The Cytokine Profile in Different Stages of Schistosomiasis Japonica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Processing
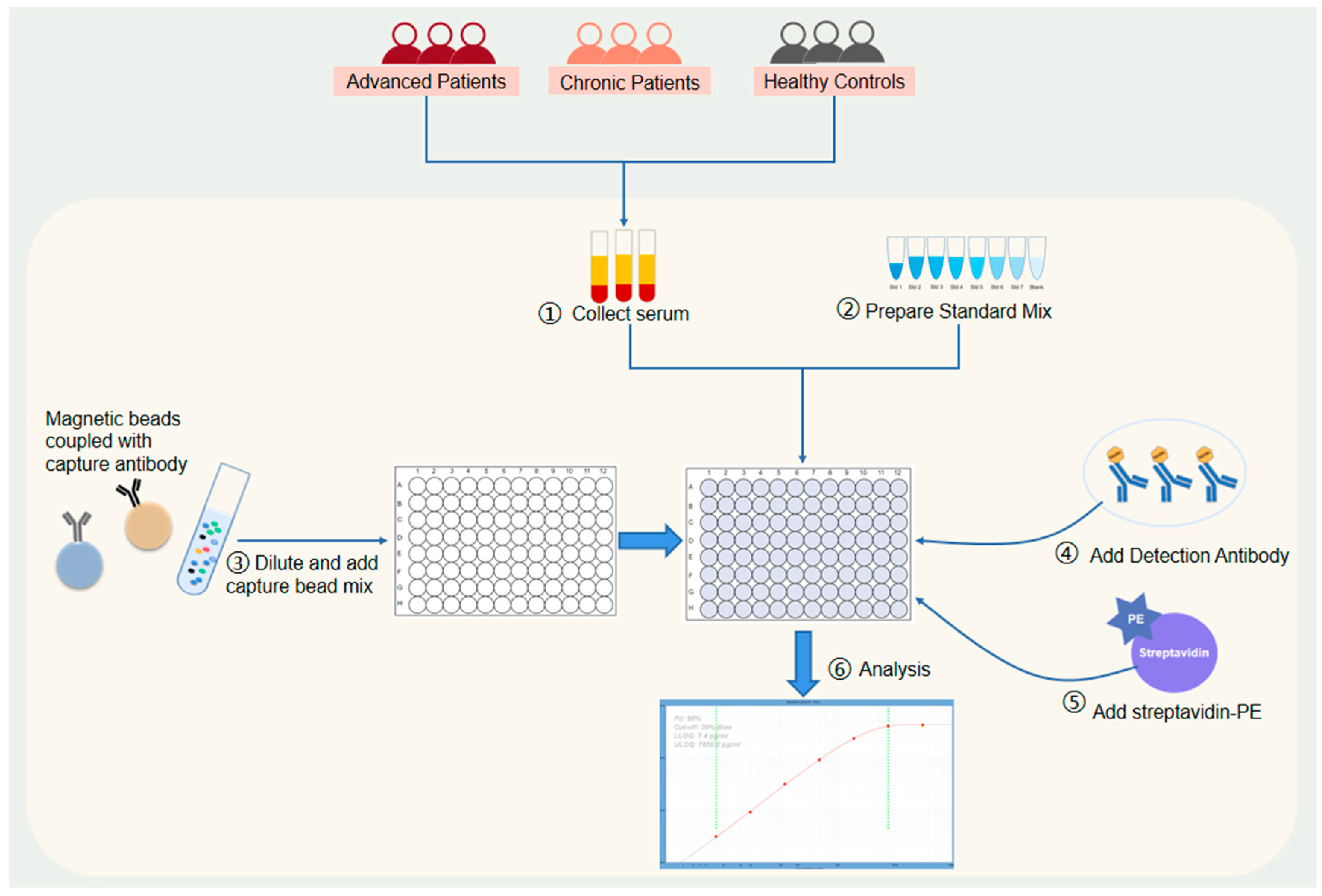
2.3. Cytokine Detection
2.4. Ethics Approval
2.5. Data Analysis
3. Results
3.1. Patient Characteristics
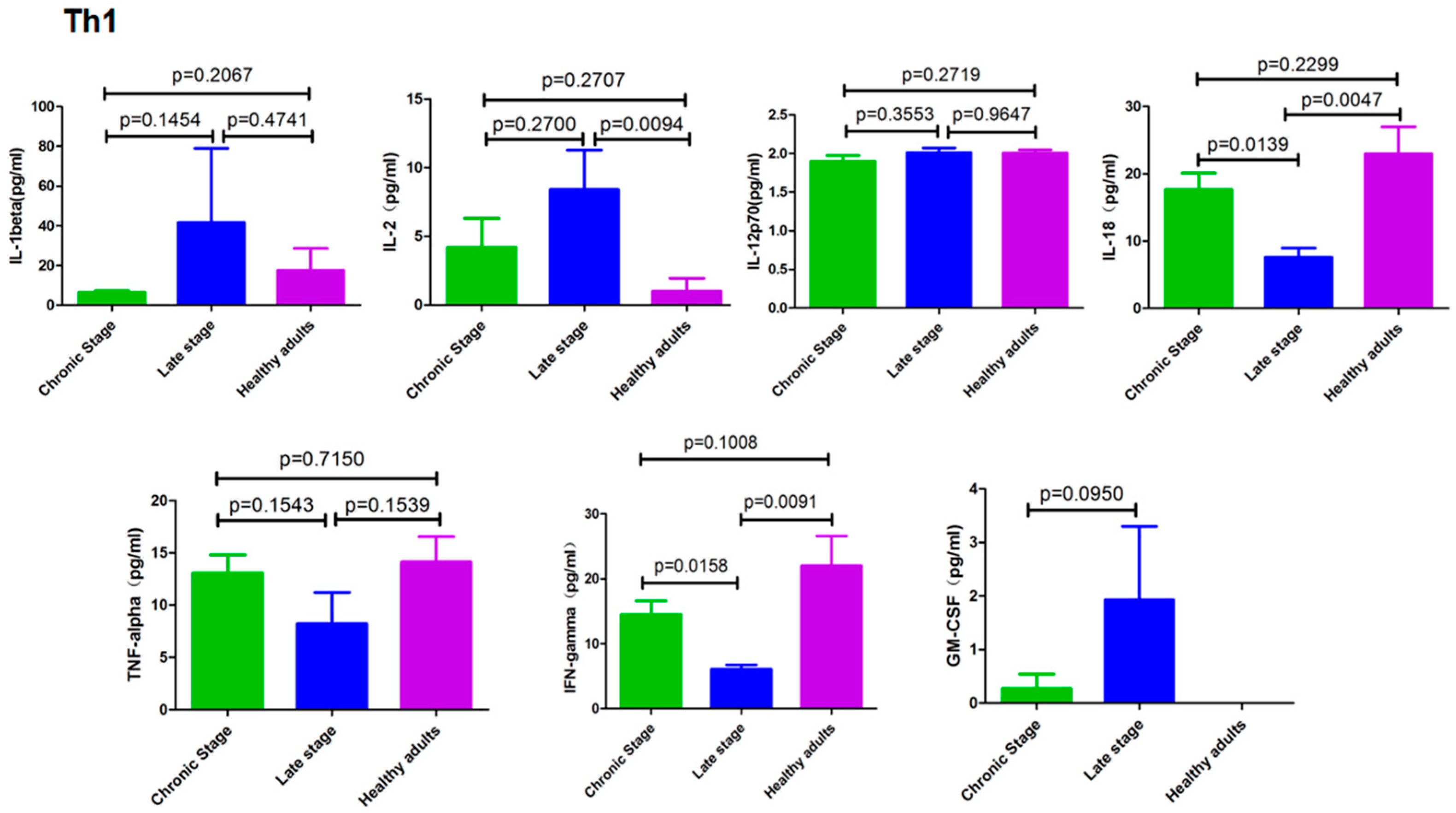
3.2. Cytokines Secreted by Th1 cells
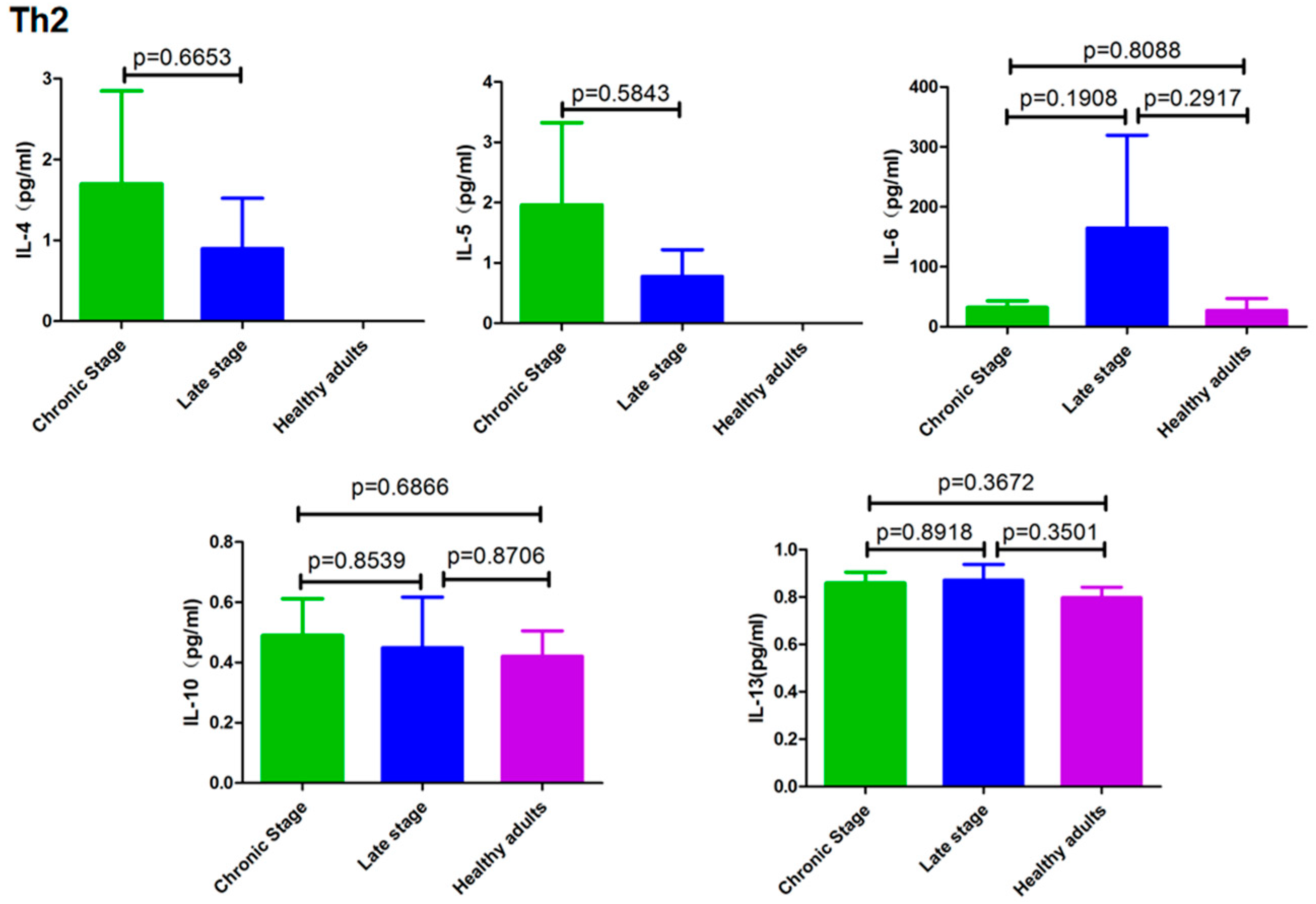
3.3. Cytokines Secreted by Th2 Cells
3.4. Cytokines Secreted by Th9/Th17/Th22/Treg Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Fact Sheet. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 16 July 2023).
- LoVerde, P.T. Schistosomiasis. Adv. Exp. Med. Biol. 2019, 1154, 45–70. [Google Scholar] [PubMed]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Bergquist, R.; Cai, P.; Ranasinghe, S.; Tebeje, B.M.; You, H. Schistosomiasis-from immunopathology to vaccines. Semin. Immunopathol. 2020, 42, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Ogongo, P.; Nyakundi, R.K.; Chege, G.K.; Ochola, L. The Road to Elimination: Current State of Schistosomiasis Research and Progress Towards the End Game. Front. Immunol. 2022, 13, 846108. [Google Scholar] [CrossRef]
- Pearce, E.J.; MacDonald, A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002, 2, 499–511. [Google Scholar] [CrossRef]
- Scott, P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J. Immunol. 1991, 147, 3149–3155. [Google Scholar] [CrossRef]
- Silva, R.A.; Flórido, M.; Appelberg, R. Interleukin-12 primes CD4+ T cells for interferon-γ production and protective immunity during Mycobacterium avium infection. Immunology 2001, 103, 368–374. [Google Scholar] [CrossRef]
- Chen, L.; Grabowski, K.A.; Xin, J.-P.; Coleman, J.; Huang, Z.; Espiritu, B.; Alkan, S.; Xie, H.B.; Zhu, Y.; White, F.A.; et al. IL-4 Induces Differentiation and Expansion of Th2 Cytokine-Producing Eosinophils. J. Immunol. 2004, 172, 2059–2066. [Google Scholar] [CrossRef]
- Muñoz-Carrillo, J.L.; Contreras-Cordero, J.F.; Gutiérrez-Coronado, O.; Villalobos-Gutiérrez, P.T.; Ramos-Gracia, L.G.; Hernández-Reyes, V.E. Cytokine Profiling Plays a Crucial Role in Activating Immune System to Clear Infectious Pathogens. In Immune Response Activation and Immunomodulation; Tyagi, R.K., Bisen, P.S., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, J.; Chen, H.; Nie, H.; Miller, H.; Gong, Q.; Liu, C. T Lymphocyte-Mediated Liver Immunopathology of Schistosomiasis. Front. Immunol. 2020, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Rutitzky, L.I.; Bazzone, L.; Shainheit, M.G.; Joyce-Shaikh, B.; Cua, D.J.; Stadecker, M.J. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17. J. Immunol. 2008, 180, 2486–2495. [Google Scholar] [CrossRef]
- Franco, K.G.S.; de Amorim, F.J.R.; Santos, M.A.; Rollemberg, C.V.V.; de Oliveira, F.A.; França, A.V.C.; Santos, C.N.O.; Magalhães, L.S.; Cazzaniga, R.A.; de Lima, F.S.; et al. Association of IL-9, IL-10, and IL-17 Cytokines with Hepatic Fibrosis in Human Schistosoma mansoni Infection. Front. Immunol. 2021, 12, 779534. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.F.; Cheever, A.W.; Wynn, T.A. IL-10 and the dangers of immune polarization: Excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 2000, 164, 6406–6416. [Google Scholar] [CrossRef]
- Hirata, M.; Fukuma, T. Cytokine regulation in experimentally-induced Schistosoma japonicum egg granuloma formation. Parasitol. Int. 2003, 52, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Shainheit, M.G.; Lasocki, K.W.; Finger, E.; Larkin, B.M.; Smith, P.M.; Sharpe, A.H.; Dinarello, C.A.; Rutitzky, L.I.; Stadecker, M.J. The pathogenic Th17 cell response to major schistosome egg antigen is sequentially dependent on IL-23 and IL-1β. J. Immunol. 2011, 187, 5328–5335. [Google Scholar] [CrossRef] [PubMed]
- Larkin, B.M.; Smith, P.M.; Ponichtera, H.E.; Shainheit, M.G.; Rutitzky, L.I.; Stadecker, M.J. Induction and regulation of pathogenic th17 cell responses in schistosomiasis. Semin. Immunopathol. 2012, 34, 873–888. [Google Scholar] [CrossRef]
- Zhu, Y.; Ni, Y.-G.; Liu, R.; Hou, M.; Yang, B.-Y.; Song, J.-W.; Sun, H.-Z.; Xu, Z.-P.; Ji, M.-J. PPAR-γ Agonist Alleviates Liver and Spleen Pathology via Inducing Treg Cells during Schistosoma japonicum Infection. J. Immunol. Res. 2018, 2018, 6398078. [Google Scholar] [CrossRef]
- Chen, X.-J.; Li, W.; Zhang, Y.; Song, X.; Xu, L.; Xu, Z.; Zhou, S.; Zhu, J.; Jin, X.; Liu, F.; et al. Distribution of Peripheral Memory T Follicular Helper Cells in Patients with Schistosomiasis Japonica. PLoS Negl. Trop. Dis. 2015, 9, e0004015. [Google Scholar] [CrossRef]
- Hiatt, R.A.; Sotomayor, Z.R.; Sanchez, G.; Zambrana, M.; Knight, W.B. Factors in the pathogenesis of acute schistosomiasis mansoni. J. Infect. Dis. 1979, 139, 659–666. [Google Scholar] [CrossRef]
- Caldas, I.R.; Campi-Azevedo, A.C.; Oliveira, L.F.; Silveira, A.M.; Oliveira, R.C.; Gazzinelli, G. Human Schistosomiasis mansoni: Immune responses during acute and chronic phases of the infection. Acta Trop. 2008, 108, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, R.; Wadhwa, M.; Bird, C.R.; Mire-Sluis, A.R. Detection and measurement of cytokines. Blood Rev. 1992, 6, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, N.; Asthana, D. Cytokine quantitation: Technologies and applications. Front. Biosci. 2007, 12, 4682–4695. [Google Scholar] [CrossRef]
- Lynch, H.E.; Sanchez, A.M.; D’Souza, M.P.; Rountree, W.; Denny, T.N.; Kalos, M.; Sempowski, G.D. Development and implementation of a proficiency testing program for Luminex bead-based cytokine assays. J. Immunol. Methods. 2014, 409, 62–71. [Google Scholar] [CrossRef]
- Kupcova Skalnikova, H.; Vodickova Kepkova, K.; Vodicka, P. Luminex xMAP Assay to Quantify Cytokines in Cancer Patient Serum. Methods Mol. Biol. 2020, 2108, 65–88. [Google Scholar] [CrossRef]
- Katz, N.; Chaves, A.; Pellegrino, J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 1972, 14, 397–400. [Google Scholar]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.; Rajko-Nenow, P.; Batten, C.; Flannery, J. Simultaneous Detection of Bluetongue Virus Serotypes Using xMAP Technology. Microorganisms 2020, 8, 1564. [Google Scholar] [CrossRef]
- Tang, C.; Chen, S.; Qian, H.; Huang, W. Interleukin-23: As a drug target for autoimmune inflammatory diseases. Immunology 2012, 135, 112–124. [Google Scholar] [CrossRef]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Front. Immunol. 2021, 12, 637829. [Google Scholar] [CrossRef]
- Eyerich, K.; Dimartino, V.; Cavani, A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur. J. Immunol. 2017, 47, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, Q.; Song, R.; Duan, B.; Bergquist, R.; Xu, J.; Li, S.; Zhou, D.; Qin, Z. Antinuclear antibodies and interleukin responses in patients with Schistosoma japonicum infection. Parasite Immunol. 2018, 40, e12577. [Google Scholar] [CrossRef] [PubMed]
- Chuah, C.; Jones, M.K.; Burke, M.L.; McManus, D.P.; Gobert, G.N. Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol. 2014, 30, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Alvarez, C.A.; Cobb, B.A. Integration of IL-2 and IL-4 signals coordinates divergent regulatory T cell responses and drives therapeutic efficacy. eLife 2021, 10, e57417. [Google Scholar] [CrossRef]
- Arnaud, V.; Li, J.; Wang, Y.; Fu, X.; Mengzhi, S.; Luo, X.; Hou, X.; Dessein, H.; Jie, Z.; Xin-Ling, Y.; et al. Regulatory role of interleukin-10 and interferon-gamma in severe hepatic central and peripheral fibrosis in humans infected with Schistosoma japonicum. J. Infect. Dis. 2008, 198, 418–426. [Google Scholar] [CrossRef]
- La Flamme, A.C.; Patton, E.A.; Bauman, B.; Pearce, E.J. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J. Immunol. 2001, 166, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Henri, S.; Chevillard, C.; Mergani, A.; Paris, P.; Gaudart, J.; Camilla, C.; Dessein, H.; Montero, F.; Elwali, N.-E.M.A.; Saeed, O.K.; et al. Cytokine regulation of periportal fibrosis in humans infected with Schistosoma mansoni: IFN-gamma is associated with protection against fibrosis and TNF-alpha with aggravation of disease. J. Immunol. 2002, 169, 929–936. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Gao, W.; Hou, X.; Gu, Y.; Gui, L.; Huang, D.; Liu, M.; Ren, C.; Wang, S.; et al. IL-17 neutralization significantly ameliorates hepatic granulomatous inflammation and liver damage in Schistosoma japonicum infected mice. Eur. J. Immunol. 2012, 42, 1523–1535. [Google Scholar] [CrossRef]
- Rutitzky, L.I.; Lopes da Rosa, J.R.; Stadecker, M.J. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J. Immunol. 2005, 175, 3920–3926. [Google Scholar] [CrossRef]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, A.; Usui, T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediat. Inflamm. 2015, 2015, 568543. [Google Scholar] [CrossRef] [PubMed]
- El-Behi, M.; Ciric, B.; Dai, H.; Yan, Y.; Cullimore, M.; Safavi, F.; Zhang, G.X.; Dittel, B.N.; Rostami, A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011, 12, 568–575. [Google Scholar] [CrossRef]
- Lotfi, N.; Thome, R.; Rezaei, N.; Zhang, G.X.; Rezaei, A.; Rostami, A.; Esmaeil, N. Roles of GM-CSF in the Pathogenesis of Autoimmune Diseases: An Update. Front. Immunol. 2019, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Postal, M.; Appenzeller, S. The role of Tumor Necrosis Factor-alpha (TNF-α) in the pathogenesis of systemic lupus erythematosus. Cytokine 2011, 56, 537–543. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Chen, D.; Xie, H.; Cha, H.; Qu, J.; Wang, M.; Li, L.; Yu, S.; Wu, C.; Tang, X.; Huang, J. Characteristics of Schistosoma japonicum infection induced IFN-γ and IL-4 co-expressing plasticity Th cells. Immunology 2016, 149, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, S.; Wang, J. IFN-γ, should not be ignored in SLE. Front. Immunol. 2022, 13, 954706. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Schmidt, T.; Schmid-Burgk, J.L.; Rapino, F.; Robertson, A.A.; Cooper, M.A.; Graf, T.; Hornung, V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity 2016, 44, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor. Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Newland, S.A.; Gibbs, S.E.; Tourlomousis, P.; Fernandes dos Santos, P.; Patel, M.N.; Hall, S.W.; Walczak, H.; Schramm, G.; Haas, H.; et al. The Schistosoma mansoni T2 ribonuclease omega-1 modulates inflammasome-dependent IL-1β secretion in macrophages. Int. J. Parasitol. 2015, 45, 809–813. [Google Scholar] [CrossRef]
- Abbas, A.K.; Trotta, E.; RSimeonov, D.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3, eaat1482. [Google Scholar] [CrossRef]
- Graßhoff, H.; Comdühr, S.; Monne, L.R.; Müller, A.; Lamprecht, P.; Riemekasten, G.; Humrich, J.Y. Low-Dose IL-2 Therapy in Autoimmune and Rheumatic Diseases. Front. Immunol. 2021, 12, 648408. [Google Scholar] [CrossRef]
- Iwaszko, M.; Biały, S.; Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef]
- Zhan, T.; Ma, H.; Jiang, S.; Zhong, Z.; Wang, X.; Li, C.; Yu, D.; Liu, L.; Xu, J.; Xia, C. Interleukin-9 blockage reduces early hepatic granuloma formation and fibrosis during Schistosoma japonicum infection in mice. Immunology 2019, 158, 296–303. [Google Scholar] [CrossRef]
- Schwartz, C.; Oeser, K.; Prazeres da Costa, C.; Layland, L.E.; Voehringer, D. T cell-derived IL-4/IL-13 protects mice against fatal Schistosoma mansoni infection independently of basophils. J. Immunol. 2014, 193, 3590–3599. [Google Scholar] [CrossRef]
- Dougan, M.; Dranoff, G.; Dougan, S.K. GM-CSF, IL-3, and IL-5 Family of Cytokines: Regulators of Inflammation. Immunity 2019, 50, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Kouro, T.; Takatsu, K. IL-5- and eosinophil-mediated inflammation: From discovery to therapy. Int. Immunol. 2009, 21, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, D.; Luo, X.; Gao, Z.; Fang, H.; Huang, J. Some characteristics of IL-5-producing T cells in mouse liver induced by Schistosoma japonicum infection. Parasitol. Res. 2013, 112, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Dienz, O.; Eaton, S.M.; Bond, J.P.; Neveu, W.; Moquin, D.; Noubade, R.; Briso, E.M.; Charland, C.; Leonard, W.J.; Ciliberto, G.; et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 2009, 206, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Saxton, R.A.; Tsutsumi, N.; Su, L.L.; Abhiraman, G.C.; Mohan, K.; Henneberg, L.T.; Aduri, N.G.; Gati, C.; Garcia, K.C. Structure-based decoupling of the pro- and anti-inflammatory functions of interleukin-10. Science 2021, 371, eabc8433. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Vignali, D.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Migliorini, P.; Italiani, P.; Pratesi, F.; Puxeddu, I.; Boraschi, D. The IL-1 family cytokines and receptors in autoimmune diseases. Autoimmun. Rev. 2020, 19, 102617. [Google Scholar] [CrossRef] [PubMed]
- Vecchié, A.; Bonaventura, A.; Toldo, S.; Dagna, L.; Dinarello, C.A.; Abbate, A. IL-18 and infections: Is there a role for targeted therapies? J. Cell Physiol. 2021, 236, 1638–1657. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Feng, Y.; Wang, Y.; Wang, J.; Zhang, Z.; Liang, J.; Xu, J. Correlation between circulating interleukin-18 level and systemic lupus erythematosus: A meta-analysis. Sci. Rep. 2021, 11, 4707. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Zhang, T.; Wang, Y.; Wang, X.; Lin, C.; Ma, H.; Duan, Z.; Li, C.; Xu, J.; Xia, C. Dynamics of Th9 cells and their potential role in immunopathogenesis of murine schistosomiasis. Parasites Vectors 2017, 10, 305. [Google Scholar] [CrossRef]
- Long, D.; Chen, Y.; Wu, H.; Zhao, M.; Lu, Q. Clinical significance and immunobiology of IL-21 in autoimmunity. J. Autoimmun. 2020, 99, 1–14, Correction in J. Autoimmun. 2020, 111, 102455. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Kumar, V.; Karnell, J.L.; Naiman, B.; Gross, P.S.; Rahman, S.; Zerrouki, K.; Hanna, R.; Morehouse, C.; et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat. Commun. 2018, 9, 1758. [Google Scholar] [CrossRef] [PubMed]
- Valeri, M.; Raffatellu, M. Cytokines IL-17 and IL-22 in the host response to infection. Pathog. Dis. 2016, 74, ftw111. [Google Scholar] [CrossRef]
- Xin, N.; Namaka, M.P.; Dou, C.; Zhang, Y. Exploring the role of interleukin-22 in neurological and autoimmune disorders. Int. Immunopharmacol. 2015, 28, 1076–1083. [Google Scholar] [CrossRef]
- Villarino, A.V.; Stumhofer, J.S.; Saris, C.J.; Kastelein, R.A.; de Sauvage, F.J.; Hunter, C.A. IL-27 limits IL-2 production during Th1 differentiation. J. Immunol. 2006, 176, 237–247. [Google Scholar] [CrossRef]
- Mei, Y.; Lv, Z.; Xiong, L.; Zhang, H.; Yin, N.; Qi, H. The dual role of IL-27 in CD4+T cells. Mol. Immunol. 2021, 138, 172–180. [Google Scholar] [CrossRef] [PubMed]

| Patient/Disease Group | Age(Mean ± s) | Gender | |
|---|---|---|---|
| Male | Female | ||
| Chronic stage | 54.8 ± 1.65 | 18 (64.3%) | 10 (35.7%) |
| Late stage | 49.7 ± 1.26 | 7 (58.3%) | 5 (41.7%) |
| Healthy adults | 47.6 ± 2.96 | 9 (50%) | 9 (50%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tang, Q.; Bergquist, R.; Zhou, X.; Qin, Z. The Cytokine Profile in Different Stages of Schistosomiasis Japonica. Pathogens 2023, 12, 1201. https://doi.org/10.3390/pathogens12101201
Wang X, Tang Q, Bergquist R, Zhou X, Qin Z. The Cytokine Profile in Different Stages of Schistosomiasis Japonica. Pathogens. 2023; 12(10):1201. https://doi.org/10.3390/pathogens12101201
Chicago/Turabian StyleWang, Xi, Qi Tang, Robert Bergquist, Xiaorong Zhou, and Zhiqiang Qin. 2023. "The Cytokine Profile in Different Stages of Schistosomiasis Japonica" Pathogens 12, no. 10: 1201. https://doi.org/10.3390/pathogens12101201
APA StyleWang, X., Tang, Q., Bergquist, R., Zhou, X., & Qin, Z. (2023). The Cytokine Profile in Different Stages of Schistosomiasis Japonica. Pathogens, 12(10), 1201. https://doi.org/10.3390/pathogens12101201






