In Vivo Efficacy of Curcumin and Curcumin Nanoparticle in Trypanosoma congolense, Broden 1904 (Kinetoplastea: Trypanosomatidae)-Infected Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Curcumin Compound
2.2. Preparation of Nanoparticles
2.2.1. Antisolvent Precipitation with a Syringe Pump (ASPS)
2.2.2. Evaporative Precipitation of Nanosuspension (EPN)
2.2.3. Wet-Milling Method (WN)
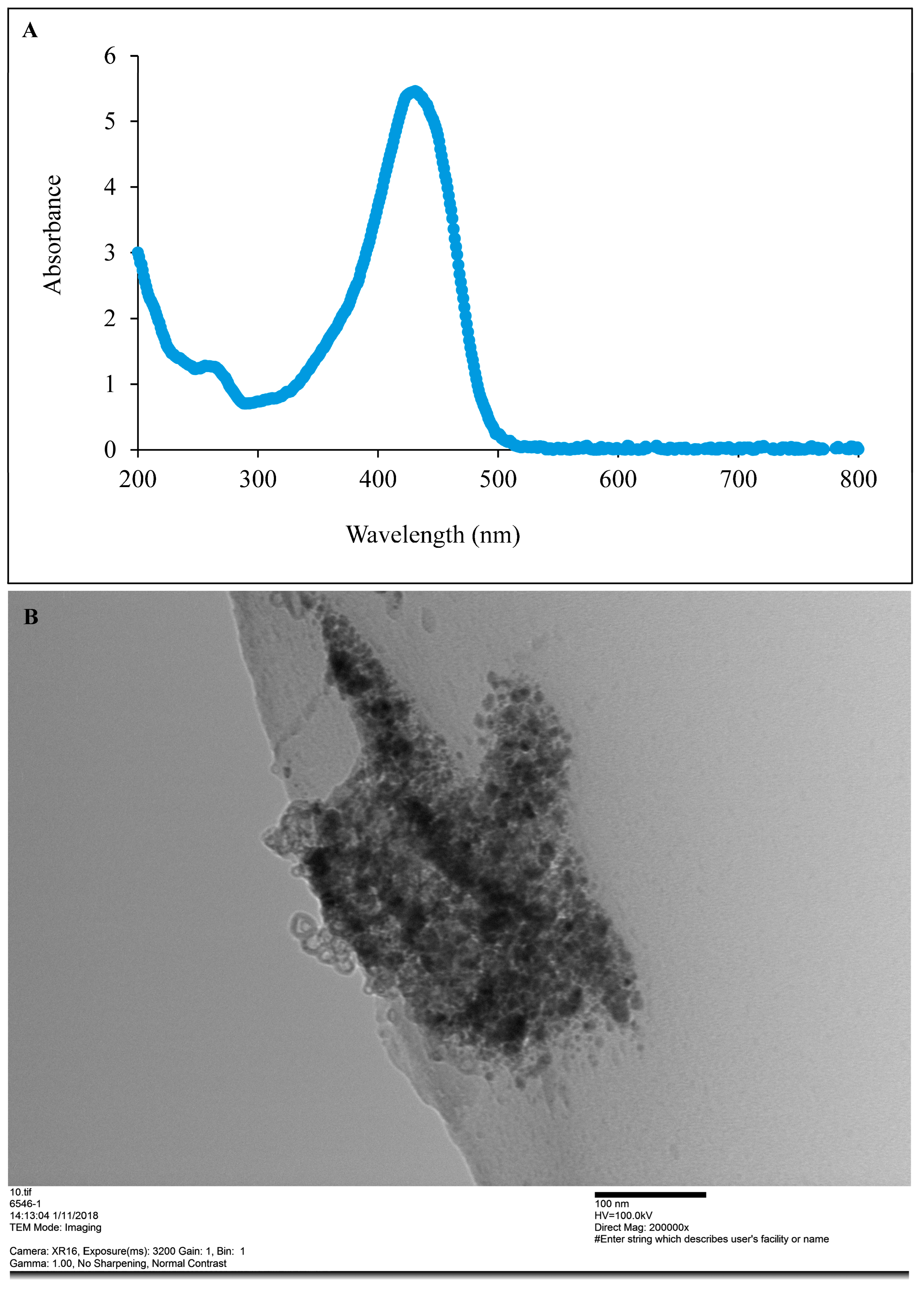
2.2.4. Preliminary Detection of CUR-Nanoparticle Using UV-Visible Spectroscopy
2.2.5. Particle Size and Shape Analysis
2.3. Trypanosome Cultures
In Vitro Anti-Trypanosoma Assay
2.4. Mammalian Cell Line Cultures
In Vitro Cytotoxicity Tests
2.5. Animal Experiments
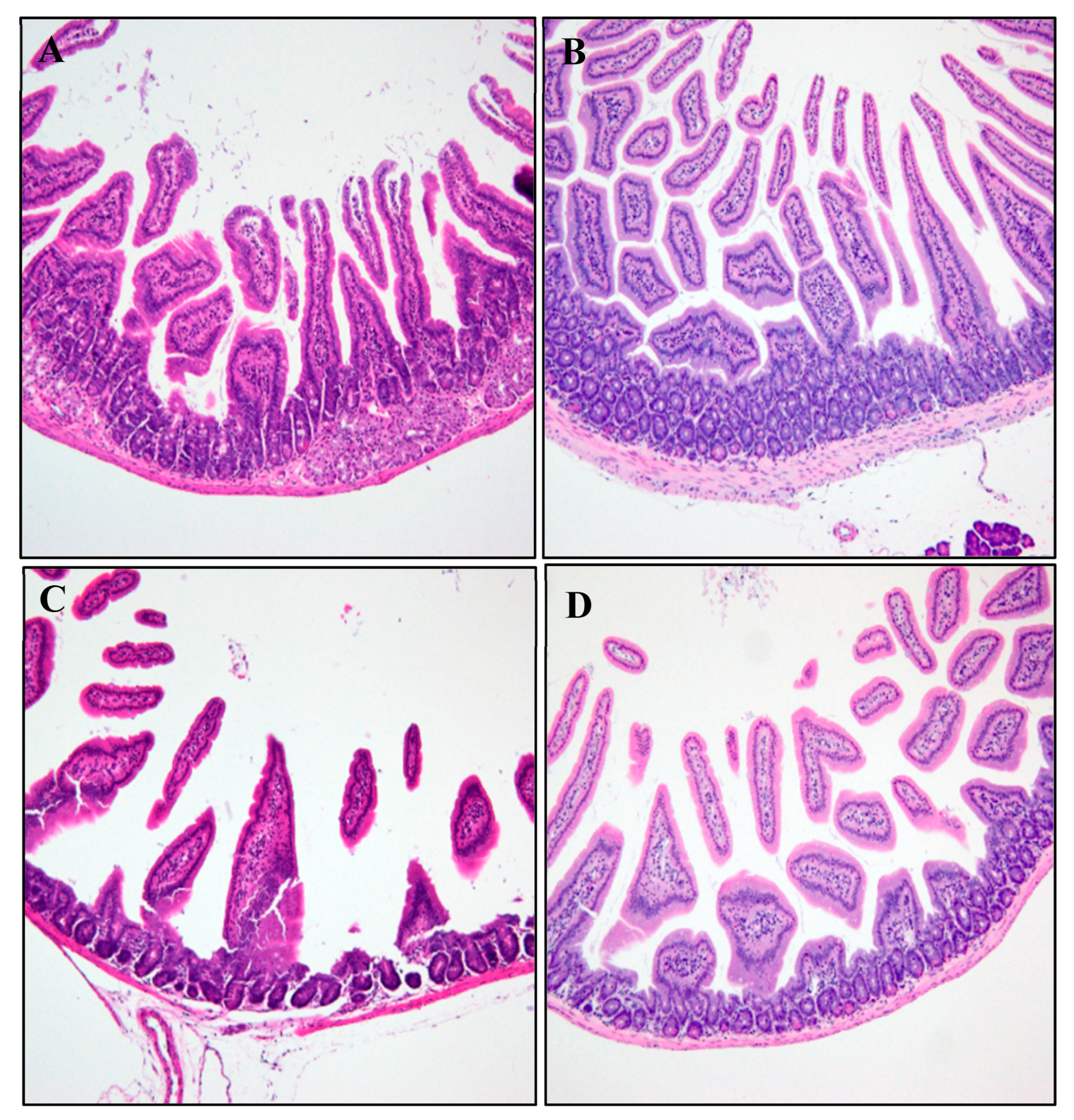
2.5.1. Histopathological Analysis
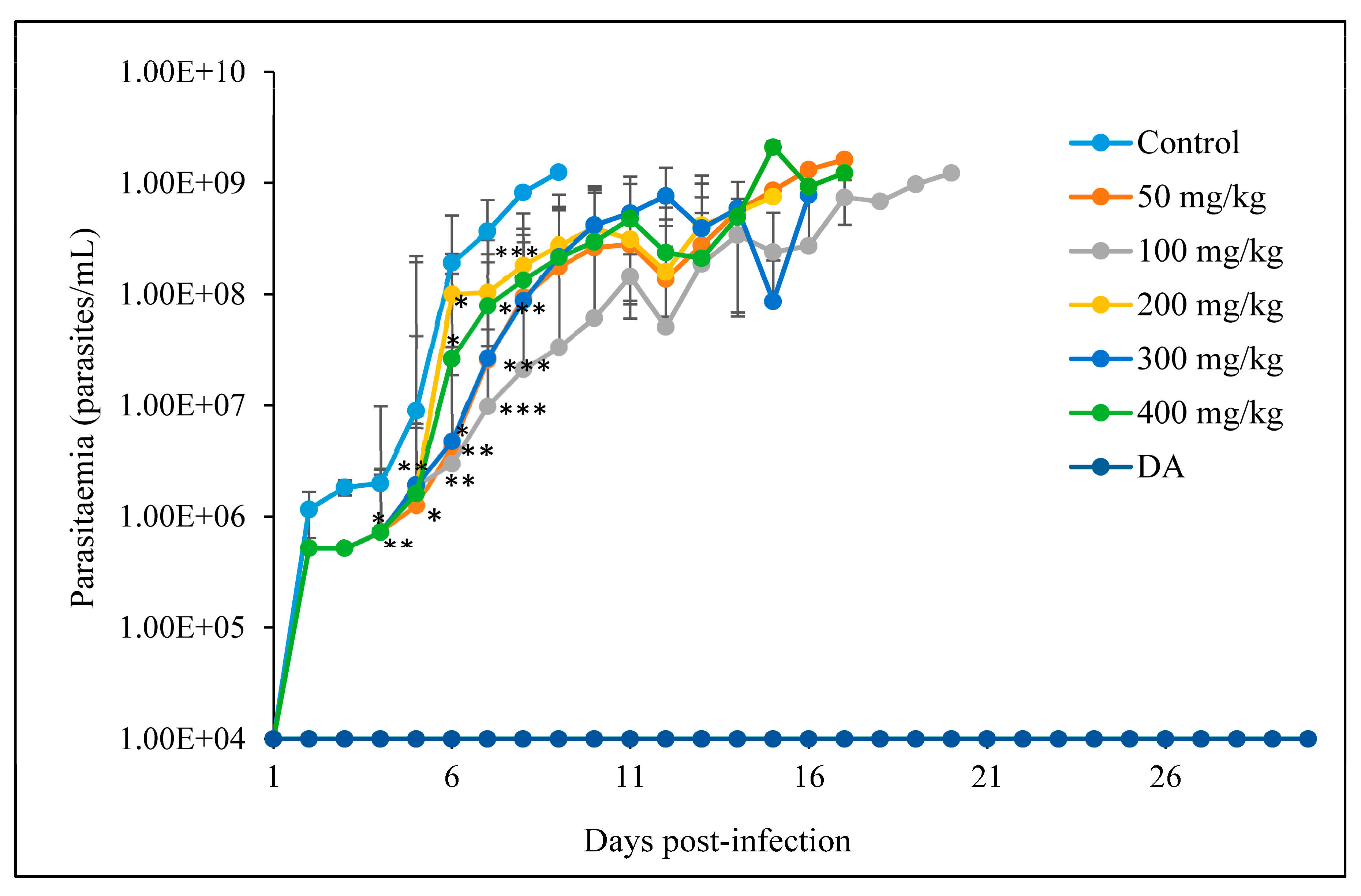
2.5.2. The In Vivo Anti-Trypanosoma Effects of CUR and CUR-Nanoparticles on Trypanosoma Congolense-Infected Mice
- A
- Oral administration
- B
- Intraperitoneal administration
2.6. Statistical Analysis
3. Results
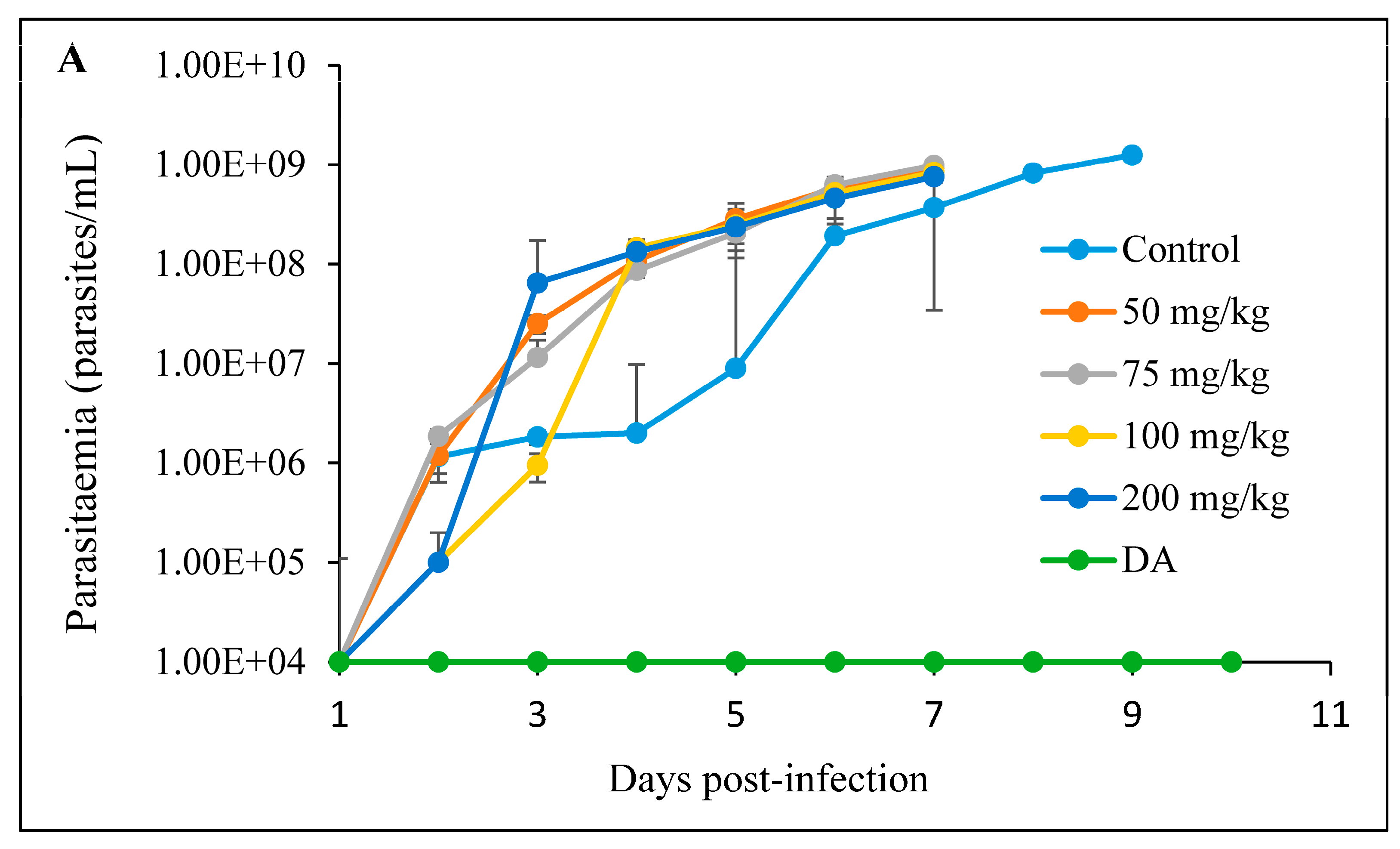
3.1. In Vitro Anti-Trypanosoma and Cytotoxic Effects
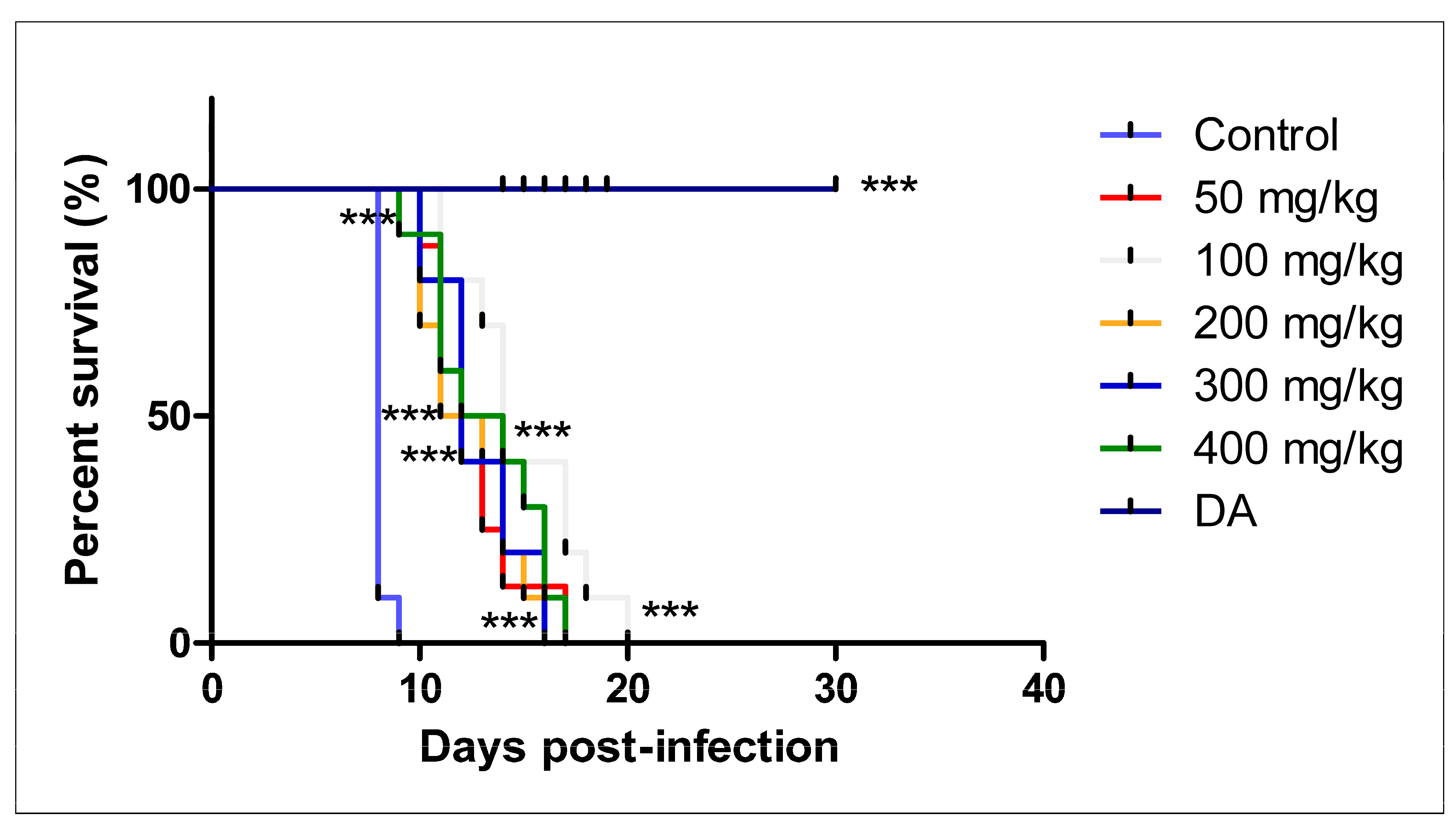
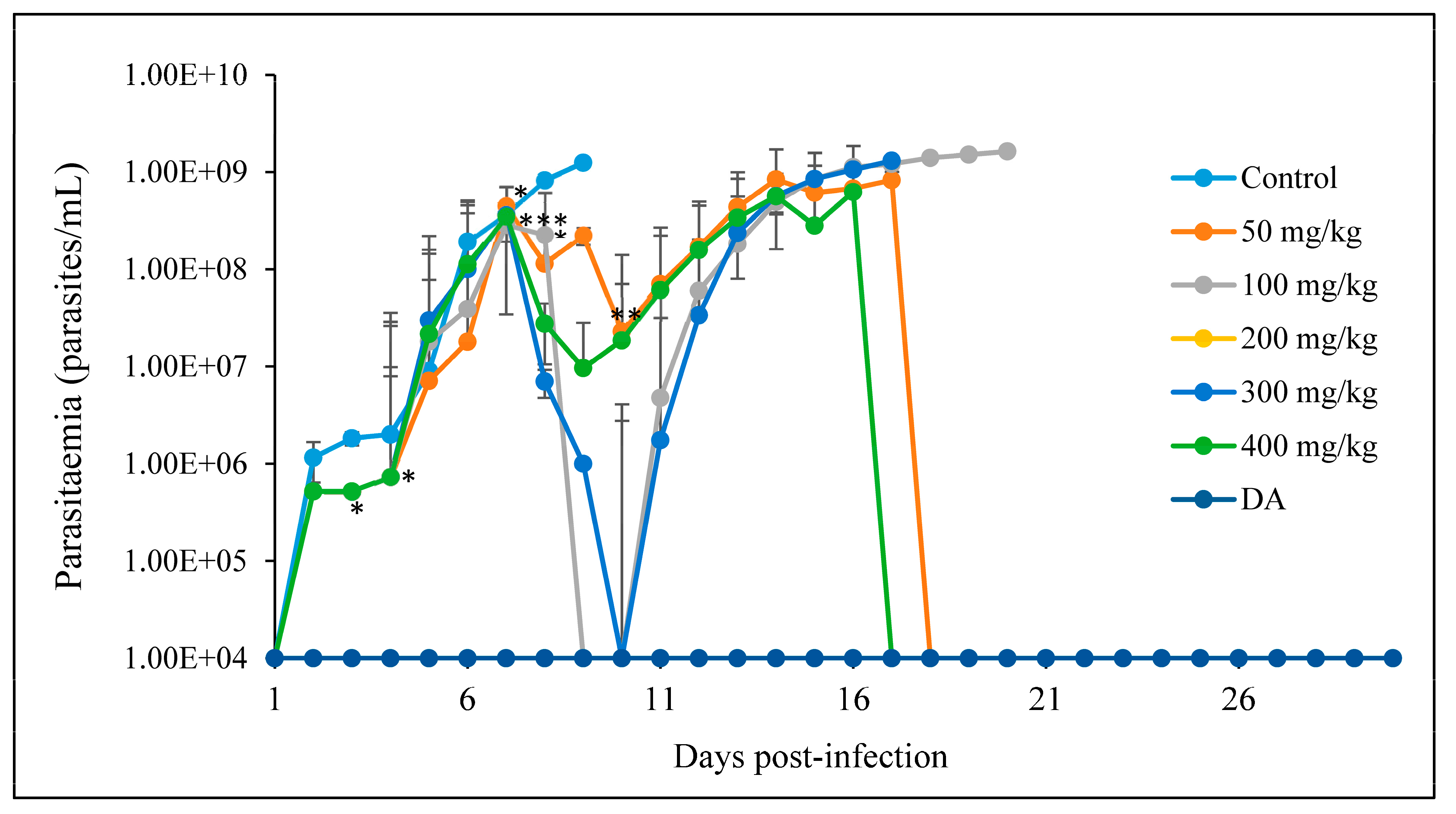
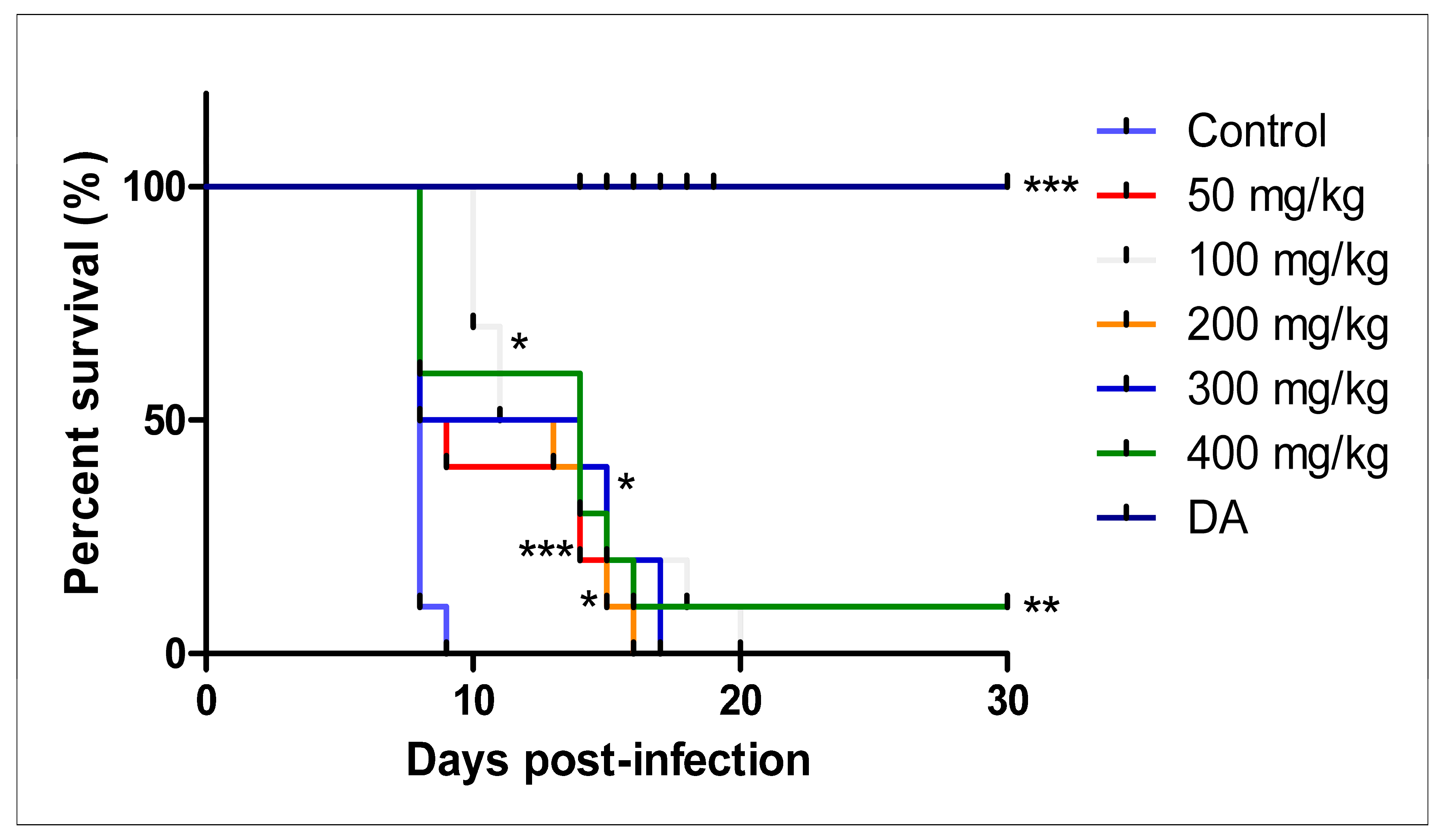
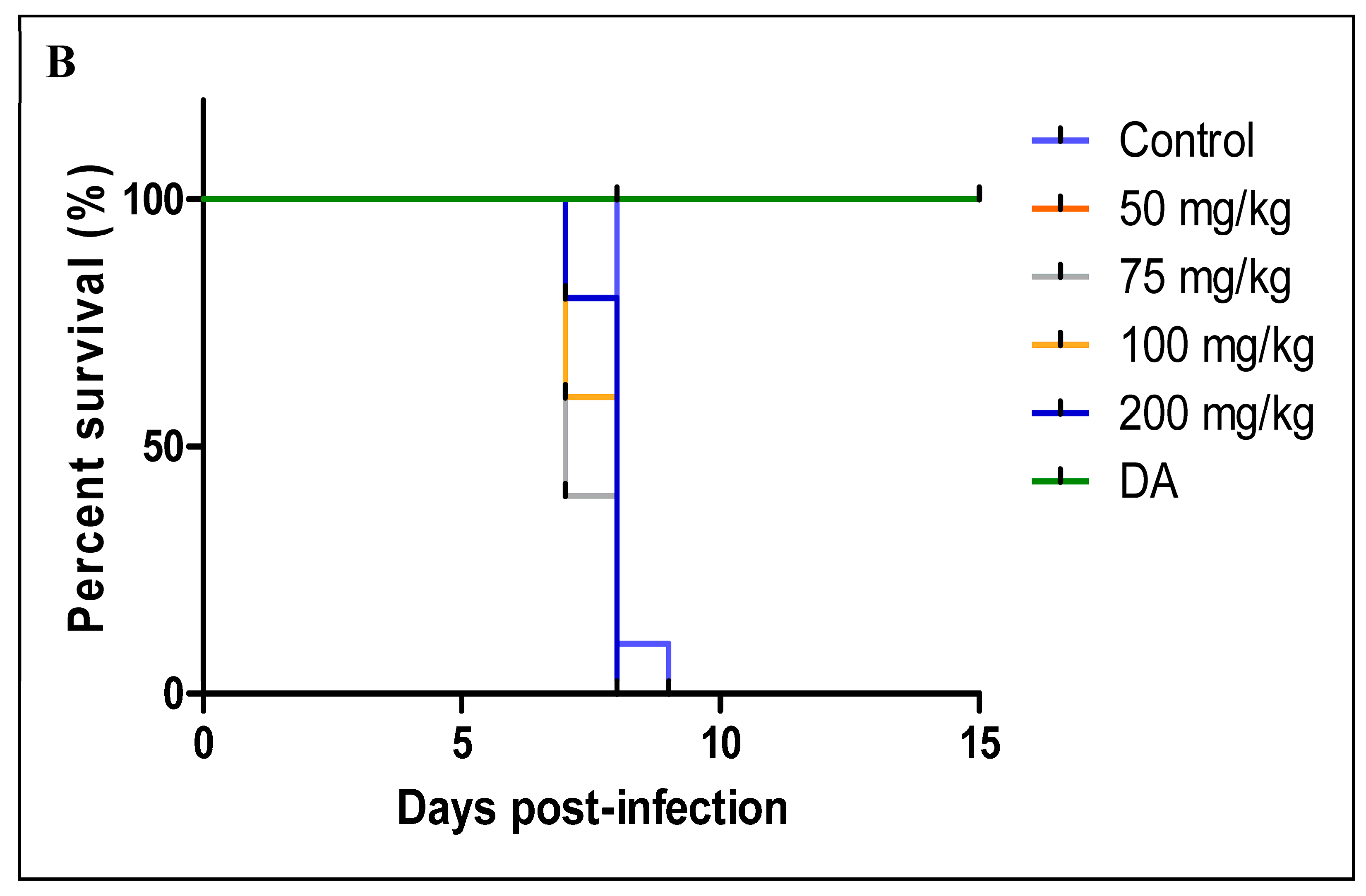
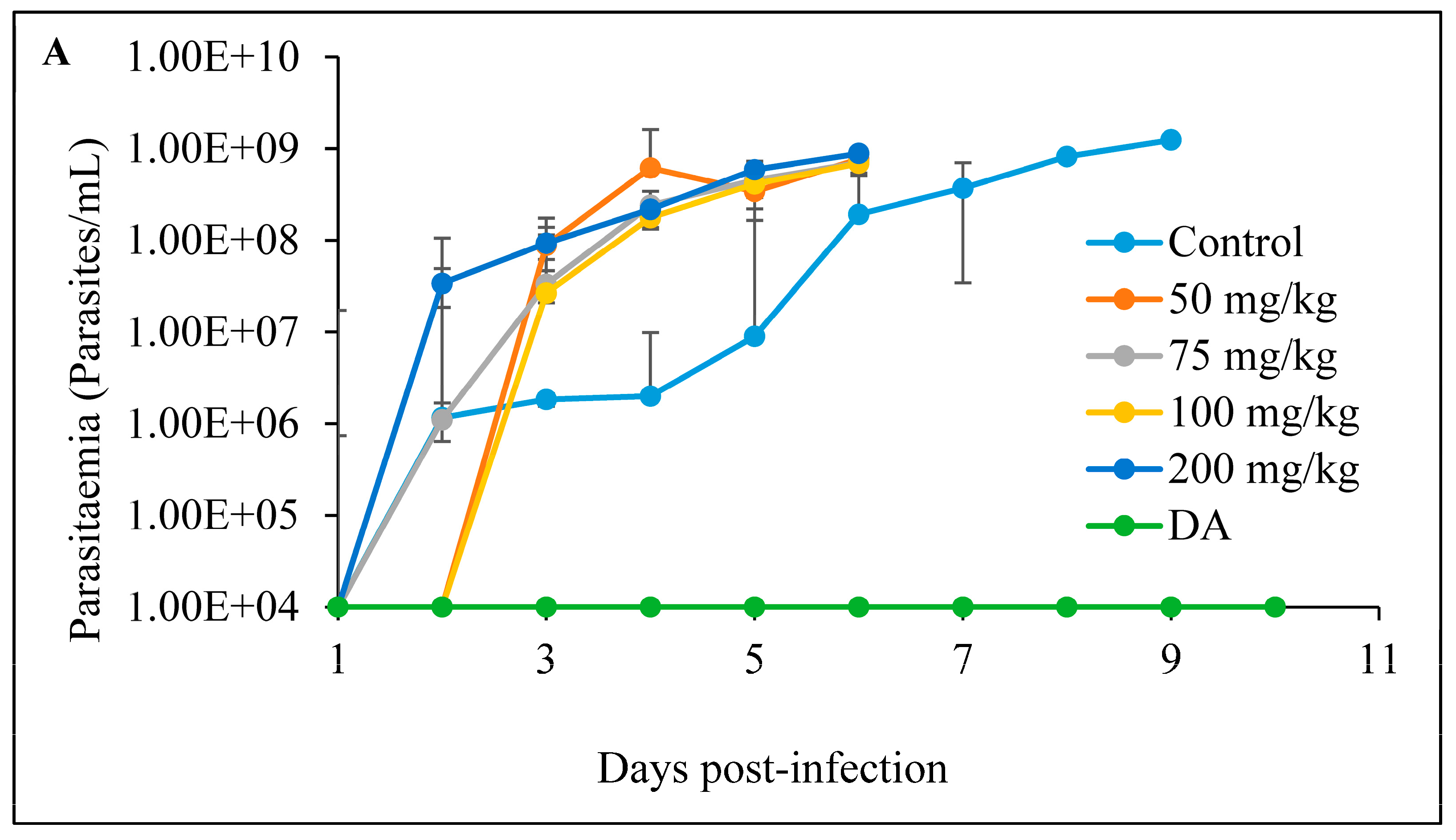
3.2. In Vivo Tests
3.2.1. Histopathological Analysis
3.2.2. Oral Administration
- A
- CUR
- B
- CUR-nanoparticles
3.2.3. Intraperitoneal Administration
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eghianruwa, K.I.; Oridupa, O.A. Chemotherapeutic control of trypanosomosis—A review of past hemotherapeutic control of trypanosomosis—A review of past measures, current status and future trends easures, current status and future trends. Vet. Arhiv. 2018, 88, 245–270. [Google Scholar] [CrossRef]
- Desquesnes, M.; Dargantes, A.; Lai, D.H.; Lun, Z.R.; Holzmuller, P.; Jittapalapong, S. Trypanosoma evansi and surra: A review and perspectives on transmission, epidemiology and control, impact, and zoonotic aspects. Biomed. Res. Int. 2013, 2013, 321237. [Google Scholar] [CrossRef] [PubMed]
- Assefa, S.; Shibeshi, W. Drug resistance in African animal trypanosomes: A review. Afr. J. Microbiol. Res. 2018, 12, 380–386. [Google Scholar]
- Stijlemans, B.; De Baetselier, P.; Magez, S.; Van Ginderachter, J.A.; De Trez, C. African Trypanosomiasis-Associated Anemia: The Contribution of the Interplay between Parasites and the Mononuclear Phagocyte System. Front. Immunol. 2018, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Mishra, K.; Dash, A.P.; Swain, B.K.; Dey, N. Anti-malarial activities of Andrographis paniculata and Hedyotis corymbosa extracts and their combination with curcumin. Malar. J. 2009, 8, 26. [Google Scholar] [CrossRef]
- Witkin, J.M.; Li, X. Curcumin, an active constiuent of the ancient medicinal herb Curcuma longa L.: Some uses and the establishment and biological basis of medical efficacy. CNS Neurol. Disord. Drug Targets 2013, 12, 487–497. [Google Scholar] [CrossRef]
- Altıntoprak, N.; Kar, M.; Acar, M.; Berkoz, M.; Muluk, N.B.; Cingi, C. Antioxidant activities of curcumin in allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2016, 273, 3765–3773. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, B.; Chu, L.; Tong, H.H.Y.; Liu, W.; Zhai, G. Evaluation in vitro and in vivo of curcumin-loaded mPEG-PLA/TPGSmixed micelles for oral administration. Colloids Surf. B Biointerfaces 2016, 141, 345–354. [Google Scholar] [CrossRef]
- Kukongviriyapan, U.; Apaijit, K.; Kukongviriyapan, V. Oxidative stress and cardiovascular dysfunction associated with cadmium exposure: Beneficial effects of curcumin and tetrahydrocurcumin. Tohoku J. Exp. Med. 2016, 239, 25–38. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Suzuki, K.; Veeraveedu, P.T.; Arumugam, S.; Lakshmanan, A.P.; Sone, H.; Watanabe, K. Molecular understanding of curcumin in diabetic nephropathy. Drug Discov. Today 2013, 18, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed. Res. Int. 2014, 2014, 186864. [Google Scholar]
- Huynh, T.P.; Mann, S.N.; Mandal, N.A. Botanical Compounds: Effects on Major Eye Diseases. Evid.-Based Compl. Alt. Med. 2013, 2013, 549174. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Naguib, Y.W.; Shi, Y.C.; Cui, Z. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016, 23, 1495–1501. [Google Scholar] [CrossRef]
- Hettiarachchi, S.S.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, R.M.G. Synthesis of curcumin nanoparticles from raw turmeric rhizome. ACS Omega 2021, 6, 8246–8252. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T. Targeted Nanoparticles for cancer therapy: Promises and challenges. J. Nanomedic. Nanotechnol. 2011, 2, 103e. [Google Scholar] [CrossRef]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef]
- Gonzalez-Martin, G.; Mkrino, I.; Rodriguez-Cabezas, M.N.; Torres, M.; Nunez, R.; Osuna, A. Characterization and trypanocidal activity of nifurtimox-containing and empty nanoparticles of polyethylcy anoacrylates. J. Pharm. Pharmacol. 1998, 50, 29–35. [Google Scholar] [CrossRef]
- Olbrich, C.; Gessner, A.; Schroder, W.; Kayser, O.; Muller, R.H. Lipid-drug conjugate nanoparticles of the hydrophilic drug diminazene-cytotoxicity testing and mouse serum adsorption. J. Control. Release 2004, 96, 425–435. [Google Scholar] [CrossRef]
- Arias, J.L.; Unciti-Broceta, J.D.; Maceira, J.; del Castillo, T.; Hernández-Quero, J.; Magez, S.; Soriano, S.; García-Salcedo, J.A. Nanobody conjugated PLGA nanoparticles for active targeting of African trypanosomiasis. J. Control. Release 2015, 197, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Ma, H.; Liu, J.; Huo, S.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C.; et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 2012, 6, 4483–4493. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Joshi, M.D.; Patravale, V.B. Parasitic diseases: Liposomes and polymeric nanoparticles versus lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Nose, M.; Koide, T.; Ogihara, Y.; Yabu, Y.; Ohta, N. Trypanocidal effects of curcumin in vitro. Biol. Pharm. Bull. 1998, 21, 643–645. [Google Scholar] [CrossRef]
- Gressler, L.T.; Oliveira, C.B.; Coradini, K.; Dalla Rosa, L.; Grando, T.H.; Baldissera, M.D.; Zimmermann, C.E.; Da Silva, A.S.; Almeida, T.C.; Hermes, C.L.; et al. Trypanocidal activity of free and nanoencapsulated curcumin on Trypanos. Evansi. Parasitol. 2015, 142, 439–448. [Google Scholar] [CrossRef]
- Novaes, R.D.; Sartini, M.V.; Rodrigues, J.P.; Goncalves, R.V.; Santos, E.C.; Souza, R.L.; Caldas, I.S. Curcumin enhances the anti-Trypanosoma cruzi activity of benznidazole-based chemotherapy in acute experimental chagas disease. Antimicrob. Agents Chemother. 2016, 60, 3355–3364. [Google Scholar] [CrossRef]
- Kakran, M.; Sahoo, N.G.; Tan, I.L.; Li, L. Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. J. Nanoparticle Res. 2012, 14, 757–767. [Google Scholar] [CrossRef]
- Pandit, R.; Gaikwad, S.C.; Agarkar, G.A.; Gade, A.K.; Rai, M. Curcumin nanoparticles: Physico-chemical fabrication and its in vitro efficacy against human pathogens. 3 Biotech 2015, 5, 991–997. [Google Scholar] [CrossRef]
- Hirumi, H.; Hirumi, K. In vitro cultivation of Trypanosoma congolense bloodstream forms in the absence of feeder cell layers. Parasitology 1991, 102, 225–236. [Google Scholar] [CrossRef]
- Bhawana, R.K.; Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef]
- Molefe, N.I.; Yamasaki, S.; Macalanda, A.M.C.; Suganuma, K.; Watanabe, K.; Xuan, X.; Inoue, N. Oral administration of azithromycin ameliorates trypanosomosis in Trypanosoma congolense-infected mice. Parasitol. Res. 2017, 116, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Molefe, N.I.; Musinguzi, P.S.; Kondoh, D.; Watanabe, K.; Thekisoe, O.M.M.; Xuan, X.; Inoue, N.; Suganuma, K. Short- and long-term effects of orally administered azithromycin on Trypanosoma brucei brucei-infected mice. Exp. Parasitol. 2019, 199, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Huang, R.; Witt, K.L.; Southall, N.; Fostel, J.; Cho, M.; Jadhav, A.; Smith, C.S.; Inglese, J.; Portier, C.J.; et al. Compound Cytotoxicity Profiling Using Quantitative High-Throughput Screening. Environ. Health Perspect. 2008, 116, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Rahiman, N.; Markina, Y.V.; Kesharwani, P.; Johnston, T.P.; Sahebkar, A. Curcumin-based nanotechnology approaches and therapeutics in restoration of autoimmune diseases. J. Control. Release 2022, 348, 264–286. [Google Scholar] [CrossRef]
- Xiao, B.; Si, X.; Zhang, M.; Merlin, D. Oral administration of pH-sensitive curcumin-loaded microparticlesfor ulcerative colitis therapy. Colloids Surf. B Biointerfaces 2015, 135, 379–385. [Google Scholar] [CrossRef]
- Jiang, M.C.; Yang-Yen, H.F.; Yen, J.J.; Lin, J.K. Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr. Cancer. 1996, 26, 111–120. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Moreno, M.; D’ávila, D.A.; Silva, M.N.; Galvão, L.M.C.; Macedo, A.M.; Chiari, E.; Gontijo, E.D.; Zingales, B. Trypanosoma cruzi benznidazole susceptibility in vitro does not predict the therapeutic outcome of human Chagas disease. Mem. Inst. Oswaldo Cruz Rio de Jan. 2010, 105, 918–924. [Google Scholar] [CrossRef]
- Feyera, T.; Terefe, G.; Shibeshi, W. Evaluation of in vivo antitrypanosomal activity of crude extracts of Artemisia abyssinica against a Trypanosoma congolense isolate. BMC Complement. Alternat. Med. 2014, 14, 117. [Google Scholar] [CrossRef]
- Kisalu, N.K.; Langousis, G.; Bentolila, L.A.; Ralston, K.S.; Hill, K.L. Mouse infection and pathogenesis by Trypanosoma brucei motility mutants. Cell Microbiol. 2014, 16, 912–924. [Google Scholar] [CrossRef]
- Cavas, M.; Beltran, D.; Navarro, J.F. Behavioural effects of dimethyl sulfoxide (DMSO): Changes in sleep architecture in rats. Toxicol. Lett. 2005, 157, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.V.; Brab, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar] [PubMed]


| Test Samples | Anti-Trypanosomal Tests (µM ± S.D.) | Cytotoxicity Tests (µM ± S.D.) | Selectivity Index | |||||
|---|---|---|---|---|---|---|---|---|
| T. congolense | T. b. brucei | T. evansi | MDBK | NIH 3T3 | T. c | T. b. b | T. e | |
| CUR | 3.67 ± 0.31 | 7.61 ± 1.22 | 6.40 ± 3.07 | 50.51 ± 1.97 | 10.96 ± 3.09 | 8.37 | 4.01 | 4.80 |
| ASPS | 1.56 ± 0.50 | 28.16 ± 9.43 | 13.12 ± 0.13 | 54.27 ± 0.3 | 78.87 ± 1.7 | 42.67 | 2.36 | 5.07 |
| WM | 1.46 ± 0.49 | 46.25 ± 2.45 | 13.18 ± 0.81 | 57.21 ± 1.082 | 72.68 ± 0.68 | 44.49 | 1.40 | 4.93 |
| EPN | 1.70 ± 0.54 | 44.49 ± 8.1 | 39.96 ± 14.43 | 56.91 ± 0.89 | 56.97 ± 2.17 | 33.49 | 1.28 | 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molefe-Nyembe, N.I.; Adeyemi, O.S.; Kondoh, D.; Kato, K.; Inoue, N.; Suganuma, K. In Vivo Efficacy of Curcumin and Curcumin Nanoparticle in Trypanosoma congolense, Broden 1904 (Kinetoplastea: Trypanosomatidae)-Infected Mice. Pathogens 2023, 12, 1227. https://doi.org/10.3390/pathogens12101227
Molefe-Nyembe NI, Adeyemi OS, Kondoh D, Kato K, Inoue N, Suganuma K. In Vivo Efficacy of Curcumin and Curcumin Nanoparticle in Trypanosoma congolense, Broden 1904 (Kinetoplastea: Trypanosomatidae)-Infected Mice. Pathogens. 2023; 12(10):1227. https://doi.org/10.3390/pathogens12101227
Chicago/Turabian StyleMolefe-Nyembe, Nthatisi Innocentia, Oluyomi Stephen Adeyemi, Daisuke Kondoh, Kentaro Kato, Noboru Inoue, and Keisuke Suganuma. 2023. "In Vivo Efficacy of Curcumin and Curcumin Nanoparticle in Trypanosoma congolense, Broden 1904 (Kinetoplastea: Trypanosomatidae)-Infected Mice" Pathogens 12, no. 10: 1227. https://doi.org/10.3390/pathogens12101227
APA StyleMolefe-Nyembe, N. I., Adeyemi, O. S., Kondoh, D., Kato, K., Inoue, N., & Suganuma, K. (2023). In Vivo Efficacy of Curcumin and Curcumin Nanoparticle in Trypanosoma congolense, Broden 1904 (Kinetoplastea: Trypanosomatidae)-Infected Mice. Pathogens, 12(10), 1227. https://doi.org/10.3390/pathogens12101227