New Insights into the Role of the Trypanosoma cruzi Aldo-Keto Reductase TcAKR
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids Construction, Recombinant Protein, and Antibody
2.2. Parasites and Cells
2.3. PGF2α Synthase Activity
2.4. Structural Comparison of AKR Proteins
2.5. Transcript Abundance Analysis
2.6. Epimastigotes Synchronization
2.7. Immunolocalization Studies
2.8. Drug Susceptibility
2.9. IC50 Determination
2.10. Phylogenetic Analysis
2.11. Statistical Analysis
3. Results
3.1. Phylogenetic Analysis of Aldo-Keto Reductase Protein Family in Trypanosomatids
3.2. TcAKR Is Located in the Mitochondria and Is Expressed in Epimastigotes and Amastigotes
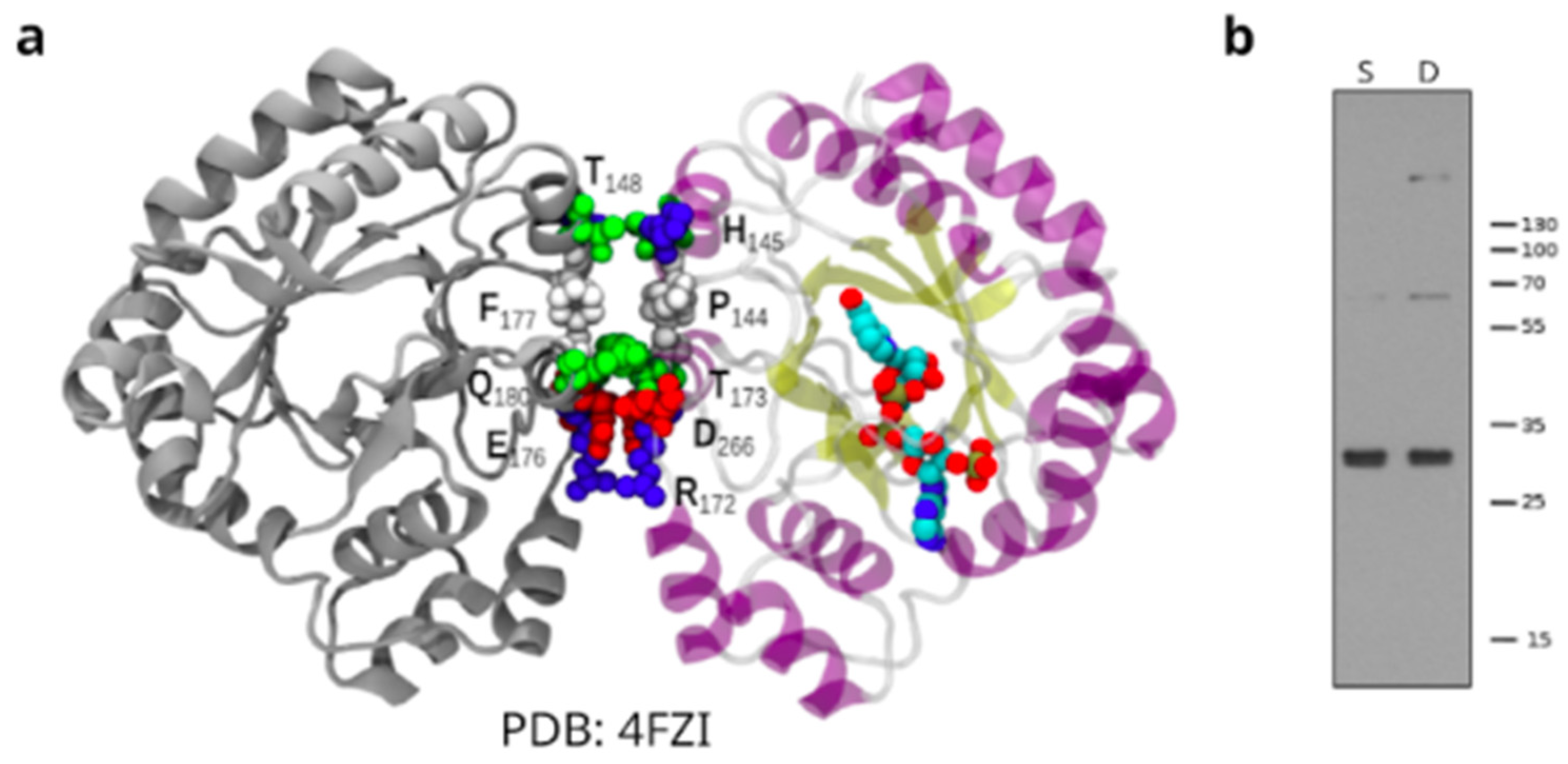
3.3. TcAKR Is Related to PGF2α Synthase Activity
3.4. Structural Analysis of TcAKR
3.5. TcAKR-Overexpressing Parasites Increases Their Susceptibility to Nifurtimox
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(American-trypanosomiasis) (accessed on 6 October 2022).
- Conners, E.E.; Vinetz, J.M.; Weeks, J.R.; Brouwer, K.C. A global systematic review of Chagas disease prevalence among migrants. Acta Trop. 2016, 156, 68–78. [Google Scholar] [CrossRef]
- Lee, B.Y.; Bacon, K.M.; Bottazzi, M.E.; Hotez, P.J. Global economic burden of Chagas disease: A computational simulation model. Lancet Infect. Dis. 2013, 13, 342–348. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Dones, W.; Morillo, C.A.; Encina, J.J.; Ribeiro, A.L. Chagas disease: An overview of clinical and epidemiological aspects. J. Am. Coll. Cardiol. 2013, 62, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Aldasoro, E.; Posada, E.; Requena-Méndez, A.; Calvo-Cano, A.; Serret, N.; Casellas, A.; Sanz, S.; Soy, D.; Pinazo, M.J.; Gascon, J. What to expect and when: Benznidazole toxicity in chronic Chagas’ disease treatment. J. Antimicrob. Chemother. 2018, 73, 1060–1067. [Google Scholar] [CrossRef]
- Jackson, Y.; Alirol, E.; Getaz, L.; Wolff, H.; Combescure, C.; Chappuis, F. Tolerance and safety of nifurtimox in patients with chronic Chagas disease. Clin. Infect. Dis. 2010, 51, e69–e75. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Bot, C.; Kelly, J.M.; Hall, B.S. Trypanocidal activity of nitroaromatic prodrugs: Current treatments and future perspectives. Curr. Top. Med. Chem. 2011, 11, 2072–2084. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 5022–5027. [Google Scholar] [CrossRef]
- Kubata, B.K.; Kabututu, Z.; Nozaki, T.; Munday, C.J.; Fukuzumi, S.; Ohkubo, K.; Lazarus, M.; Maruyama, T.; Martin, S.K.; Duszenko, M.; et al. A key role for old yellow enzyme in the metabolism of drugs by Trypanosoma cruzi. J. Exp. Med. 2022, 196, 1241–1252. [Google Scholar] [CrossRef]
- Murta, S.M.; Krieger, M.A.; Montenegro, L.R.; Campos, F.F.; Probst, C.M.; Avila, A.R.; Muto, N.H.; de Oliveira, R.C.; Nunes, L.R.; Nirdé, P.; et al. Deletion of copies of the gene encoding old yellow enzyme (TcOYE), a NAD(P)H flavin oxidoreductase, associates with in vitro-induced benznidazole resistance in Trypanosoma cruzi. Mol. Biochem. Parasitol. 2006, 146, 151–162. [Google Scholar] [CrossRef]
- Díaz-Viraqué, F.; Chiribao, M.L.; Trochine, A.; Gonzalez, F.; Castillo, C.; Liempi, A.; Kemmerling, U.; Maya, J.M.; Robello, C. Old Yellow Enzyme from Trypanosoma cruzi exhibits in vivo prostaglandin F2α synthase activity and has a key role in parasite infection and drug susceptibility. Front. Immunol. 2018, 9, 456. [Google Scholar] [CrossRef]
- Santi, A.M.M.; Ribeiro, J.M.; Reis-Cunha, J.L.; de Assis Burle-Caldas, G.; Santos, I.F.M.; Silva, P.A.; Resende, D.M.; Bartholomeu, D.C.; Teixeira, S.M.R. Disruption of multiple copies of the Prostaglandin F2alpha synthase gene affects oxidative stress response and infectivity in Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2022, 16, e0010845. [Google Scholar] [CrossRef] [PubMed]
- Garavaglia, P.A.; Cannata, J.J.; Ruiz, A.M.; Maugeri, D.; Duran, R.; Galleano, M.; García, G.A. Identification, cloning and characterization of an aldo-keto reductase from Trypanosoma cruzi with quinone oxido-reductase activity. Mol. Biochem. Parasitol. 2010, 173, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Garavaglia, P.A.; Laverrière, M.; Cannata, J.J.; García, G.A. Putative role of the aldo-keto reductase from Trypanosoma cruzi in benznidazole metabolism. Antimicrob. Agents Chemother. 2016, 60, 2664–2670. [Google Scholar] [CrossRef]
- Trochine, A.; Alvarez, G.; Corre, S.; Faral-Tello, P.; Durán, R.; Batthyany, C.; Cerecetto, H.; González, M.; Robello, C. Trypanosoma cruzi chemical proteomics using immobilized benznidazole. Exp. Parasitol. 2014, 140, 33–38. [Google Scholar] [CrossRef]
- Roberts, A.J.; Dunne, J.; Scullion, P.; Norval, S.; Fairlamb, A.H. A role for Trypanosomatid aldo-keto reductases in methylglyoxal, prostaglandin and isoprostane metabolism. Biochem. J. 2018, 475, 2593–2610. [Google Scholar] [CrossRef]
- Jez, J.M.; Bennett, M.J.; Schlegel, B.P.; Lewis, M.; Penning, T.M. Comparative anatomy of the aldo-keto reductase superfamily. Biochem. J. 1997, 326 Pt 3, 625. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M. The aldo-keto reductases (AKRs): Overview. Chem.-Biol. Interact. 2015, 234, 236–246. [Google Scholar] [CrossRef]
- Kubata, B.K.; Duszenko, M.; Kabututu, Z.; Rawer, M.; Szallies, A.; Fujimori, K.; Inui, T.; Nozaki, T.; Yamashita, K.; Horii, T.; et al. Identification of a novel prostaglandin F2α synthase in Trypanosoma brucei. J. Exp. Med. 2000, 192, 1327–1338. [Google Scholar] [CrossRef]
- Kabututu, Z.; Martin, S.K.; Nozaki, T.; Kawazu, S.I.; Okada, T.; Munday, C.J.; Duszenko, M.; Lazarus, M.; Thuita, L.W.; Urade, Y.; et al. Prostaglandin production from arachidonic acid and evidence for a 9, 11-endoperoxide prostaglandin H2 reductase in Leishmania. Int. J. Parasitol. 2002, 32, 1693–1700. [Google Scholar] [CrossRef]
- Alves-Ferreira, E.V.C.; Ferreira, T.R.; Walrad, P.; Kaye, P.M.; Cruz, A.K. Leishmania braziliensis prostaglandin F2α synthase impacts host infection. Parasit Vectors 2020, 13, 9. [Google Scholar] [CrossRef]
- Kabututu, Z.; Manin, M.; Pointud, J.C.; Maruyama, T.; Nagata, N.; Lambert, S.; Lefrançois-Martinez, A.M.; Martinez, A.; Urade, Y. Prostaglandin f2α synthase activities of aldo–keto reductase 1b1, 1b3 and 1b7. J. Biochem. 2008, 145, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ashton, A.W.; Mukherjee, S.; Nagajyothi, F.N.U.; Huang, H.; Braunstein, V.L.; Desruisseaux, M.S.; Factor, S.M.; Lopez, L.; Berman, J.W.; Wittner, M.; et al. Thromboxane A2 is a key regulator of pathogenesis during Trypanosoma cruzi infection. J. Exp. Med. 2007, 204, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.; Levin, M.J. Functional analysis of the intergenic regions of Tc 2β gene loci allowed the construction of an improved Trypanosoma cruzi expression vector. Gene 1999, 239, 217–225. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Simizu, B.; Rhim, J.S.; Wiebenga, N.H. Characterization of the Tacaribe Group of Arboviruses. 1. Propagation and Plaque Assay of Tacaribe Virus in a Line of African Green Monkey Kidney Cells (Vero). Proc. Soc. Exp. Biol. Med. 1967, 125, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Contreras, V.T.; Araújo-Jorge , T.C.D.; Bonaldo, M.C.; Thomaz, N.; Barbosa, H.S.; Roberts, E.; Meirelles, M.d.S.; Goldenberg, S. Biological aspects of the DM28C clone of Trypanosoma cruzi after metacyclogenesis in chemically defined media. Mem. Do Inst. Oswaldo Cruz 1988, 83, 123–133. [Google Scholar] [CrossRef]
- Roberts, E.; Eargle, J.; Wright, D.; Luthey-Schulten, Z. MultiSeq: Unifying sequence and structure data for evolutionary analysis. BMC Bioinform. 2006, 7, 382. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Russell, R.B.; Barton, G.J. Multiple protein sequence alignment from tertiary structure comparison: Assignment of global and residue confidence levels. Proteins Struct. Funct. Bioinform. 1992, 14, 309–323. [Google Scholar] [CrossRef]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N.A. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007, 35 (Suppl. S2), W522–W525. [Google Scholar] [CrossRef]
- Li, H.; Robertson, A.D.; Jensen, J.H. Very fast empirical prediction and rationalization of protein pKa values. Proteins Struct. Funct. Bioinform. 2005, 61, 704–721. [Google Scholar] [CrossRef] [PubMed]
- Krissinel, E.ñ.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Berná, L.; Chiribao, M.L.; Greif, G.; Rodriguez, M.; Alvarez-Valin, F.; Robello, C. Transcriptomic analysis reveals metabolic switches and surface remodeling as key processes for stage transition in Trypanosoma cruzi. PeerJ 2017, 8, e3017. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Galanti, N.; Dvorak, J.A.; Grenet, J.; McDaniel, J.P. Hydroxyurea-induced synchrony of DNA replication in the Kinetoplastida. Exp. Cell Res. 1994, 214, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.C.Q.; Faria, M.; Mortara, R.A.; Motta, M.C.M.; de Souza, W.; Thiry, M.; Schenkman, S. Chromosome localization changes in the Trypanosoma cruzi nucleus. Eukaryot. Cell 2002, 1, 944–953. [Google Scholar] [CrossRef]
- Elias, M.C.Q.; da Cunha, J.P.; de Faria, F.P.; Mortara, R.A.; Freymüller, E.; Schenkman, S. Morphological events during the Trypanosoma cruzi cell cycle. Protist 2007, 158, 147–157. [Google Scholar] [CrossRef]
- Alonso, V.L. Ultrastructure Expansion Microscopy (U-ExM) in Trypanosoma cruzi: Localization of tubulin isoforms and isotypes. Parasitol. Res. 2022, 121, 3019–3024. [Google Scholar] [CrossRef]
- Tomasina, R.; Gonzalez, F.C.; Martins-Duarte, É.S.; Bastin, P.; Gissot, M.; Francia, M.E. Separate to operate: The centriole-free inner core of the centrosome regulates the assembly of the intranuclear spindle in Toxoplasma gondii. Mbio 2022, 13, e0185922. [Google Scholar] [CrossRef]
- Rolón, M.; Vega, C.; Escario, J.A.; Gómez-Barrio, A. Development of resazurin microtiter assay for drug sensibility testing of Trypanosoma cruzi epimastigotes. Parasitol. Res. 2006, 99, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Berná, L.; Rodriguez, M.; Chiribao, M.L.; Parodi-Talice, A.; Pita, S.; Rijo, G.; Alvarez-Valin, F.; Robello, C. Expanding an expanded genome: Long-read sequencing of Trypanosoma cruzi. Microb. Genom. 2018, 4, e000177. [Google Scholar] [CrossRef]
- Piñeyro, M.D.; Parodi-Talice, A.; Arcari, T.; Robello, C. Peroxiredoxins from Trypanosoma cruzi: Virulence factors and drug targets for treatment of Chagas disease? Gene 2008, 408, 45–50. [Google Scholar] [CrossRef]
- Moen, S.O.; Fairman, J.W.; Barnes, S.R.; Sullivan, A.; Nakazawa-Hewitt, S.; Van Voorhis, W.C.; Staker, B.L.; Lorimer, D.D.; Myler, P.J.; Edwards, T.E. Structures of prostaglandin F synthase from the protozoa Leishmania major and Trypanosoma cruzi with NADP. Acta Cryst. F Struct. Biol. Commun. 2015, 71 Pt 5, 609–614. [Google Scholar] [CrossRef]
- Trochine, A.; Creek, D.J.; Faral-Tello, P.; Barrett, M.P.; Robello, C. Benznidazole biotransformation and multiple targets in Trypanosoma cruzi revealed by metabolomics. PLoS Negl. Trop. Dis. 2014b, 8, e2844. [Google Scholar] [CrossRef]
- González, L.; García-Huertas, P.; Triana-Chávez, O.; García, G.A.; Murta, S.M.F.; Mejía-Jaramillo, A.M. Aldo-keto reductase and alcohol dehydrogenase contribute to benznidazole natural resistance in Trypanosoma cruzi. Mol. Microbiol. 2017, 106, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.E.; Englund, P.T. Network news: The replication of kinetoplast DNA. Annu. Rev. Microbiol. 2012, 66, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Molecular and Functional Characteristics of DNA Polymerase Beta-Like Enzymes from Trypanosomatids. Front. Cell Infect. Microbiol. 2021, 11, 670564. [Google Scholar] [CrossRef]
- Klingbeil, M.M.; Englund, P.T. Closing the gaps in kinetoplast DNA network replication. Proc. Natl. Acad. Sci. USA 2004, 101, 4333–4334. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, Y.; Motyka, S.A.; Agbo, E.E.C.; Englund, P.T. Fellowship of the rings: The replication of kinetoplast DNA. Trends Paras. 2005, 21, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems--role in ageing and disease. Drug Metab. Drug Interact 2008, 23, 125–150. [Google Scholar] [CrossRef] [PubMed]
- Murata-Kamiya, N.; Kamiya, H. Methylglyoxal, an endogenous aldehyde, crosslinks DNA polymerase and the substrate DNA. Nucleic Acids Res. 2001, 29, 3433–3438. [Google Scholar] [CrossRef]
- Araújo-Santos, T.; Rodríguez, N.E.; Moura-Pontes, S.; Dixt, U.G.; Abánades, D.R.; Bozza, P.T.; Wilson, M.E.; Borges, V.M. Role of prostaglandin F2α production in lipid bodies from Leishmania infantum chagasi: Insights on virulence. J. Infect. Dis. 2014, 210, 1951–1961. [Google Scholar] [CrossRef]
- Schlegel, B.P.; Jez, J.M.; Penning, T.M. Mutagenesis of 3α-Hydroxysteroid Dehydrogenase Reveals a “Push− Pull” Mechanism for Proton Transfer in Aldo-Keto Reductases. Biochemistry 1998, 37, 3538–3548. [Google Scholar] [CrossRef]
- Toogood, H.S.; Gardiner, J.M.; Scrutton, N.S. Biocatalytic reductions and chemical versatility of the old yellow enzyme family of flavoprotein oxidoreductases. ChemCatChem 2010, 2, 892–914. [Google Scholar] [CrossRef]
- Hall, B.S.; Wilkinson, S.R. Activation of benznidazole by Trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob. Agents Chemother. 2012, 56, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.S.; Bot, C.; Wilkinson, S.R. Nifurtimox activation by Trypanosomal type I nitroreductases generates cytotoxic nitrile metabolites. J. Biol. Chem. 2011, 286, 13088–13095. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Viraqué, F.; Chiribao, M.L.; Paes-Vieira, L.; Machado, M.R.; Faral-Tello, P.; Tomasina, R.; Trochine, A.; Robello, C. New Insights into the Role of the Trypanosoma cruzi Aldo-Keto Reductase TcAKR. Pathogens 2023, 12, 85. https://doi.org/10.3390/pathogens12010085
Díaz-Viraqué F, Chiribao ML, Paes-Vieira L, Machado MR, Faral-Tello P, Tomasina R, Trochine A, Robello C. New Insights into the Role of the Trypanosoma cruzi Aldo-Keto Reductase TcAKR. Pathogens. 2023; 12(1):85. https://doi.org/10.3390/pathogens12010085
Chicago/Turabian StyleDíaz-Viraqué, Florencia, María Laura Chiribao, Lisvane Paes-Vieira, Matias R. Machado, Paula Faral-Tello, Ramiro Tomasina, Andrea Trochine, and Carlos Robello. 2023. "New Insights into the Role of the Trypanosoma cruzi Aldo-Keto Reductase TcAKR" Pathogens 12, no. 1: 85. https://doi.org/10.3390/pathogens12010085
APA StyleDíaz-Viraqué, F., Chiribao, M. L., Paes-Vieira, L., Machado, M. R., Faral-Tello, P., Tomasina, R., Trochine, A., & Robello, C. (2023). New Insights into the Role of the Trypanosoma cruzi Aldo-Keto Reductase TcAKR. Pathogens, 12(1), 85. https://doi.org/10.3390/pathogens12010085





