Scholar] [CrossRef]
- Menna-Barreto, R.F.; de Castro, S.L. The double-edged sword in pathogenic trypanosomatids: The pivotal role of mitochondria in oxidative stress and bioenergetics. Biomed. Res. Int. 2014, 2014, 614014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Gélvez, A.; Diniz Junior, J.; Brígida, R.; Rodrigues, A. AgNP-PVP-meglumine antimoniate nanocomposite reduces Leishmania amazonensis infection in macrophages. BMC Microbiol. 2021, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Fernández, O.L.; Diaz-Toro, Y.; Ovalle, C.; Valderrama, L.; Muvdi, S.; Rodríguez, I.; Gomez, M.A.; Saravia, N.G. Miltefosine and antimonial drug susceptibility of Leishmania Viannia species and populations in regions of high transmission in Colombia. PLoS Negl. Trop. Dis. 2014, 8, e2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santi, A.M.M.; Murta, S.M.F. Impact of genetic diversity and genome plasticity of Leishmania spp. in treatment and the search for novel chemotherapeutic targets. Front. Cell. Infect. Microbiol. 2022, 12, 826287. [Google Scholar] [CrossRef]
- Becco, L.; Rodríguez, A.; Bravo, M.E.; Prieto, M.J.; Ruiz-Azuara, L.; Garat, B.; Moreno, V.; Gambino, D. New achievements on biological aspects of copper complexes Casiopeínas®: Interaction with DNA and proteins and anti-Trypanosoma cruzi activity. J Inorg. Biochem. 2012, 109, 49–56. [Google Scholar] [CrossRef]
- Méndez-Arriaga, J.M.; Oyarzabal, I.; Escolano, G.; Rodríguez-Diéguez, A.; Sánchez-Moreno, M.; Salas, J.M. In vitro leishmanicidal and trypanocidal evaluation and magnetic properties of 7-amino-1,2,4-triazolo[1,5-a]pyrimidine Cu(II) complexes. J. Inorg. Biochem. 2018, 180, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
- Masuri, S.; Vaňhara, P.; Cabiddu, M.G.; Moráň, L.; Havel, J.; Cadoni, E.; Pivetta, T. Copper(II) phenanthroline-based complexes as potential anticancer drugs: A walkthrough on the mechanisms of action. Molecules 2021, 27, 49. [Google Scholar] [CrossRef]
- Riaz Ahmed, K.B.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. In Vitro. 2017, 38, 179–192. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Bhaumik, S.K.; Karmakar, S.; Paul, J.; Sawoo, S.; Majumder, H.K.; Roy, A. Copper salisylaldoxime (CuSAL) imparts protective efficacy against visceral leishmaniasis by targeting Leishmania donovani topoisomerase IB. Exp. Parasitol. 2017, 175, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Zahir, A.A.; Chauhan, I.S.; Bagavan, A.; Kamaraj, C.; Elango, G.; Shankar, J.; Arjaria, N.; Roopan, S.M.; Rahuman, A.A.; Singh, N. Green synthesis of silver and titanium dioxide nanoparticles using Euphorbia prostrata extract shows shift from apoptosis to G0/G1 arrest followed by necrotic cell death in Leishmania donovani. Antimicrob. Agents Chemother. 2015, 59, 4782–4799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matassov, D.; Kagan, T.; Leblanc, J.; Sikorska, M.; Zakeri, Z. Measurement of apoptosis by DNA fragmentation. Methods Mol. Biol. 2004, 282, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Galdino, A.; Viganor, L.; Pereira, M.M.; Devereux, M.; McCann, M.; Branquinha, M.H.; Molphy, Z.; O’Carroll, S.; Bain, C.; Menounou, G.; et al. Copper(II) and silver(I)-1,10-phenanthroline-5,6-dione complexes interact with double-stranded DNA: Further evidence of their apparent multi-modal activity towards Pseudomonas aeruginosa. J. Biol. Inorg. Chem. 2022, 27, 201–213. [Google Scholar] [CrossRef]
- Koonin, E.V.; Aravind, L. Origin and evolution of eukaryotic apoptosis: The bacterial connection. Cell Death Differ. 2002, 9, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Weingärtner, A.; Kemmer, G.; Müller, F.D.; Zampieri, R.A.; Gonzaga dos Santos, M.; Schiller, J.; Pomorski, T.G. Leishmania promastigotes lack phosphatidylserine but bind annexin V upon permeabilization or miltefosine treatment. PLoS ONE 2012, 7, e42070. [Google Scholar] [CrossRef] [Green Version]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 1995, 184, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Zaki, L.; KarimiPourSaryazdi, A.; Tavakoli, P.; Tavajjohi, A.; Poursalehi, R.; Delavari, H.; Ghaffarifar, F. Efficacy of green synthesized silver nanoparticles via ginger rhizome extract against Leishmania major in vitro. PLoS ONE 2021, 16, e0255571. [Google Scholar] [CrossRef]
- Das, M.; Mukherjee, S.B.; Shaha, C. Hydrogen peroxide induces apoptosis-like death in Leishmania donovani promastigotes. J. Cell Sci. 2001, 114, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Bertholet, S.; Debrabant, A.; Muller, J.; Duncan, R.; Nakhasi, H.L. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ. 2002, 9, 53–64. [Google Scholar] [CrossRef]
- Baiocco, P.; Ilari, A.; Ceci, P.; Orsini, S.; Gramiccia, M.; Di Muccio, T.; Colotti, G. Inhibitory effect of silver nanoparticles on trypanothione reductase activity and Leishmania infantum proliferation. ACS Med. Chem. Lett. 2010, 2, 230–233. [Google Scholar] [CrossRef] [Green Version]
- Basmaciyan, L.; Casanova, M. Cell death in Leishmania. Parasite 2019, 26, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galdino, A.; Viganor, L.; de Castro, A.A.; da Cunha, E.; Mello, T.P.; Mattos, L.M.; Pereira, M.D.; Hunt, M.C.; O’Shaughnessy, M.; Howe, O.; et al. Disarming Pseudomonas aeruginosa virulence by the inhibitory action of 1,10-phenanthroline-5,6-dione-based compounds: Elastase b (lasb) as a chemotherapeutic target. Front. Microbiol. 2019, 10, 1701. [Google Scholar] [CrossRef] [PubMed]







| IC50 Values (nM) | ||
|---|---|---|
| Compounds | L. amazonensis | L. chagasi |
| Cu-phendione | 7.5 | 20.0 |
| Ag-phendione | 7.8 | 24.5 |
| Phendione | 19.1 | 30.0 |
| Phen | 870.0 | 1120.0 |
| THP-1 Cells | L. amazonensis | L. chagasi | |||
|---|---|---|---|---|---|
| Compounds | CC50 (nM) | IC50 (nM) | SI | IC50 (nM) | SI |
| Cu-phendione | 1470 | 35 | 42 | 51 | 28.8 |
| Ag-phendione | 1870 | 43 | 43.4 | 88 | 21.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, S.S.C.; Santos, V.S.; Devereux, M.; McCann, M.; Santos, A.L.S.; Branquinha, M.H. The Anti-Leishmania amazonensis and Anti-Leishmania chagasi Action of Copper(II) and Silver(I) 1,10-Phenanthroline-5,6-dione Coordination Compounds. Pathogens 2023, 12, 70. https://doi.org/10.3390/pathogens12010070
Oliveira SSC, Santos VS, Devereux M, McCann M, Santos ALS, Branquinha MH. The Anti-Leishmania amazonensis and Anti-Leishmania chagasi Action of Copper(II) and Silver(I) 1,10-Phenanthroline-5,6-dione Coordination Compounds. Pathogens. 2023; 12(1):70. https://doi.org/10.3390/pathogens12010070
Chicago/Turabian StyleOliveira, Simone S. C., Vanessa S. Santos, Michael Devereux, Malachy McCann, André L. S. Santos, and Marta H. Branquinha. 2023. "The Anti-Leishmania amazonensis and Anti-Leishmania chagasi Action of Copper(II) and Silver(I) 1,10-Phenanthroline-5,6-dione Coordination Compounds" Pathogens 12, no. 1: 70. https://doi.org/10.3390/pathogens12010070
APA StyleOliveira, S. S. C., Santos, V. S., Devereux, M., McCann, M., Santos, A. L. S., & Branquinha, M. H. (2023). The Anti-Leishmania amazonensis and Anti-Leishmania chagasi Action of Copper(II) and Silver(I) 1,10-Phenanthroline-5,6-dione Coordination Compounds. Pathogens, 12(1), 70. https://doi.org/10.3390/pathogens12010070








