The Presence of a Parasite in the Head Tissues of a Threatened Fish (Bidyanus bidyanus, Terapontidae) from South-Eastern Australia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
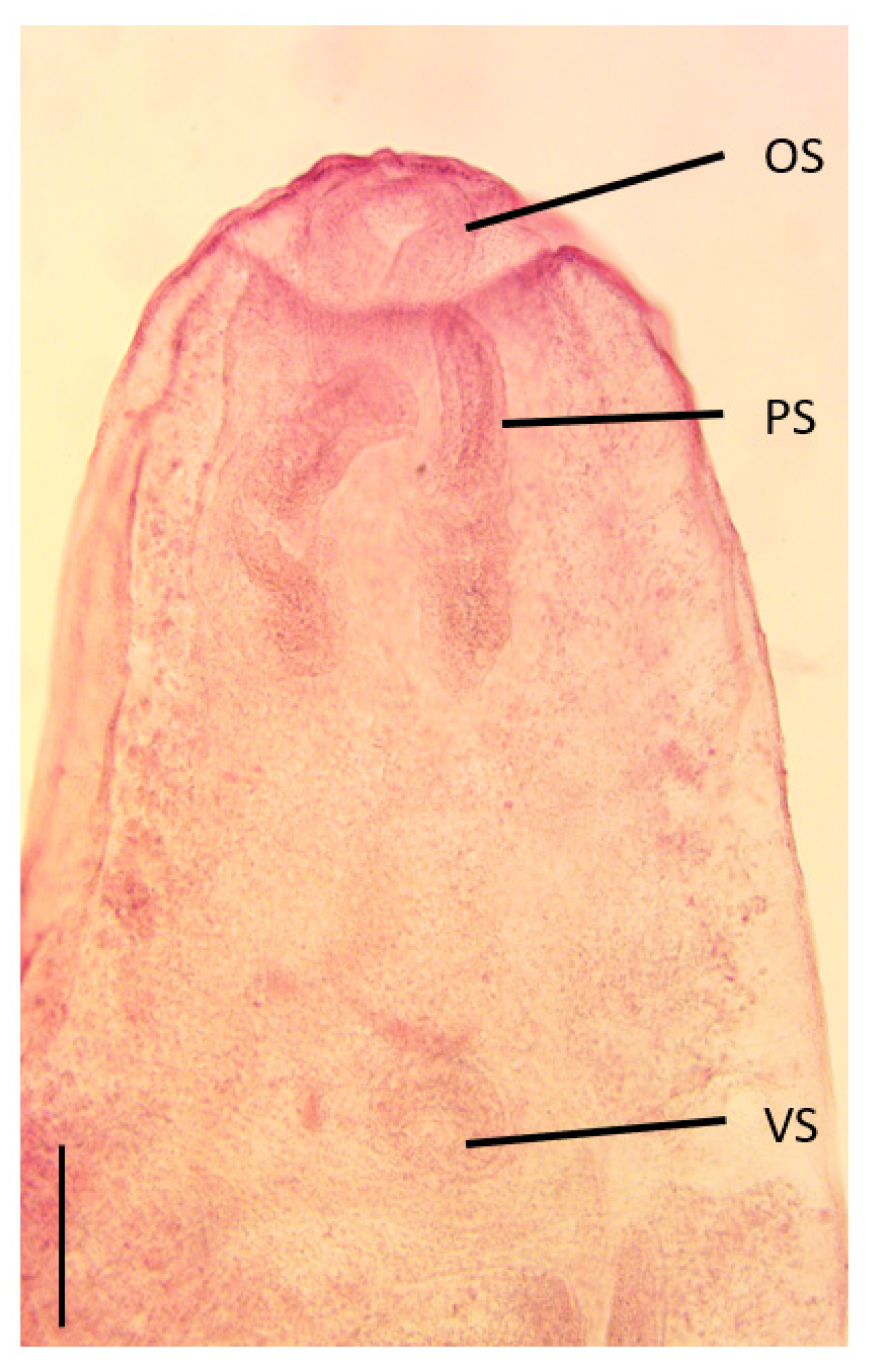
3.1. Morphology of Larval Stage
Morphological Remarks
3.2. Molecular Sequencing
Genetic Remarks
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, G.R.; Midgley, S.H.; Allen, M. Freshwater Fishes of Australia; Western Australian Museum: Perth, Australia, 2003. [Google Scholar]
- DCCEEW. Species Profile and Threats Database. EPBC Act List of Threatened Fauna 2022. Available online: https://www.environment.gov.au/cgi-bin/sprat/public/publicthreatenedlist.pl (accessed on 5 May 2023).
- Rowland, S.J. Review of aquaculture research and development of the Australian freshwater fish silver perch, Bidyanus bidyanus. J. World Aquac. Soc. 2009, 40, 291–324. [Google Scholar] [CrossRef]
- Tonkin, Z.; Stuart, I.; Kitchingman, A.; Thiem, J.D.; Zampatti, B.; Hackett, G.; Koster, W.; Koehn, J.; Morrongiello, J.; Mallen-Cooper, M.; et al. Hydrology and water temperature influence recruitment dynamics of the threatened silver perch Bidyanus bidyanus in a regulated lowland river. Mar. Freshw. Res. 2019, 70, 1333–1344. [Google Scholar] [CrossRef]
- Kopf, R.K.; Humphries, P.; Bond, N.R.; Sims, N.C.; Watts, R.J.; Thompson, R.M.; Hladyz, S.; Koehn, J.D.; King, A.J.; McCasker, N.; et al. Macroecology of fish community biomass–size structure: Effects of invasive species and river regulation. Can. J. Fish. Aquat. Sci. 2019, 76, 109–122. [Google Scholar] [CrossRef]
- Timi, J.T.; Poulin, R. Why ignoring parasites in fish ecology is a mistake. Int. J. Parasitol. 2020, 50, 755–761. [Google Scholar] [CrossRef]
- Freire, R.; Rogers, L.; Creece, D.; Shamsi, S. Neophobic behavioural responses of parasitised fish to a potential predator and baited hook. Appl. Anim. Behav. Sci. 2022, 254, 105722. [Google Scholar] [CrossRef]
- Shamsi, S.; Rogers, L.; Sales, E.; Kopf, R.K.; Freire, R. Do parasites influence behavioural traits of wild and hatchery-reared Murray cod, Maccullochella peelii? Parasitol. Res. 2021, 120, 515–523. [Google Scholar] [CrossRef]
- Rowland, S.J.; Landos, M.; Callinan, R.B.; Allan, G.L.; Read, P.; Mifsud, C.; Nixon, M.; Boyd, P.; Tully, P. Development of a health management strategy for the silver perch aquaculture industry; Report to Fisheries Research and Development Corporation on Projects 2000/267 and 2004/089. In NSW Department of Primary Industries; Fisheries Final Report Series; NSW Department of Primary Industries: Cronulla, NSW, Australia, 2007. [Google Scholar]
- Barton, D.P.; Shamsi, S. Freshwater fish as hosts for parasites in Australia—How much do we really know? Ecol. Freshw. Fish 2023, 1–12. [Google Scholar] [CrossRef]
- Beumer, J.; Ashburner, L.D.; Burbury, M.E.; Jette, E.; Latham, D.J. A Checklist of the Parasites of Fishes from Australia and its Adjacent Antarctic Territories; Technical Communication No. 48; Bureaux, C.A., Ed.; Thanet Press: Margate, FL, USA, 1983. [Google Scholar]
- Angel, L.M.; Manter, H.W. Pretestis australianus gen. et sp. nov. (Digenea: Paramphistomatidae) from Australian fish, and a closely related cercaria, Cercaria acetabulapapillosa sp. nov., with notes on the life history. An. Inst. Biol. Univ. Nac. Auton. Mex. Zool. 1970, 41, 1–10. [Google Scholar]
- Johnston, T.H.; Angel, L.M. Life cycle of the trematode, Diplostomum murrayense J. and C. Trans. R. Soc. South Aust. 1941, 65, 140–144. [Google Scholar]
- Barber, I.; Hoare, D.; Krause, J. Effects of parasites on fish behaviour: A review and evolutionary perspective. Rev. Fish Biol. Fish. 2000, 10, 131–165. [Google Scholar] [CrossRef]
- Aghlmandi, F.; Habibi, F.; Afraii, M.A.; Abdoli, A.; Shamsi, S. Infection with metacercaria of Clinostomum complanatum (Trematoda: Clinostomidae) in freshwater fishes from Southern Caspian Sea Basin. Rev. Med. Vet. 2018, 169, 147–151. [Google Scholar]
- Coleman, R.A.; Hoffmann, A.A. Digenean trematode cysts within the heads of threatened Galaxiella species (Teleostei: Galaxiidae) from south-eastern Australia. Aust. J. Zool. 2016, 64, 285–291. [Google Scholar] [CrossRef]
- Negm-Eldin, M.; Davies, R.W. Morphology and life cycle of Apatemon hypseleotris species novum from Australia including metacercariae viability and excystment. Dtsch. Tieraerztliche Wochenschr. 2001, 109, 306–314. [Google Scholar]
- Chapman, A.; Hobbs, R.P.; Morgan, D.L.; Gill, H.S. Helminth parasitism of Galaxias maculatus (Jenyns 1842) in southwestern Australia. Ecol. Freshw. Fish 2006, 15, 559–564. [Google Scholar] [CrossRef]
- Niewiadomska, K. Superfamily Diplostomoidea Poirier, 1886. In Keys to the Trematoda; Gibson, D.I., Jones, A., Bray, R.A., Eds.; CAB International: Wollingford, UK, 2002; Volume 1, pp. 159–166. [Google Scholar]
- Niewiadomska, K. Family Strigeidae Railliet, 1919. In Keys to the Trematoda; Gibson, D.I., Jones, A., Bray, R.A., Eds.; CAB International: Wollingford, UK, 2002; Volume 1, pp. 231–241. [Google Scholar]
- Shamsi, S.; Chen, Y.; Poupa, A.; Ghadam, M.; Justine, J.-L. Occurrence of anisakid parasites in marine fishes and whales off New Caledonia. Parasitol. Res. 2018, 117, 3195–3204. [Google Scholar] [CrossRef]
- Hillis, D.M.; Dixon, M.T. Ribosomal DNA: Molecular Evolution and Phylogenetic Inference. Q. Rev. Biol. 1991, 66, 411–453. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Schmidt, G.D.; Roberts, L.S. Foundations of Parasitology, 4th ed.; Times Mirror/Mosby College Publishing: London, UK, 1989. [Google Scholar]
- Shamsi, S.; Day, S.; Zhu, X.; McLellan, M.; Barton, D.P.; Dang, M.; Nowak, B.F. Wild fish as reservoirs of parasites on Australian Murray cod farms. Aquaculture 2021, 539, 736584. [Google Scholar] [CrossRef]
- Anon. Atlas of Living Australia. 2021. Available online: http://www.ala.org.au (accessed on 22 August 2021).
- Hoffman, G.L. Synopsis of Strigeoidae (Trematoda) of fishes and their life cycles. U.S. Fish Wildl. Publ. 1960, 90, 439–469. [Google Scholar]
- Locke, S.A.; McLaughlin, J.D.; Lapierre, A.R.; Johnson, P.T.; Marcogliese, D.J. Linking larvae and adults of Apharyngostrigea cornu, Hysteromorpha triloba, and Alaria mustelae (Diplostomoidea: Digenea) using molecular data. J. Parasitol. 2011, 97, 846–851. [Google Scholar] [CrossRef]
- Shamsi, S.; Barton, D.P.; Day, S.; Masiga, J.; Zhu, X.; McLellan, M. Characterization of Clinostomum sp. (Trematoda: Clinostomidae) infecting cormorants in south-eastern Australia. Parasitol. Res. 2021, 120, 2793–2803. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Capuano, S.; Simoni, E.; Giari, L.; Shinn, A. Histopathological and ultrastructural observations of metacercarial infections of Diplostomum phoxini (Digenea) in the brain of minnows Phoxinus phoxinus. Dis. Aquat. Org. 2007, 75, 51–59. [Google Scholar] [CrossRef]
- Conn, D.B.; Goater, C.P.; Bray, D. Developmental and functional ultrastructure of Ornithodiplostomum ptychocheilus diplostomula (Trematoda: Strigeoidea) during invasion of the brain of the fish intermediate host, Pimephales promelas. J. Parasitol. 2008, 94, 635–642. [Google Scholar] [CrossRef]
- Bosma, N.J. The life history of the trematode Alaria mustelae, Bosma, 1931. Trans. Am. Microsc. Society 1934, 53, 116–153. [Google Scholar] [CrossRef]
- Mawson, P.M.; Angel, L.M.; Edmonds, S.J. A checklist of helminths from Australian birds. Rec. South Aust. Mus. 1986, 19, 219–325. [Google Scholar]
- Atlas of Living Australia. Atlas of Living Australia. 2020. Available online: http://www.ala.org.au (accessed on 16 November 2020).
- Spratt, D.M.; Beveridge, I. Wildlife Parasitology in Australia: Past, present and future. Aust. J. Zool. 2018, 66, 286–305. [Google Scholar] [CrossRef]
- Rowland, S.J.; Ingram, B.A. Diseases of Australian Native freshwater Fishes with Particular Emphases on the Ectoparasitic and Fungal Diseases of Murray Cod (Maccullochella peeli), Golden Perch (Macquaria ambigua) and Silver Perch (Bidyanus bidyanus); NSW Fisheries, Ed.; NSWcAgriculture & Fisheries: Sydney, Australia, 1991. [Google Scholar]
- Cribb, T.H. Two new digenetic trematodes from Australian freshwater fishes with notes on previously described species. J. Nat. Hist. 1988, 22, 27–43. [Google Scholar] [CrossRef]
- Chong, R.S.-M. Digenetic trematode infections. In Aquaculture Pathophysiology: Finfish Diseases; Kibenge, F.S.B., Baldoisserotto, B., Chong, R.S.-M., Eds.; Academic Press: London, UK, 2022; Volume 1, pp. 569–590. [Google Scholar]
- Ruehle, B.; Poulin, R. Risky business: Influence of eye flukes on use of risky microhabitats and conspicuousness of a fish host. Parasitol. Res. 2020, 119, 423–430. [Google Scholar] [CrossRef]
- Poulin, R. Modification of host social networks by manipulative parasites. Behaviour 2018, 155, 671–688. [Google Scholar] [CrossRef]
- Seppälä, O.; Karvonen, A.; Valtonen, T. Shoaling behaviour of fish under parasitism and predation risk. Anim. Behav. 2008, 75, 145–150. [Google Scholar] [CrossRef]
- Cunningham, A.A.; Daszak, P. Extinction of a species of land snail due to infection with a microsporidian parasite. Conserv. Biol. 1998, 12, 1139–1141. [Google Scholar] [CrossRef]
- Achatz, T.J.; Pulis, E.E.; González-Acuña, D.; Tkach, V.V. Phylogenetic Relationships of Cardiocephaloides spp. (Digenea, Diplostomoidea) and the Genetic Characterization of Cardiocephaloides physalis from Magellanic Penguin, Spheniscus magellanicus, in Chile. Acta Parasitol. 2020, 65, 525–534. [Google Scholar] [CrossRef]
- Gordy, M.A.; Locke, S.A.; Rawlings, T.A.; Lapierre, A.R.; Hanington, P.C. Molecular and morphological evidence for nine species in North American Australapatemon (Sudarikov, 1959): A phylogeny expansion with description of the zygocercous Australapatemon mclaughlini n. sp. Parasitol. Res. 2017, 116, 2181–2198. [Google Scholar] [CrossRef]
- Hernández-Mena, D.I.; García-Prieto, L.; García-Varela, M. Morphological and molecular differentiation of Parastrigea (Trematoda: Strigeidae) from Mexico, with the description of a new species. Parasitol. Int. 2014, 63, 315–323. [Google Scholar] [CrossRef]
- Hoogendoorn, C.; Smit, N.J.; Kudlai, O. Molecular and morphological characterisation of four diplostomid metacercariae infecting Tilapia sparrmanii (Perciformes: Cichlidae) in the North West Province, South Africa. Parasitol. Res. 2019, 118, 1403–1416. [Google Scholar] [CrossRef]
- Locke, S.A.; Van Dam, A.; Caffara, M.; Pinto, H.A.; López-Hernández, D.; Blanar, C.A. Validity of the Diplostomoidea and Diplostomida (Digenea, Platyhelminthes) upheld in phylogenomic analysis. Int. J. Parasitol. 2018, 48, 1043–1059. [Google Scholar] [CrossRef]
- López-Hernández, D.; Locke, S.A.; de Assis, J.C.A.; Drago, F.B.; de Melo, A.L.; Rabelo, M.L.; Pinto, H.A. Molecular, morphological and experimental-infection studies of cercariae of five species in the superfamily Diplostomoidea (Trematoda: Digenea) infecting Biomphalaria straminea (Mollusca: Planorbidae) in Brazil. Acta Trop. 2019, 199, 105082. [Google Scholar] [CrossRef]
- Sinsch, U.; Heneberg, P.; Těšínský, M.; Balczun, C.; Scheid, P. Helminth endoparasites of the smooth newt Lissotriton vulgaris: Linking morphological identification and molecular data. J. Helminthol. 2018, 93, 332–341. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barton, D.P.; Kopf, R.K.; Zhu, X.; Shamsi, S. The Presence of a Parasite in the Head Tissues of a Threatened Fish (Bidyanus bidyanus, Terapontidae) from South-Eastern Australia. Pathogens 2023, 12, 1296. https://doi.org/10.3390/pathogens12111296
Barton DP, Kopf RK, Zhu X, Shamsi S. The Presence of a Parasite in the Head Tissues of a Threatened Fish (Bidyanus bidyanus, Terapontidae) from South-Eastern Australia. Pathogens. 2023; 12(11):1296. https://doi.org/10.3390/pathogens12111296
Chicago/Turabian StyleBarton, Diane P., R. Keller Kopf, Xiaocheng Zhu, and Shokoofeh Shamsi. 2023. "The Presence of a Parasite in the Head Tissues of a Threatened Fish (Bidyanus bidyanus, Terapontidae) from South-Eastern Australia" Pathogens 12, no. 11: 1296. https://doi.org/10.3390/pathogens12111296
APA StyleBarton, D. P., Kopf, R. K., Zhu, X., & Shamsi, S. (2023). The Presence of a Parasite in the Head Tissues of a Threatened Fish (Bidyanus bidyanus, Terapontidae) from South-Eastern Australia. Pathogens, 12(11), 1296. https://doi.org/10.3390/pathogens12111296







