Genetic Underpinnings of Carotenogenesis and Light-Induced Transcriptome Remodeling in the Opportunistic Pathogen Mycobacterium kansasii
Abstract
1. Introduction
2. Results and Discussion
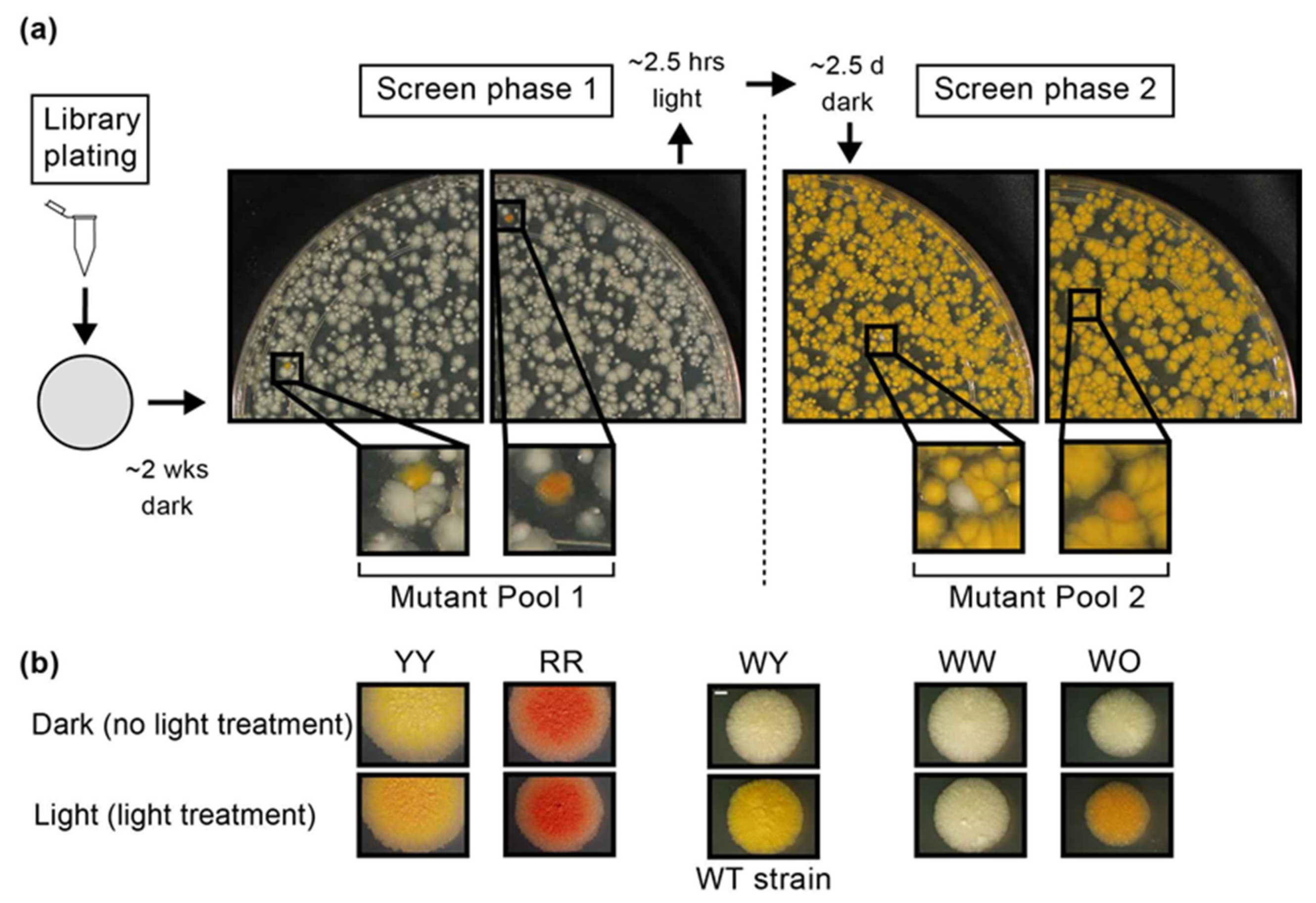
2.1. The Screen of an M. kansasii Transposon Library for Pigmentation Mutants Led to Identification of Four Distinct Mutant Phenotype Groups
2.2. The Insertion Sites in the Pigmentation Mutant Collection Clustered in Three Chromosomal Regions
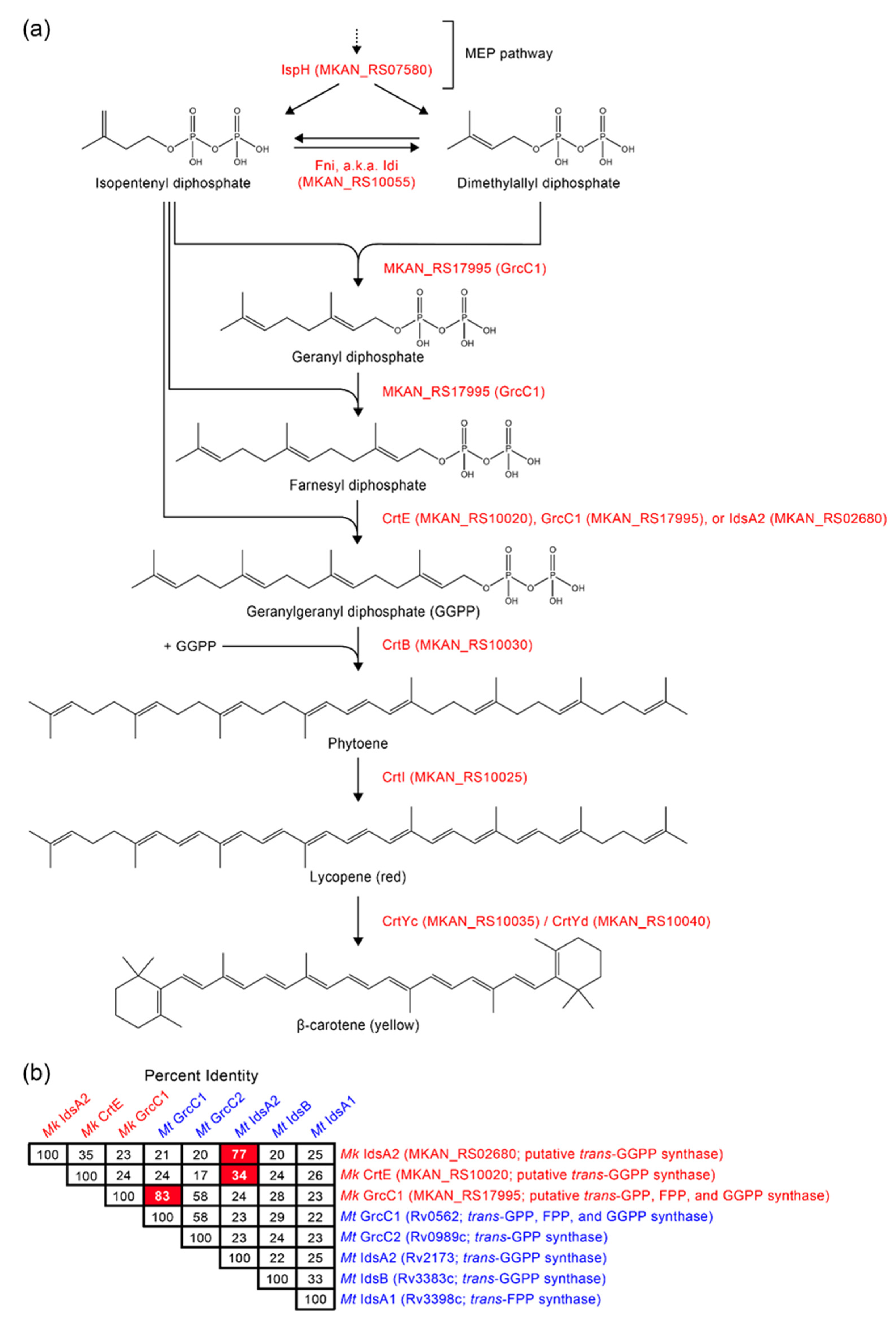
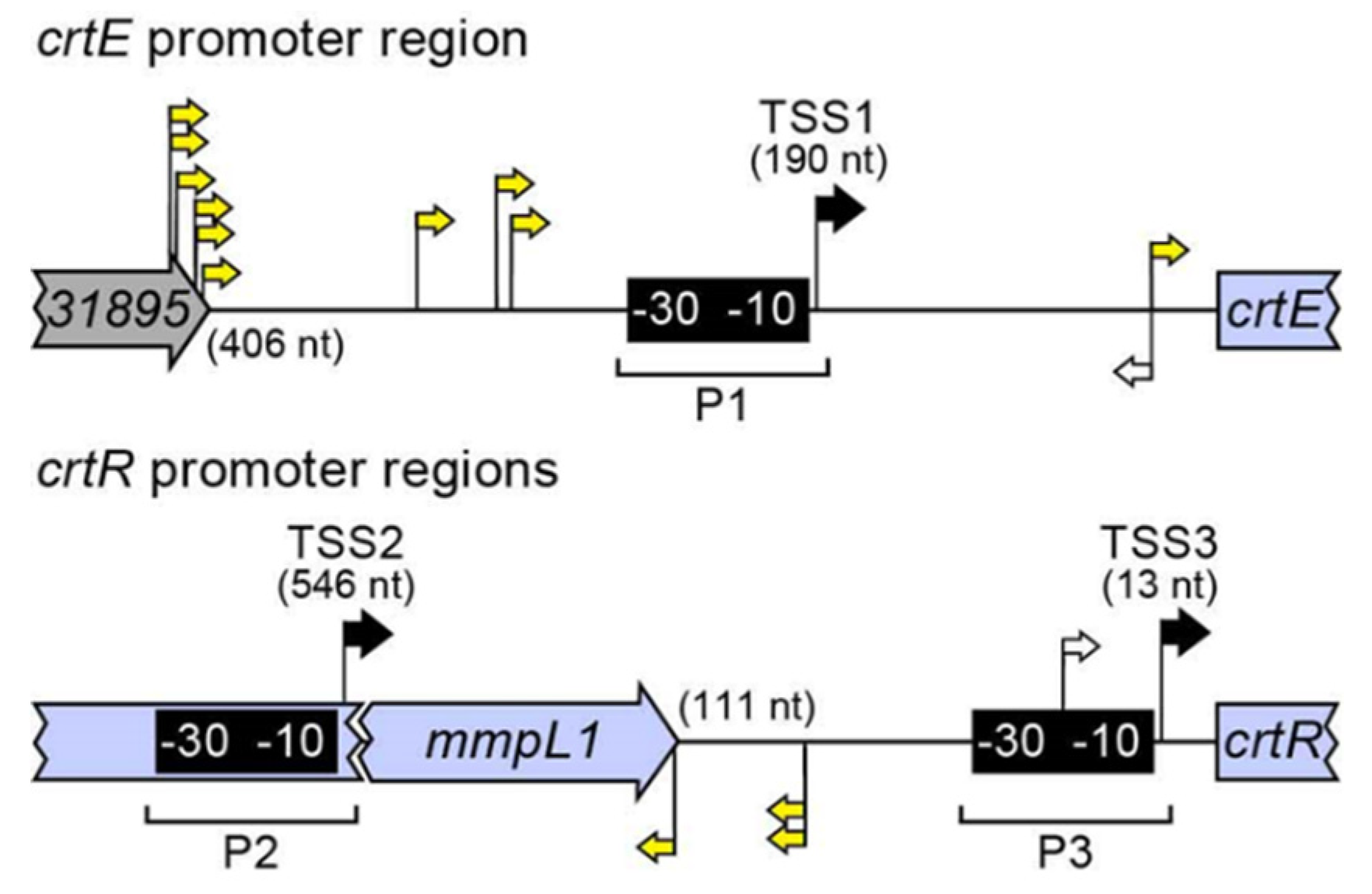
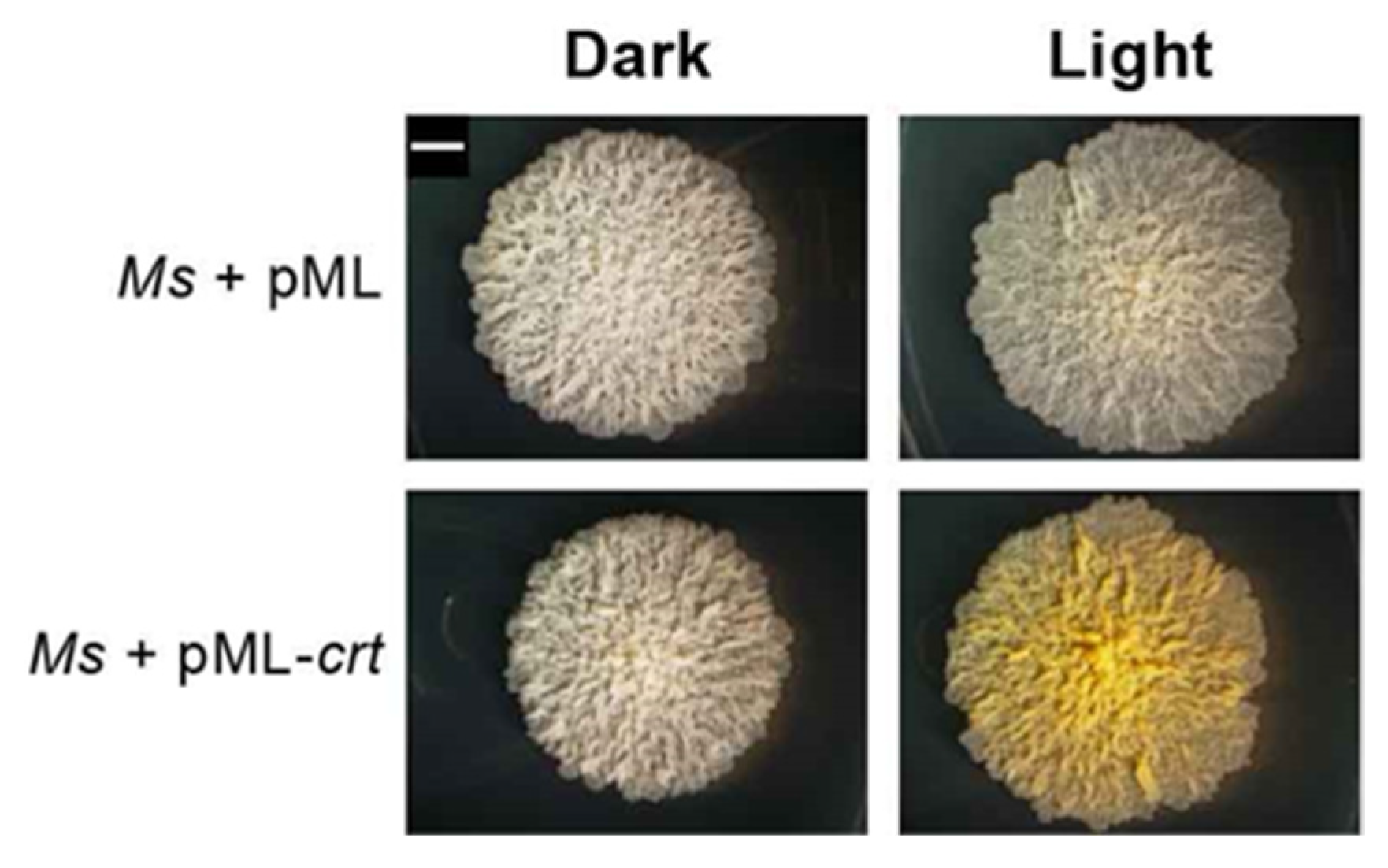
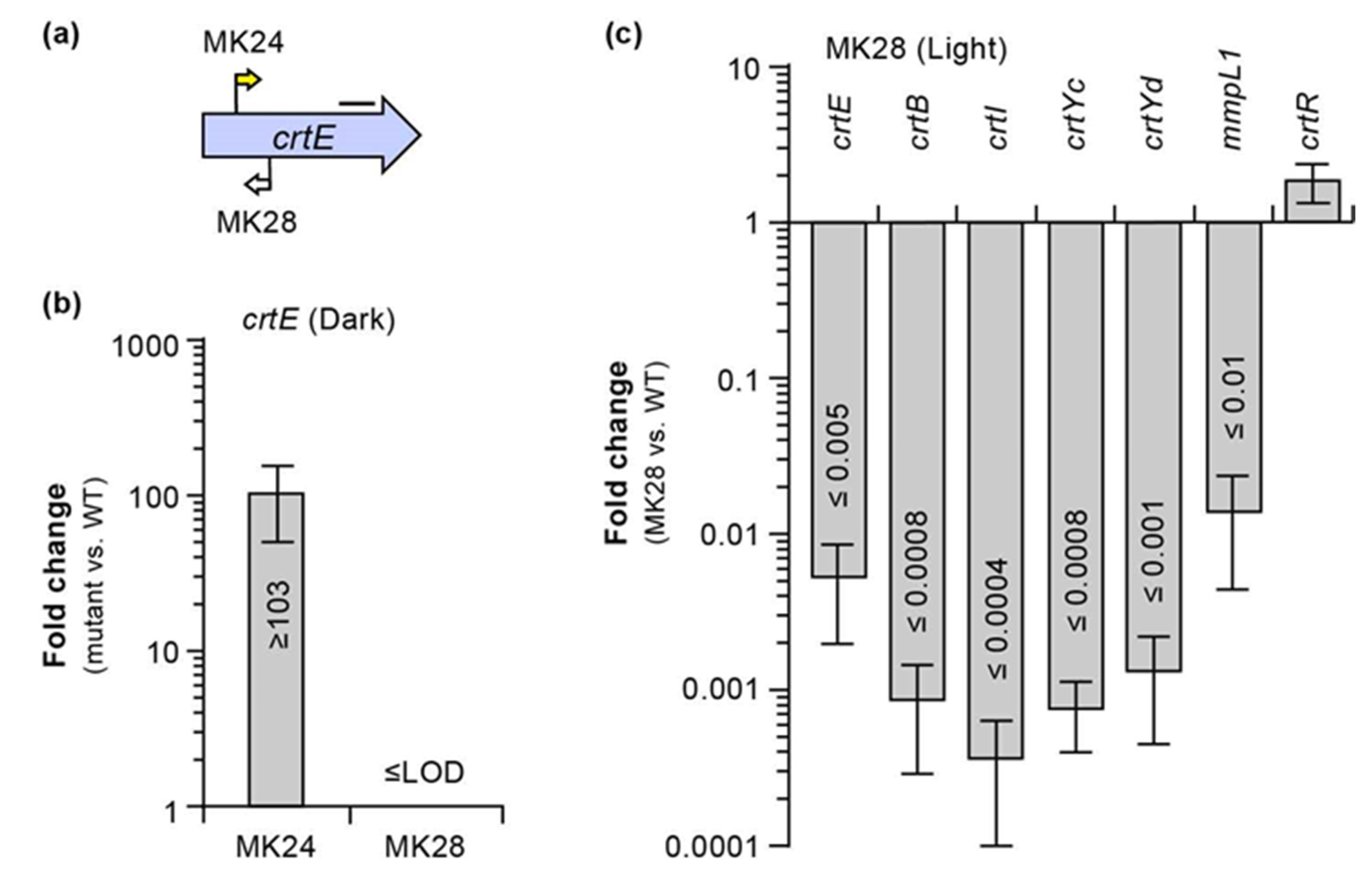
2.3. Sequence Bioinformatics and Insertional Analysis of the Carotenoid Biosynthesis Locus
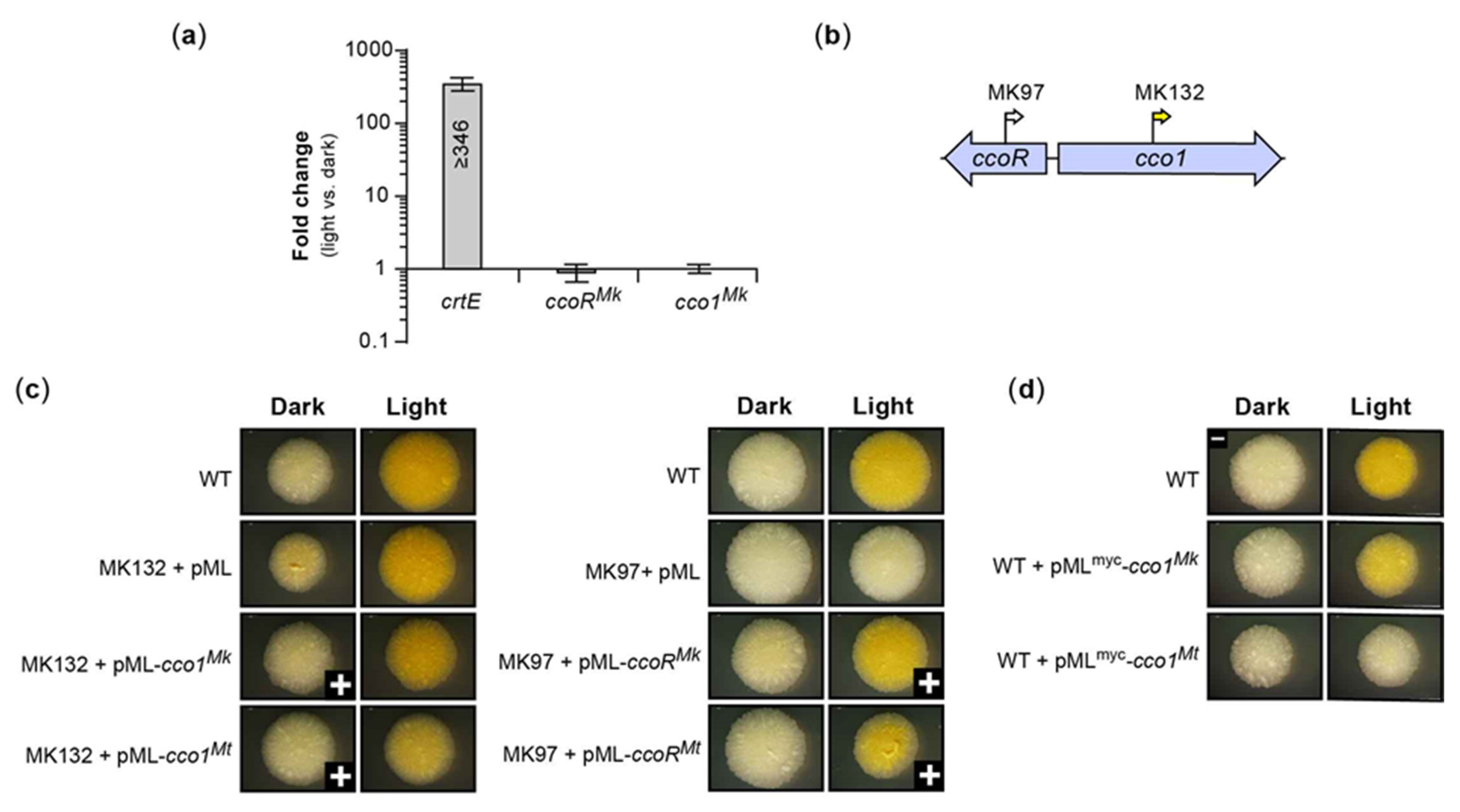
2.4. Bioinformatic and Insertional Analysis of the Carotenoid Cleavage Oxygenase Locus
2.5. Bioinformatic and Insertional Analysis of the fnr1-desA3 Locus
2.6. Transcriptome Changes Induced by Light in M. kansasii
3. Materials and Methods
3.1. Routine Culturing Conditions, Molecular Biology Techniques, and Reagents
3.2. Preparation of the M. kansasii Transposon Library and Screening for Pigmentation Mutants
3.3. Insertion Site Determination and Southern Blot Hybridization Analysis
3.4. Construction of Plasmids
3.5. Pigmentation Phenotype Assessment
3.6. Preparation of RNA for RT-qPCR and RNA-Seq Analyses
3.7. RT-qPCR Analysis
3.8. RNA-Seq Analysis
3.9. Routine Sequence Bioinformatics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jagielski, T.; Borówka, P.; Bakuła, Z.; Lach, J.; Marciniak, B.; Brzostek, A.; Dziadek, J.; Dziurzyński, M.; Pennings, L.; van Ingen, J.; et al. Genomic insights into the Mycobacterium kansasii complex: An update. Front. Microbiol. 2019, 10, 2918. [Google Scholar] [CrossRef]
- Tagini, F.; Aeby, S.; Bertelli, C.; Droz, S.; Casanova, C.; Prod’hom, G.; Jaton, K.; Greub, G. Phylogenomics reveal that Mycobacterium kansasii subtypes are species-level lineages. Description of Mycobacterium pseudokansasii sp. nov., Mycobacterium innocens sp. nov. and Mycobacterium attenuatum sp. nov. Int. J. Syst. Evol. Microbiol. 2019, 69, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.C.; Chiang, L.; Elwood, K. Mycobacterium kansasii. Microbiol. Spectr. 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Maliwan, N.; Zvetina, J.R. Clinical features and follow up of 302 patients with Mycobacterium kansasii pulmonary infection: A 50 year experience. Postgrad. Med. J. 2005, 81, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.J.; Crisp, A.J.; Hubbard, R.B.; Colville, A.; Evans, S.A.; Johnston, I.D. Pulmonary Mycobacterium kansasii infection: Comparison of radiological appearances with pulmonary tuberculosis. Thorax 1996, 51, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Matveychuk, A.; Fuks, L.; Priess, R.; Hahim, I.; Shitrit, D. Clinical and radiological features of Mycobacterium kansasii and other NTM infections. Respir. Med. 2012, 106, 1472–1477. [Google Scholar] [CrossRef]
- Ehsani, L.; Reddy, S.C.; Mosunjac, M.; Kraft, C.S.; Guarner, J. Fatal aortic pseudoaneurysm from disseminated Mycobacterium kansasii infection: Case report. Hum. Pathol. 2015, 46, 467–470. [Google Scholar] [CrossRef]
- Hirashima, T.; Nagai, T.; Shigeoka, H.; Tamura, Y.; Yoshida, H.; Kawahara, K.; Kondoh, Y.; Sakai, K.; Hashimoto, S.; Fujishima, M.; et al. Comparison of the clinical courses and chemotherapy outcomes in metastatic colorectal cancer patients with and without active Mycobacterium tuberculosis or Mycobacterium kansasii infection: A retrospective study. BMC Cancer 2014, 14, 770. [Google Scholar] [CrossRef]
- Nei, T.; Okabe, M.; Mikami, I.; Koizumi, Y.; Mase, H.; Matsuda, K.; Yamamoto, T.; Takeda, S.; Tanaka, K.; Dan, K. A non-HIV case with disseminated Mycobacterium kansasii disease associated with strong neutralizing autoantibody to interferon-gamma. Respir. Med. Case Rep. 2013, 8, 10–13. [Google Scholar]
- Queipo, J.A.; Broseta, E.; Santos, M.; Sanchez-Plumed, J.; Budia, A.; Jimenez-Cruz, F. Mycobacterial infection in a series of 1261 renal transplant recipients. Clin. Microbiol. Infect. 2003, 9, 518–525. [Google Scholar] [CrossRef]
- Ulmann, V.; Kracalikova, A.; Dziedzinska, R. Mycobacteria in water used for personal hygiene in heavy industry and collieries: A potential risk for employees. Int. J. Environ. Res. Public Health 2015, 12, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.M.; Carter, R.; Tolson, C.; Coulter, C.; Huygens, F.; Hargreaves, M. Factors associated with the isolation of nontuberculous mycobacteria (NTM) from a large municipal water system in Brisbane, Australia. BMC Microbiol. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Ashbolt, N.J. Environmental (saprozoic) pathogens of engineered water systems: Understanding their ecology for risk assessment and management. Pathogens 2015, 4, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.C.; Elwood, K. Mycobacterium kansasii. In Tuberculosis and Nontuberculous Mycobacterial Infections, 6th ed.; Schlossberg, D., Ed.; ASM Press: Washington, DC, USA, 2011; pp. 578–585. [Google Scholar]
- Henkle, E.; Winthrop, K.L. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin. Chest Med. 2015, 36, 91–99. [Google Scholar] [CrossRef]
- Flor, A.; Capdevila, J.A.; Martin, N.; Gavalda, J.; Pahissa, A. Nontuberculous mycobacterial meningitis: Report of two cases and review. Clin. Infect. Dis. 1996, 23, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Brown-Elliott, B.A.; Nash, K.A.; Wallace, R.J., Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 2012, 25, 545–582. [Google Scholar] [CrossRef]
- Basille, D.; Jounieaux, V.; Andréjak, C. Treatment of other nontuberculous mycobacteria. Semin. Respir. Crit. Care. Med. 2018, 39, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Van Ingen, J.; Boeree, M.J.; van Soolingen, D.; Iseman, M.D.; Heifets, L.B.; Daley, C.L. Are phylogenetic position, virulence, drug susceptibility and in vivo response to treatment in mycobacteria interrelated? Infect. Genet. Evol. 2012, 12, 832–837. [Google Scholar] [CrossRef]
- Van Ingen, J.; Boeree, M.J.; van Soolingen, D.; Mouton, J.W. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug. Resist. Updat. 2012, 15, 149–161. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Koh, W.-J. Nontuberculous mycobacteria—Overview. Microbiol. Spectr. 2017, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.M.; Odell, J.A. Nontuberculous mycobacterial pulmonary infections. J. Thorac. Dis. 2014, 6, 210–220. [Google Scholar] [PubMed]
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Angeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef]
- Simons, S.; van Ingen, J.; Hsueh, P.R.; van Hung, N.; Dekhuijzen, P.N.; Boeree, M.J.; van Soolingen, D. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg. Infect. Dis. 2011, 17, 343–349. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Farshidpour, M.; Allen, M.B.; Ebrahimi, G.; Falkinham, J.O. Highlight on advances in nontuberculous mycobacterial disease in North America. BioMed Res. Int. 2014, 2014, 919474. [Google Scholar] [CrossRef]
- Davies, B.S.; Roberts, C.H.; Kaul, S.; Klein, J.L.; Milburn, H.J. Non-tuberculous slow-growing mycobacterial pulmonary infections in non-HIV-infected patients in south London. Scand. J. Infect. Dis. 2012, 44, 815–819. [Google Scholar] [CrossRef]
- Cook, J.L. Nontuberculous mycobacteria: Opportunistic environmental pathogens for predisposed hosts. Br. Med. Bull. 2010, 96, 45–59. [Google Scholar] [CrossRef]
- Wang, P.H.; Pan, S.W.; Wang, S.M.; Shu, C.C.; Chang, C.H. The Impact of nontuberculous mycobacteria species on mortality inpatients with nontuberculous mycobacterial lung disease. Front. Microbiol. 2022, 13, 909274. [Google Scholar]
- Gopinath, K.; Singh, S. Non-tuberculous mycobacteria in TB-endemic countries: Are we neglecting the danger? PLoS Negl. Trop. Dis. 2010, 4, e615. [Google Scholar] [CrossRef]
- McQuaid, C.F.; Vassall, A.; Cohen, T.; Fiekert, K.; White, R.G. The impact of COVID-19 on TB: A review of the data. Int. J. Tuberc. Lung Dis. 2021, 25, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Nikolayevskyy, V.; Holicka, Y.; van Soolingen, D.; van der Werf, M.J.; Ködmön, C.; Surkova, E.; Hillemann, D.; Groenheit, R.; ERLTB-Net-2 Study Participants; Cirillo, D. Impact of the COVID-19 pandemic on tuberculosis laboratory services in Europe. Eur. Respir. J. 2021, 57, 2003890. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, V.; Pang, P.C.; Haslam, S.M.; Veerapen, N.; Minnikin, D.E.; Dell, A.; Besra, G.S.; Bhatt, A. MKAN27435 is required for the biosynthesis of higher subclasses of lipooligosaccharides in Mycobacterium kansasii. PLoS ONE 2015, 10, e0122804. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klein, J.L.; Brown, T.J.; French, G.L. Rifampin resistance in Mycobacterium kansasii is associated with rpoB mutations. Antimicrob. Agents Chemother. 2001, 45, 3056–3058. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Y. Reduced pyrazinamidase activity and the natural resistance of Mycobacterium kansasii to the antituberculosis drug pyrazinamide. Antimicrob. Agents Chemother. 1999, 43, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Onwueme, K.C.; Vos, C.J.; Zurita, J.; Soll, C.E.; Quadri, L.E. Identification of phthiodiolone ketoreductase, an enzyme required for production of mycobacterial diacyl phthiocerol virulence factors. J. Bacteriol. 2005, 187, 4760–4766. [Google Scholar] [CrossRef][Green Version]
- Budell, W.C.; Germain, G.A.; Janisch, N.; McKie-Krisberg, Z.; Jayaprakash, A.D.; Resnick, A.E.; Quadri, L.E.N. Transposon mutagenesis in Mycobacterium kansasii links a small RNA gene to colony morphology and biofilm formation and identifies 9885 intragenic insertions that do not compromise colony outgrowth. Microbiologyopen 2020, 9, e988. [Google Scholar] [CrossRef]
- Buhler, V.B.; Pollak, A. Human infection with atypical acid-fast organisms: Report of two cases with pathologic findings. Am. J. Clin. Pathol. 1953, 23, 363–374. [Google Scholar] [CrossRef]
- Runyon, E.H. Anonymous mycobacteria in pulmonary disease. Med. Clin. N. Am. 1959, 43, 273–290. [Google Scholar] [CrossRef]
- David, H.L. Carotenoid pigments of Mycobacterium kansasii. Appl. Microbiol. 1974, 28, 696–699. [Google Scholar] [CrossRef]
- Siegrist, M.S.; Rubin, E.J. Phage transposon mutagenesis. Methods Mol. Biol. 2009, 465, 311–323. [Google Scholar] [PubMed]
- Moise, A.R.; Al-Babili, S.; Wurtzel, E.T. Mechanistic aspects of carotenoid biosynthesis. Chem. Rev. 2014, 114, 164–193. [Google Scholar] [CrossRef]
- Gao, L.Y.; Groger, R.; Cox, J.S.; Beverley, S.M.; Lawson, E.H.; Brown, E.J. Transposon mutagenesis of Mycobacterium marinum identifies a locus linking pigmentation and intracellular survival. Infect. Immun. 2003, 71, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Daffé, M.; Crick, D.C.; Jackson, M. Genetics of capsular polysaccharides and cell envelope (glyco)lipids. Microbiol. Spectr. 2014, 2, 14. [Google Scholar] [CrossRef]
- Berthelot, K.; Estevez, Y.; Deffieux, A.; Peruch, F. Isopentenyl diphosphate isomerase: A checkpoint to isoprenoid biosynthesis. Biochimie 2012, 94, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Eoh, H.; Brennan, P.J.; Crick, D.C. The Mycobacterium tuberculosis MEP (2C-methyl-D-erythritol 4-phosphate) pathway as a new drug target. Tuberculosis 2009, 89, 1–11. [Google Scholar] [CrossRef]
- Viljoen, A.; Dubois, V.; Girard-Misguich, F.; Blaise, M.; Herrmann, J.-L.; Kremer, L. The diverse family of MmpL transporters in mycobacteria: From regulation to antimicrobial developments. Mol. Microbiol. 2017, 104, 889–904. [Google Scholar] [CrossRef]
- Mann, F.M.; Thomas, J.A.; Peters, R.J. Rv0989c encodes a novel (E)-geranyl diphosphate synthase facilitating decaprenyl diphosphate biosynthesis in Mycobacterium tuberculosis. FEBS Lett. 2011, 585, 549–554. [Google Scholar] [CrossRef]
- Mann, F.M.; Xu, M.; Davenport, E.K.; Peters, R.J. Functional characterization and evolution of the isotuberculosinol operon in Mycobacterium tuberculosis and related mycobacteria. Front Microbiol 2012, 3, 368. [Google Scholar] [CrossRef]
- Dhiman, R.K.; Schulbach, M.C.; Mahapatra, S.; Baulard, A.R.; Vissa, V.; Brennan, P.J.; Crick, D.C. Identification of a novel class of omega,E,E-farnesyl diphosphate synthase from Mycobacterium tuberculosis. J. Lipid. Res. 2004, 45, 1140–1147. [Google Scholar] [CrossRef]
- Levendosky, K.; Janisch, N.; Quadri, L.E.N. Comprehensive essentiality analysis of the Mycobacterium kansasii genome by saturation transposon mutagenesis and deep sequencing. 2022; Unpublished results (manuscript in preparation). [Google Scholar]
- Williams, N.K.; Dichtl, B. Co-translational control of protein complex formation: A fundamental pathway of cellular organization? Biochem. Soc. Trans. 2018, 46, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Fournié, M.; Truan, G. Multiplicity of carotene patterns derives from competition between phytoene desaturase diversification and biological environments. Sci. Rep. 2020, 10, 21106. [Google Scholar] [CrossRef] [PubMed]
- Ravanello, M.P.; Ke, D.; Alvarez, J.; Huang, B.; Shewmaker, C.K. Coordinate expression of multiple bacterial carotenoid genes in canola leading to altered carotenoid production. Metab. Eng. 2003, 5, 255–263. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.; Kimura, M. Harvestplus handbook for carotenoid analysis. In Harvest Plus Handbook for Carotenoid Analysis; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2004; pp. 2–11. [Google Scholar]
- Ahrazem, O.; Gómez-Gómez, L.; Rodrigo, M.J.; Avalos, J.; Limón, M.C. Carotenoid cleavage oxygenases from microbes and photosynthetic organisms: Features and functions. Int. J. Mol. Sci. 2016, 17, 1781. [Google Scholar] [CrossRef]
- Di Capua, C.B.; Doprado, M.; Belardinelli, J.M.; Morbidoni, H.R. Complete auxotrophy for unsaturated fatty acids requires deletion of two sets of genes in Mycobacterium smegmatis. Mol. Microbiol. 2017, 106, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Fox, B.G. Identification of Rv3230c as the NADPH oxidoreductase of a two-protein DesA3 acyl-CoA desaturase in Mycobacterium tuberculosis H37Rv. Biochemistry 2006, 45, 13476–13486. [Google Scholar] [CrossRef]
- Phetsuksiri, B.; Jackson, M.; Scherman, H.; McNeil, M.; Besra, G.S.; Baulard, A.R.; Slayden, R.A.; DeBarber, A.E.; Barry, C.E.; Baird, M.S.; et al. Unique mechanism of action of the thiourea drug isoxyl on Mycobacterium tuberculosis. J. Biol. Chem. 2003, 278, 53123–53130. [Google Scholar] [CrossRef]
- Chang, Y.; Wesenberg, G.E.; Bingman, C.A.; Fox, B.G. In vivo inactivation of the mycobacterial integral membrane stearoyl coenzyme A desaturase DesA3 by a C-terminus-specific degradation process. J. Bacteriol. 2008, 190, 6686–6696. [Google Scholar] [CrossRef]
- Pushparajan, A.R.; Edison, L.K.; Ajay Kumar, R. Mycobacterium tuberculosis transcriptional regulator Rv1019 is upregulated in hypoxia, and negatively regulates Rv3230c-Rv3229c operon encoding enzymes in the oleic acid biosynthetic pathway. bioRxiv 2022. [Google Scholar] [CrossRef]
- Koepff, J.; Morschett, H.; Busche, T.; Winkler, A.; Kalinowski, J.; Wiechert, W.; Oldiges, M. Differential transcriptomic analysis reveals hidden light response in Streptomyces lividans. Biotechnol. Prog. 2018, 34, 287–292. [Google Scholar] [CrossRef]
- Hiser, C.; Montgomery, B.L.; Ferguson-Miller, S. TSPO protein binding partners in bacteria, animals, and plants. J. Bioenerg. Biomembr. 2021, 53, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Bonsack, F.; Sukumari-Ramesh, S. TSPO: An evolutionarily conserved protein with elusive functions. Int. J. Mol. Sci. 2018, 19, 1694. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.W.U.; Montgomery, B.L. The tryptophan-rich sensory protein (TSPO) is involved in stress-related and light-dependent processes in the Cyanobacterium Fremyella diplosiphon. Front. Microbiol. 2015, 6, 1393. [Google Scholar] [CrossRef]
- Yeliseev, A.A.; Kaplan, S. A sensory transducer homologous to the mammalian peripheral-type benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 1995, 270, 21167–21175. [Google Scholar] [CrossRef]
- Yeliseev, A.A.; Krueger, K.E.; Kaplan, S. A mammalian mitochondrial drug receptor functions as a bacterial “oxygen” sensor. Proc. Natl. Acad. Sci. USA 1997, 94, 5101–5106. [Google Scholar] [CrossRef] [PubMed]
- Wiker, H.G. MPB70 and MPB83—Major antigens of Mycobacterium bovis. Scand. J. Immunol. 2009, 69, 492–499. [Google Scholar] [CrossRef]
- Said-Salim, B.; Mostowy, S.; Kristof, A.S.; Behr, M.A. Mutations in Mycobacterium tuberculosis Rv0444c, the gene encoding anti-SigK, explain high level expression of MPB70 and MPB83 in Mycobacterium bovis. Mol. Microbiol. 2006, 62, 1251–1263. [Google Scholar] [CrossRef]
- Veyrier, F.; Saïd-Salim, B.; Behr, M.A. Evolution of the mycobacterial SigK regulon. J. Bacteriol. 2008, 190, 1891–1899. [Google Scholar] [CrossRef][Green Version]
- Juárez, M.D.; Torres, A.; Espitia, C. Characterization of the Mycobacterium tuberculosis region containing the mpt83 and mpt70 genes. FEMS Microbiol. Lett. 2001, 203, 95–102. [Google Scholar] [CrossRef]
- Goldstone, D.C.; Metcalf, P.; Baker, E.N. Structure of the ectodomain of the electron transporter Rv2874 from Mycobacterium tuberculosis reveals a thioredoxin-like domain combined with a carbohydrate-binding module. Acta Crystallogr. D Struct. Biol. 2016, 72, 40–48. [Google Scholar] [CrossRef]
- Shukla, J.; Gupta, R.; Thakur, K.G.; Gokhale, R.; Gopal, B. Structural basis for the redox sensitivity of the Mycobacterium tuberculosis SigK-RskA sigma-anti-sigma complex. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- David, H.L. Biogenesis of beta-carotene in Mycobacterium kansasii. J. Bacteriol. 1974, 119, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.F.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbour Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Mohandas, P.; Budell, W.C.; Mueller, E.; Au, A.; Bythrow, G.V.; Quadri, L.E. Pleiotropic consequences of gene knockouts in the phthiocerol dimycocerosate and phenolic glycolipid biosynthetic gene cluster of the opportunistic human pathogen Mycobacterium marinum. FEMS Microbiol. Lett. 2016, 363, fnw016. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.T.; Schwartzman, J.A.; Gilmore, M.S. Mapping transposon insertions in bacterial genomes by arbitrarily primed PCR. Curr. Protoc. Mol. Biol. 2017, 118, 15.15.11–15.15.15. [Google Scholar] [CrossRef] [PubMed]
- Belisle, J.T.; Sonnenberg, M.G. Isolation of genomic DNA from mycobacteria. Methods Mol. Biol. 1998, 101, 31–44. [Google Scholar]
- Billi, D.; Grilli Caiola, M.; Paolozzi, L.; Ghelardini, P. A method for DNA extraction from the desert Cyanobacterium Chroococcidiopsis and its application to identification of ftsZ. Appl. Environ. Microbiol. 1998, 64, 4053–4056. [Google Scholar] [CrossRef]
- Huff, J.; Czyz, A.; Landick, R.; Niederweis, M. Taking phage integration to the next level as a genetic tool for mycobacteria. Gene 2010, 468, 8–19. [Google Scholar] [CrossRef]
- Ferreras, J.A.; Stirrett, K.L.; Lu, X.; Ryu, J.S.; Soll, C.E.; Tan, D.S.; Quadri, L.E. Mycobacterial phenolic glycolipid virulence factor biosynthesis: Mechanism and small-molecule inhibition of polyketide chain initiation. Chem. Biol. 2008, 15, 51–61. [Google Scholar] [CrossRef]
- Budell, W.C. Transposon Mutagenesis Facilitates Discovery of Genotype-Phenotype Associations and Functional Interrogation of the Mycobacterium kansasii Genome. Ph.D. Thesis, The City University of New York, New York, NY, USA, 2019. [Google Scholar]
- Ehrt, S.; Guo, X.V.; Hickey, C.M.; Ryou, M.; Monteleone, M.; Riley, L.W.; Schnappinger, D. Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res. 2005, 33, e21. [Google Scholar] [CrossRef]
- Bythrow, G.V.; Farhat, M.F.; Levendosky, K.; Mohandas, P.; Germain, G.A.; Yoo, B.; Quadri, L.E.N. Mycobacterium abscessus mutants with a compromised functional link between the type VII ESX-3 system and an iron uptake mechanism reliant on an unusual mycobactin siderophore. Pathogens 2022, 11, 953. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdottir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Salmeron, J.E.; Moreno-Hagelsieb, G. Progress in quickly finding orthologs as reciprocal best hits: Comparing blast, last, diamond and MMseqs2. BMC Genom. 2020, 21, 741. [Google Scholar] [CrossRef]
- Oberto, J. SyntTax: A web server linking synteny to prokaryotic taxonomy. BMC Bioinform. 2013, 14, 4. [Google Scholar] [CrossRef]
- Sumi, S.; Suzuki, Y.; Matsuki, T.; Yamamoto, T.; Tsuruta, Y.; Mise, K.; Kawamura, T.; Ito, Y.; Shimada, Y.; Watanabe, E.; et al. Light-inducible carotenoid production controlled by a MarR-type regulator in Corynebacterium glutamicum. Sci. Rep. 2019, 9, 13136. [Google Scholar] [CrossRef]
- Henke, N.A.; Heider, S.A.E.; Hannibal, S.; Wendisch, V.F.; Peters-Wendisch, P. Isoprenoid pyrophosphate-dependent transcriptional regulation of carotenogenesis in Corynebacterium glutamicum. Front. Microbiol. 2017, 8, 633. [Google Scholar] [CrossRef]
- Gökalsın, B.; Aksoydan, B.; Erman, B.; Sesal, N.C. Reducing virulence and biofilm of Pseudomonas aeruginosa by potential quorum sensing inhibitor carotenoid: Zeaxanthin. Microb. Ecol. 2017, 74, 466–473. [Google Scholar] [CrossRef]
- Tran, T.; Dawrs, S.N.; Norton, G.J.; Virdi, R.; Honda, J.R. Brought to you courtesy of the red, white and blue—Pigments of nontuberculous mycobacteria. AIMS Microbiol. 2020, 6, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Sivanesan, I.; Keum, Y.S. Emerging roles of carotenoids in the survival and adaptations of microbes. Indian J. Microbiol. 2019, 59, 125–127. [Google Scholar] [CrossRef] [PubMed]






| Locus Tag 1 (Gene Name) | Predicted Gene Product Function 2 | Dark Condition Readcount 3 | Light Condition Readcount | Fold Change 4 | Q-Value 5 | |
|---|---|---|---|---|---|---|
| CRT locus genes | MKAN_RS10020 (crtE) | GGPP synthase | 8 | 1278 | 160 | 1.5 × 10−278 |
| MKAN_RS10025 (crtI) | Phytoene dehydrogenase | 11 | 1771 | 161 | 0 | |
| MKAN_RS10030 (crtB) | Phytoene synthase | 8 | 1124 | 141 | 7.9 × 10−249 | |
| MKAN_RS10035 (crtYc) | Lycopene cyclase | <1 | 29 | 221 | 3.6 × 10−5 | |
| MKAN_RS10040 (crtYd) | Lycopene cyclase | <1 | 88 | 334 | 1.5 × 10−16 | |
| MKAN_RS10045 (mmpL1) | MmpL family transporter | 33 | 1339 | 41 | 0 | |
| MKAN_RS10050 (crtR) | MarR-type regulator | 127 | 331 | 3 | 3.5 × 10−18 | |
| MKAN_RS10055 (fni) | Isopentenyl diphosphate isomerase | 124 | 1007 | 8 | 3.4 × 10−162 | |
| Photolyase locus genes | MKAN_RS22245 (phrB) | Deoxyribodipyrimidine photolyase | 15 | 474 | 32 | 2.7 × 10−106 |
| MKAN_RS22250 (tspO) | Tryptophan-rich sensory protein | 9 | 258 | 29 | 5.1 × 10−57 | |
| MKAN_RS22255 (mmpL2) | MmpL family transporter | 84 | 2277 | 27 | 0 | |
| Loci of unknown function | MKAN_RS11600 (mpk83) | Fasciclin domain-containing protein | 6 | 36 | 6 | 8.1 × 10−4 |
| MKAN_RS11610 (mpk70) | Fasciclin domain-containing protein | 21 | 64 | 3 | 1.0 × 10−3 | |
| MKAN_RS19770 | PE family protein | 121 | 601 | 5 | 3.2 × 10−71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janisch, N.; Levendosky, K.; Budell, W.C.; Quadri, L.E.N. Genetic Underpinnings of Carotenogenesis and Light-Induced Transcriptome Remodeling in the Opportunistic Pathogen Mycobacterium kansasii. Pathogens 2023, 12, 86. https://doi.org/10.3390/pathogens12010086
Janisch N, Levendosky K, Budell WC, Quadri LEN. Genetic Underpinnings of Carotenogenesis and Light-Induced Transcriptome Remodeling in the Opportunistic Pathogen Mycobacterium kansasii. Pathogens. 2023; 12(1):86. https://doi.org/10.3390/pathogens12010086
Chicago/Turabian StyleJanisch, Niklas, Keith Levendosky, William C. Budell, and Luis E. N. Quadri. 2023. "Genetic Underpinnings of Carotenogenesis and Light-Induced Transcriptome Remodeling in the Opportunistic Pathogen Mycobacterium kansasii" Pathogens 12, no. 1: 86. https://doi.org/10.3390/pathogens12010086
APA StyleJanisch, N., Levendosky, K., Budell, W. C., & Quadri, L. E. N. (2023). Genetic Underpinnings of Carotenogenesis and Light-Induced Transcriptome Remodeling in the Opportunistic Pathogen Mycobacterium kansasii. Pathogens, 12(1), 86. https://doi.org/10.3390/pathogens12010086







