Synergistic Strategies of Heat and Peroxyacetic Acid Disinfection Treatments for Salmonella Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Consumables
2.2. Devices
2.3. Bacterial Strains
2.4. Preparation of Bacterial Suspension
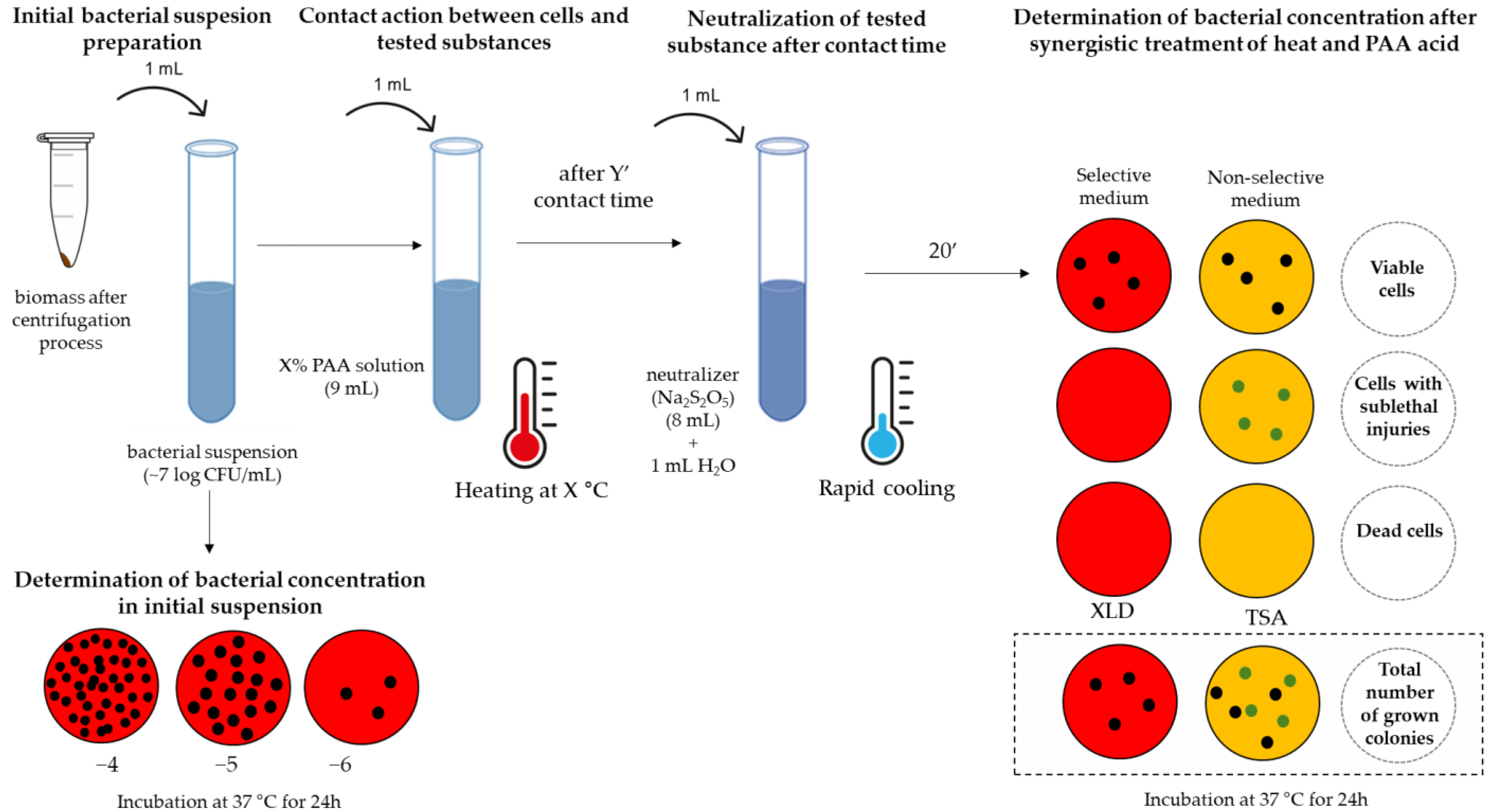
2.5. The Concept of Testing Biocidal Effects
- step
- II.
- step
- III.
- step
2.6. Mathematical Analysis
3. Results
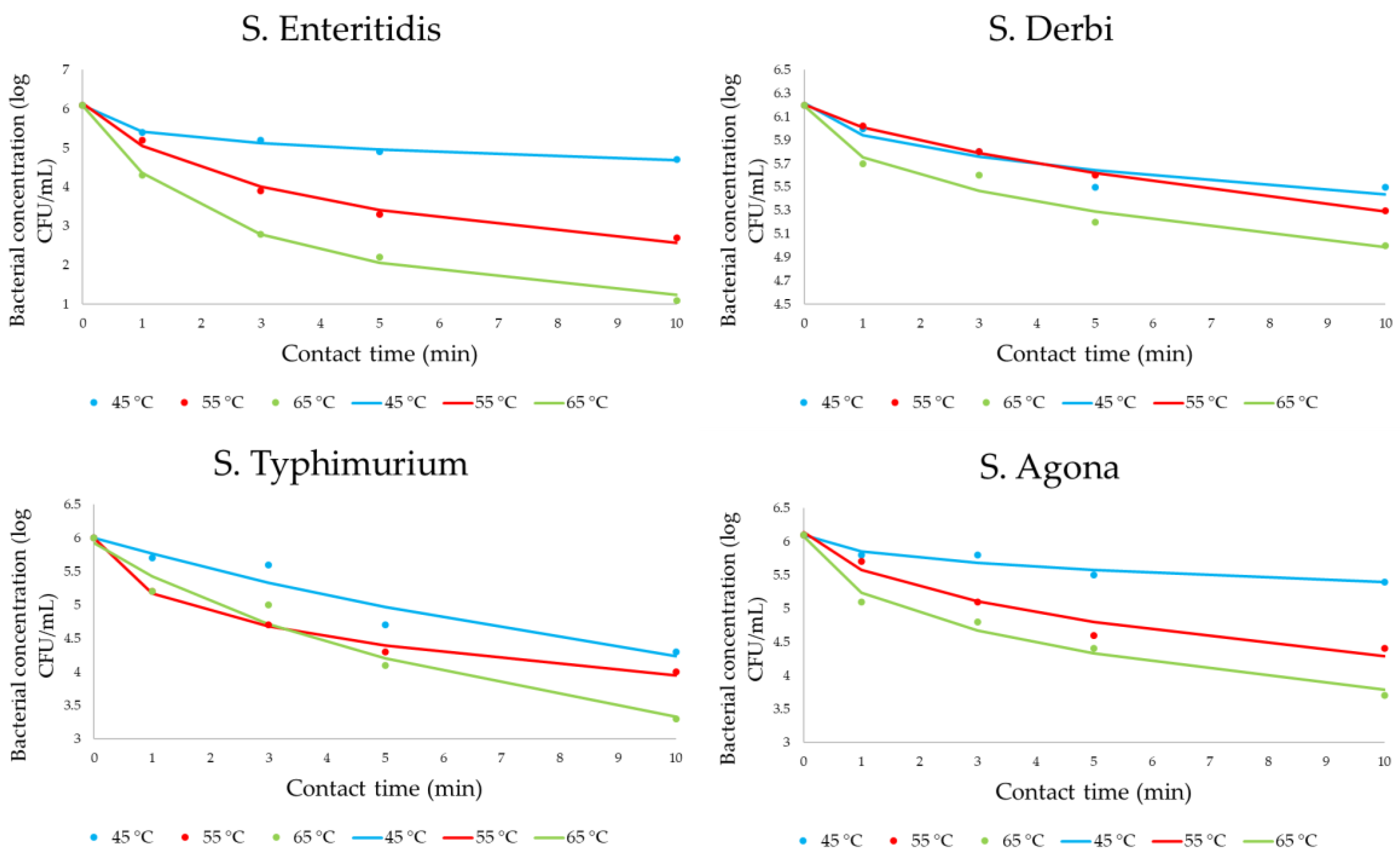
3.1. Effect of Heat on the Inactivation of Salmonella Cells
3.2. Effect of Peroxyacetic Acid on the Inactivation of Salmonella Cells
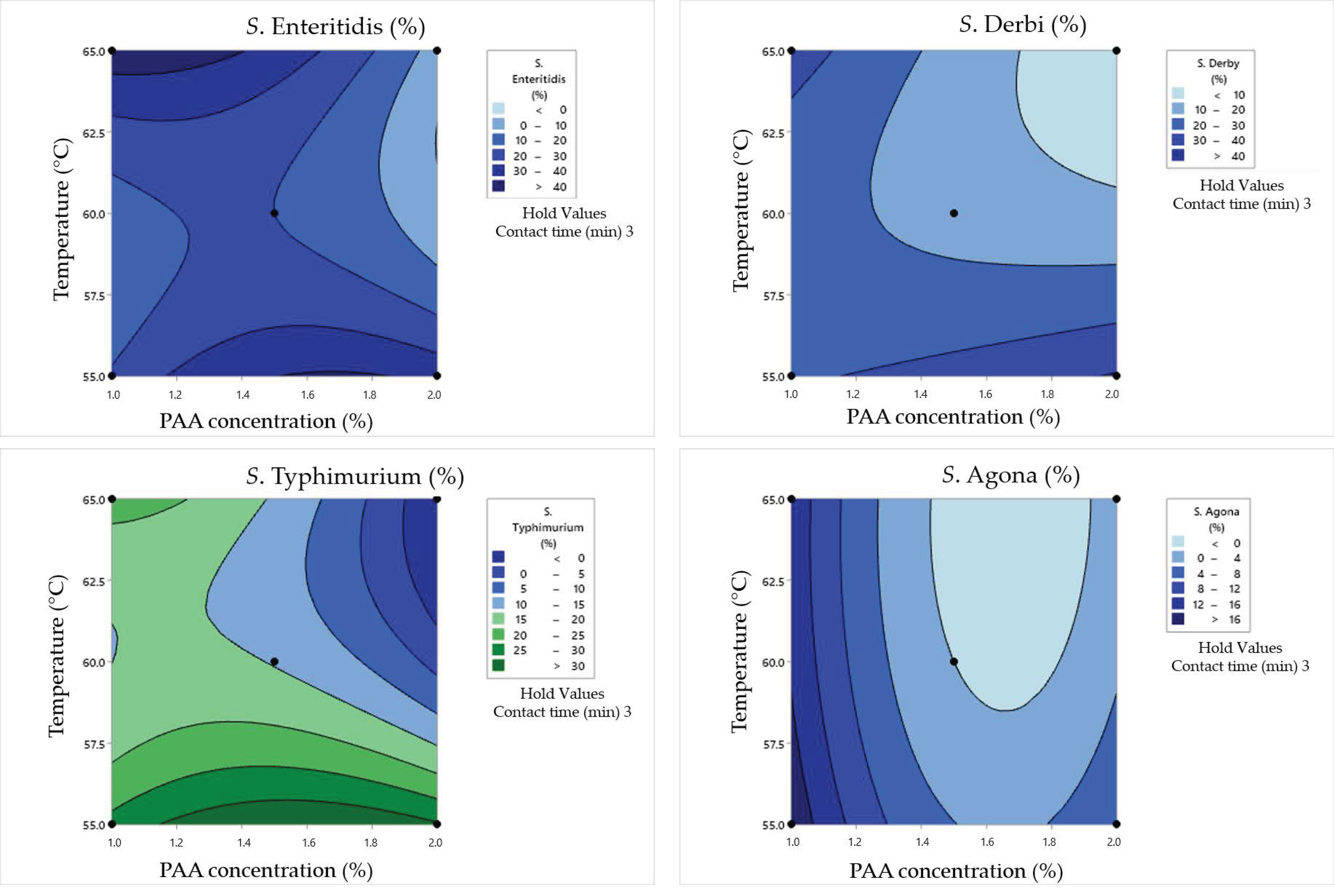
3.3. Synergistic Effect of Heat and Peroxyacetic Acid on the Inactivation of Salmonella Cells in Clean Conditions
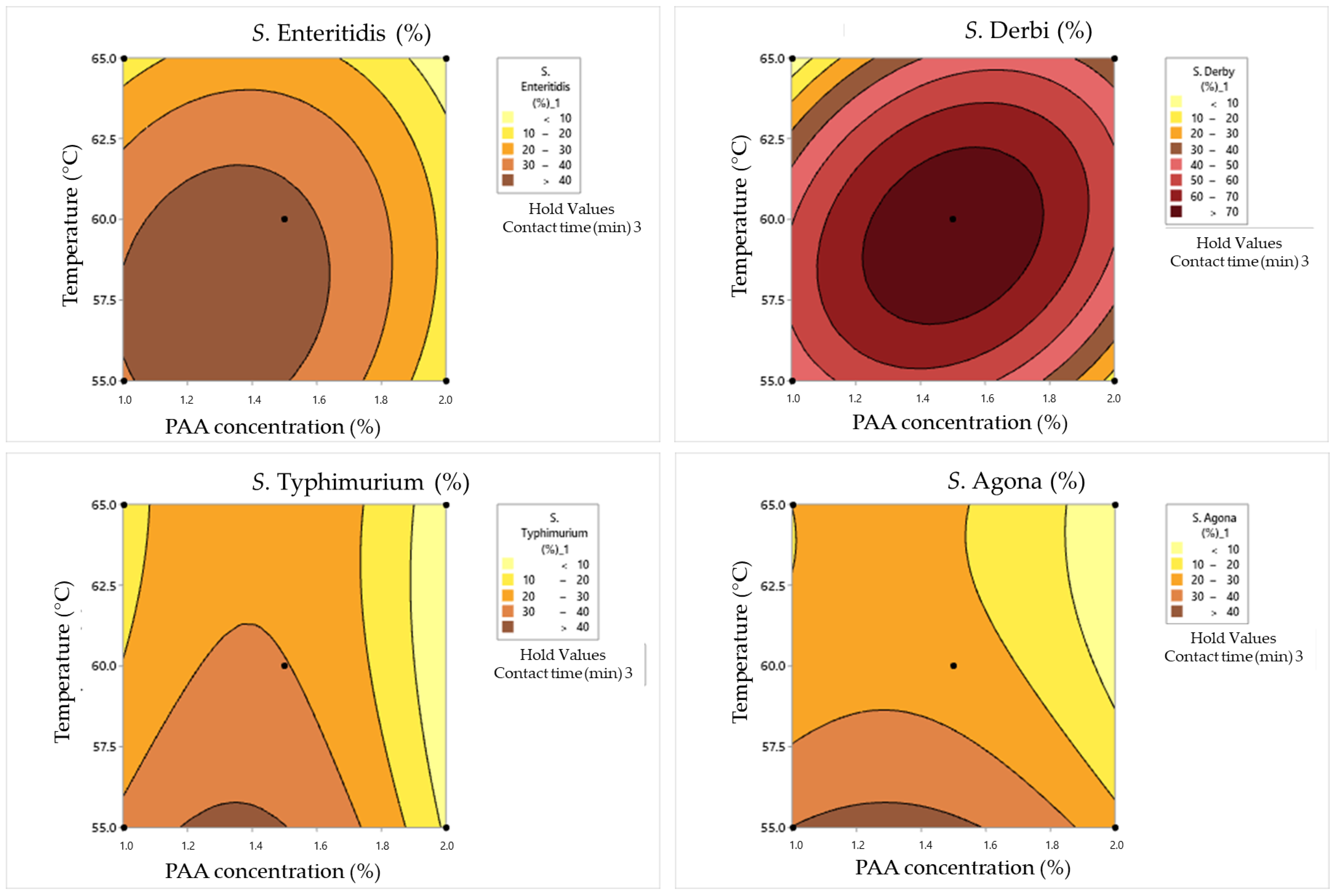
3.4. Synergistic Effect of Heat and Peroxyacetic Acid on the Inactivation of Salmonella Cells in Dirty Conditions
4. Discussion
4.1. Effect of Heat on the Inactivation of Salmonella Cells
4.2. Effects of Peroxyacetic Acid on the Inactivation of Salmonella Cells
4.3. Synergistic Effects of Heat and Peroxyacetic Acid on the Inactivation of Salmonella Cells in Clean Conditions
4.4. Synergistic Effects of Heat and Peroxyacetic Acid on the Inactivation of Salmonella Cells in Dirty Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef] [PubMed]
- Uçar, A.; Yilmaz, M.V.; Çakiroglu, F.P. Food Safety–Problems and Solutions; InTech: London, UK, 2016. [Google Scholar]
- McFarland, P.; Checinska Sielaff, A.; Rasco, B.; Smith, S. Efficacy of Food Safety Training in Commercial Food Service. J. Food Sci. 2019, 84, 1239–1246. [Google Scholar] [CrossRef]
- Aladhadh, M. A Review of Modern Methods for the Detection of Foodborne Pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef]
- Tomić, A.; Šovljanski, O.; Erceg, T. Insight on Incorporation of Essential Oils as Antimicrobial Substances in Biopolymer-Based Active Packaging. Antibiotics 2023, 12, 1473. [Google Scholar] [CrossRef]
- Almansour, A.M.; Alhadlaq, M.A.; Alzahrani, K.O.; Mukhtar, L.E.; Alharbi, A.L.; Alajel, S.M. The Silent Threat: Antimicrobial-Resistant Pathogens in Food-Producing Animals and Their Impact on Public Health. Microorganisms 2023, 11, 2127. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Tropea, A. Microbial Contamination and Public Health: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7441. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main Groups of Microorganisms of Relevance for Food Safety and Stability: General Aspects and Overall Description. Innov. Technol. Food Preserv. 2018, 53–107. [Google Scholar]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef] [PubMed]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, Food Safety and Food Handling Practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Willson, N.L.; Chousalkar, K. Dominant Salmonella Serovars in Australian Broiler Breeder Flocks and Hatcheries: A Longitudinal Study. Appl. Environ. Microbiol. 2023, 89, e0062723. [Google Scholar] [CrossRef]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018. [Google Scholar] [CrossRef]
- Andino, A.; Hanning, I. Salmonella enterica: Survival, colonization, and virulence differences among serovars. Sci. World J. 2015, 2015, 520179. [Google Scholar] [CrossRef]
- Fàbrega, A.; Vila, J. Salmonella enterica serovar Typhimurium skills to succeed in the host: Virulence and regulation. Clin. Microbiol. Rev. 2013, 26, 308–341. [Google Scholar] [CrossRef]
- Hoelzer, K.; Moreno Switt, A.; Wiedmann, M. Animal contact as a source of human non-typhoidal salmonellosis. Vet. Res. 2011, 42, 34. [Google Scholar] [CrossRef]
- Back, D.S.; Shin, G.W.; Wendt, M.; Heo, G.J. Prevalence of Salmonella spp. in pet turtles and their environment. Lab. Anim. Res. 2016, 32, 166–170. [Google Scholar] [CrossRef]
- Cox, N.A.; Cason, J.A.; Richardson, L.J. Minimization of Salmonella Contamination on Raw Poultry. Annu. Rev. Food Sci. Technol. 2011, 2, 75–95. [Google Scholar] [CrossRef]
- Bacon, R.T.; Sofos, J.N.; Belk, K.E.; Hyatt, D.R.; Smith, G.C. Prevalence and antibiotic susceptibility of Salmonella isolated from beef animal hides and carcasses. J. Food Prot. 2002, 65, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; De Reu, K.; Gabriël, S.; Mattheus, W.; De Zutter, L.; Rasschaert, G. Salmonella prevalence and persistence in industrialized poultry slaughterhouses. Poult. Sci. 2021, 100, 100991. [Google Scholar] [CrossRef] [PubMed]
- Reiter, M.G.R.; Fiorese, M.L.; Moretto, G.; López, M.C.; Jordano, R. Prevalence of Salmonella in a poultry slaughterhouse. J. Food Prot. 2007, 70, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.R.; Cavicchioli, V.Q.; Camargo, A.C.; Lanna, F.G.P.A.; Pinto, P.S.D.A.; Bersot, L.D.S.; Nero, L.A. Molecular tracking of Salmonella spp. in chicken meat chain: From slaughterhouse reception to end cuts. J. Food Sci. Technol. 2016, 53, 1084–1091. [Google Scholar]
- Botteldoorn, N.; Heyndrickx, M.; Rijpens, N.; Grijspeerdt, K.; Herman, L. Salmonella on pig carcasses: Positive pigs and cross contamination in the slaughterhouse. J. Appl. Microbiol. 2003, 95, 891–903. [Google Scholar] [CrossRef]
- Olsen, J.E.; Brown, D.J.; Madsen, M.; Bisgaard, M. Cross-contamination with Salmonella on a broiler slaughterhouse line demonstrated by use of epidemiological markers. J. Appl. Microbiol. 2003, 94, 826–835. [Google Scholar] [CrossRef]
- Reta, G.G.; Lopes, S.M.; de Aquino, N.S.M.; Tondo, E.C. Quantification of Salmonella transfer in cross-contamination scenarios found in chicken slaughterhouses. Food Microbiol. 2023, 116, 104347. [Google Scholar] [CrossRef]
- Bhandari, R.; Singh, A.K.; Bhatt, P.R.; Timalsina, A.; Bhandari, R.; Thapa, P.; Baral, J.; Adhikari, S.; Poudel, P.; Chiluwal, S.; et al. Factors associated with meat hygiene-practices among meat-handlers in Metropolitan City of Kathmandu, Nepal. PLoS Glob Public Health 2022, 2, e0001181. [Google Scholar] [CrossRef]
- Montoro-Dasi, L.; Lorenzo-Rebenaque, L.; Marco-Fuertes, A.; Vega, S.; Marin, C. Holistic Strategies to Control Salmonella Infantis: An Emerging Challenge in the European Broiler Sector. Microorganisms 2023, 11, 1765. [Google Scholar] [CrossRef]
- Liu, S.; Tang, J.; Tadapaneni, R.K.; Yang, R.; Zhu, M.J. Exponentially Increased Thermal Resistance of Salmonella spp. and Enterococcus faecium at Reduced Water Activity. Appl. Environ. Microbiol. 2018, 84, e02742-17. [Google Scholar] [CrossRef]
- Finn, S.; Condell, O.; McClure, P.; Amézquita, A.; Fanning, S. Mechanisms of survival, responses and sources of Salmonella in low-moisture environments. Front. Microbiol. 2013, 4, 331. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, M.; Cosby, D.E.; Cox, N.A.; Thippareddi, H. Efficacy of peroxy acetic acid in reducing Salmonella and Campylobacter spp. populations on chicken breast fillets. Poult. Sci. 2020, 99, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- de Rezende, H.C.; de Lima, M.; Santos, L.D. Peracetic acid application as an antimicrobial and its residual (HEDP): A holistic approach on the technological characteristics of chicken meat. Poult. Sci. 2023, 102, 103003. [Google Scholar] [CrossRef]
- Kitis, M. Disinfection of wastewater with peracetic acid: A review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Huertas, J.P.; Aznar, A.; Esnoz, A.; Fernández, P.S.; Iguaz, A.; Periago, P.M.; Palop, A. High Heating Rates Affect Greatly the Inactivation Rate of Escherichia coli. Front. Microbiol. 2016, 7, 1256. [Google Scholar] [CrossRef]
- Rui, L.; Xiaoxi, K.; Lihui, Z.; Shaojin, W. Inactivation kinetics of food-borne pathogens subjected to thermal treatments: A review. Int. J. Hyperth. 2018, 34, 177–188. [Google Scholar]
- Holah, J.T. Cleaning and disinfection. In Hygiene in Food Processing; Lelieveld, H.L.M., Mostert, M.A., Holah, J., White, B., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 235–278. [Google Scholar]
- Meyer, B.; Morin, V.N.; Rödger, H.-J.; Holah, J.; Bird, C. Do European Standard Disinfectant tests truly simulate in-use microbial and organic soiling conditions on food preparation surfaces? J. Appl. Microbiol. 2010, 108, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.; Macdonald, T.J.; Gavriilidis, A.; Allan, E.; MacRobert, A.J.; Parkin, I.P. Effects of bovine serum albumin on light activated antimicrobial surfaces. RSC Adv. 2018, 8, 34252–34258. [Google Scholar] [CrossRef]
- Mastusaka, Y.; Kawabata, J. Evaluation of Antioxidant Capacity of Non-Edible Parts of Some Selected Tropical Fruits. Food Sci. Technol. Res. 2010, 16, 467–472. [Google Scholar]
- Moore, G.; Blair, I.S.; McDowell, D.A. Recovery and Transfer of Salmonella Typhimurium from Four Different Domestic Food Contact Surfaces. J. Food Prot. 2007, 70, 2273–2280. [Google Scholar] [CrossRef]
- Tomić, A.; Šovljanski, O.; Nikolić, V.; Pezo, L.; Aćimović, M.; Cvetković, M.; Stanojev, J.; Kuzmanović, N.; Markov, S. Screening of Antifungal Activity of Essential Oils in Controlling Biocontamination of Historical Papers in Archives. Antibiotics 2023, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Šovljanski, O.; Pezo, L.; Tomić, A.; Ranitović, A.; Cvetković, D.; Markov, S. Formation of Predictive-Based Models for Monitoring the Microbiological Quality of Beef Meat Processed for Fast-Food Restaurants. Int. J. Environ. Res. Public Health 2022, 19, 16727. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Šovljanski, O.; Šeregelj, V.; Pezo, L.; Zheljazkov, V.D.; Ljujić, J.; Tomić, A.; Ćetković, G.; Čanadanović-Brunet, J.; Miljković, A.; et al. Chemical Composition, Antioxidant, and Antimicrobial Activity of Dracocephalum moldavica L. Essential Oil and Hydrolate. Plants 2022, 11, 941. [Google Scholar] [CrossRef]
- Šovljanski, O.; Pezo, L.; Stanojev, J.; Bajac, B.; Kovač, S.; Tóth, E.; Ristić, I.; Tomić, A.; Ranitović, A.; Cvetković, D.; et al. Comprehensive Profiling of Microbiologically Induced CaCO3 Precipitation by Ureolytic Bacillus Isolates from Alkaline Soils. Microorganisms 2021, 9, 1691. [Google Scholar] [CrossRef]
- Mazzola, P.G.; Penna, T.C.; Martins, A.M. Determination of decimal reduction time (D value) of chemical agents used in hospitals for disinfection purposes. BMC Infect Dis. 2003, 3, 24. [Google Scholar] [CrossRef]
- Cebrián, G.; Condón, S.; Mañas, P. Physiology of the Inactivation of Vegetative Bacteria by Thermal Treatments: Mode of Action, Influence of Environmental Factors and Inactivation Kinetics. Foods 2017, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Dash, K.K.; Fayaz, U.; Dar, A.H.; Shams, R.; Manzoor, S.; Sundarsingh, A.; Deka, P.; Khan, S.A. A comprehensive review on heat treatments and related impact on the quality and microbial safety of milk and milk-based products. Food Chem. Adv. 2022, 1, 100041. [Google Scholar] [CrossRef]
- Hassan, H.; Iskandar, C.F.; Hamzeh, R.; Malek, N.J.; Khoury, A.E.; Abiad, M.G. Heat resistance of Staphylococcus aureus, Salmonella sp., and Escherichia coli isolated from frequently consumed foods in the Lebanese market. Int. J. Food Prop. 2022, 25, 2435–2444. [Google Scholar] [CrossRef]
- James, C.; Dixon, R.; Talbot, L.; James, S.J.; Williams, N.; Onarinde, B.A. Assessing the Impact of Heat Treatment of Food on Antimicrobial Resistance Genes and Their Potential Uptake by Other Bacteria-A Critical Review. Antibiotics 2021, 10, 1440. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; Guerra, N.P.; Pastrana, L. Thermal resistance of Salmonella enterica, Escherichia coli and Staphylococcus aureus isolated from vegetable feed ingredients. J. Sci. Food Agric. 2014, 94, 2274–2281. [Google Scholar] [CrossRef]
- Stopforth, J.D.; Suhalim, R.; Kottapalli, B.; Hill, W.E.; Samadpour, M. Thermal Inactivation D- and z-Values of Multidrug-Resistant and Non–Multidrug-Resistant Salmonella Serotypes and Survival in Ground Beef Exposed to Consumer-Style Cooking. J. Food Prot. 2008, 71, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Rajkowski, K.T. Thermal inactivation of Escherichia coli O157:H7 and Salmonella on catfish and tilapia. Food Microbiol. 2012, 30, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Muriana, P.M. Efficacy of Commercial Sanitizers Used in Food Processing Facilities for Inactivation of Listeria Monocytogenes, E. Coli O157:H7, and Salmonella Biofilms. Foods 2019, 8, 639. [Google Scholar] [CrossRef]
- Nagel, G.M.; Bauermeister, L.J.; Bratcher, C.L.; Singh, M.; McKee, S.R. Salmonella and Campylobacter reduction and quality characteristics of poultry carcasses treated with various antimicrobials in a post-chill immersion tank. Int. J. Food Microbiol. 2013, 165, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.A.d.S.; Paula, O.F.P.; de Silva, C.R.G.; Leao, M.V.P.; dos Santos, S.S.F. Stability of antimicrobial activity of peracetic acid solutions used in the final disinfection process. Braz. Oral Res. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Suo, B.; Shi, C.; Shi, X. Inactivation and occurrence of sublethal injury of Salmonella Typhimurium under mild heat stress in broth. J. Für Verbraucherschutz Und Leb. 2012, 7, 125–131. [Google Scholar] [CrossRef]
- Elpers, L.; Deiwick, J.; Hensel, M. Effect of Environmental Temperatures on Proteome Composition of Salmonella enterica Serovar Typhimurium. Mol Cell Proteom. 2022, 21, 100265. [Google Scholar] [CrossRef]
- Abee, T. Microbial stress response in minimal processing. Int. J. Food Microbiol. 1999, 50, 65–91. [Google Scholar] [CrossRef]




| Code of the Strains | Serotype | Location of Isolation |
|---|---|---|
| A1 | Salmonella Enteritidis | Livestock slaughter and cutting department (slaughterhouse sector A) |
| A6 | S. Derby | Livestock slaughter and cutting department (slaughterhouse sector A) |
| B1 | S. Typhimurium | Stomach and intestinal cleansing department (slaughterhouse sector B) |
| B4 | S. Agona | Stomach and intestinal cleansing department (slaughterhouse sector B) |
| Experiment No. | Coded Values * | Numeric Values | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | PAA Concentration (%) | Temperature (°C) | Contact Time (min) | |
| 1 | −1 | −1 | 0 | 1 | 55 | 3 |
| 2 | 1 | −1 | 0 | 5 | 55 | 3 |
| 3 | −1 | 1 | 0 | 1 | 65 | 3 |
| 4 | 1 | 1 | 0 | 5 | 65 | 3 |
| 5 | −1 | 0 | −1 | 1 | 60 | 1 |
| 6 | 1 | 0 | −1 | 5 | 60 | 1 |
| 7 | −1 | 0 | 1 | 1 | 60 | 5 |
| 8 | 1 | 0 | 1 | 5 | 60 | 5 |
| 9 | 0 | −1 | −1 | 1.5 | 55 | 1 |
| 10 | 0 | 1 | −1 | 1.5 | 65 | 1 |
| 11 | 0 | −1 | 1 | 1.5 | 55 | 5 |
| 12 | 0 | 1 | 1 | 1.5 | 65 | 5 |
| 13 | 0 | 0 | 0 | 1.5 | 60 | 3 |
| 14 | 0 | 0 | 0 | 1.5 | 60 | 3 |
| 15 | 0 | 0 | 0 | 1.5 | 60 | 3 |
| Serotype | S. Enteritidis | S. Derbi | S. Typhimurium | S. Agona | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 45 | 55 | 65 | 45 | 55 | 65 | 45 | 55 | 65 | 45 | 55 | 65 |
| d | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.001 ± 0.000 |
| a | 6.099 ± 0.006 | 6.141 ± 0.004 | 6.085 ± 0.006 | 6.214 ± 0.006 | 6.204 ± 0.008 | 6.194 ± 0.003 | 6.005 ± 0.010 | 6.007 ± 0.006 | 5.929 ± 0.005 | 6.094 ± 0.009 | 6.135 ± 0.003 | 6.074 ± 0.008 |
| c | 232.710 ± 19.237 | 6.618 ± 0.016 | 2.536 ± 0.001 | 482.502 ± 51.734 | 110.423 ± 2.009 | 172.619 ± 2.464 | 23.934 ± 2.246 | 36.232 ± 1.549 | 13.058 ± 0.176 | 621.091 ± 7.072 | 37.867 ± 0.472 | 24.060 ± 0.264 |
| b | 0.383 ± 0.004 | 0.807 ± 0.010 | 0.990 ± 0.000 | 0.501 ± 0.003 | 0.732 ± 0.006 | 0.499 ± 0.011 | 0.998 ± 0.022 | 0.506 ± 0.001 | 0.926 ± 0.009 | 0.494 ± 0.003 | 0.631 ± 0.004 | 0.577 ± 0.009 |
| Serotype | Temp. (°C) | χ2 | RMSE | MBE | MPE |
|---|---|---|---|---|---|
| S. Enteritidis | 45 | 0.002 | 0.043 | 0.000 | 0.644 |
| 55 | 0.018 | 0.120 | 0.003 | 3.063 | |
| 65 | 0.011 | 0.094 | −0.006 | 4.293 | |
| S. Derbi | 45 | 0.007 | 0.076 | 0.000 | 1.113 |
| 55 | 0.000 | 0.012 | 0.000 | 0.175 | |
| 65 | 0.007 | 0.075 | 0.000 | 1.061 | |
| S. Typhimurium | 45 | 0.037 | 0.173 | 0.000 | 2.623 |
| 55 | 0.003 | 0.051 | 0.000 | 0.923 | |
| 65 | 0.036 | 0.171 | −0.001 | 2.898 | |
| S. Agona | 45 | 0.005 | 0.066 | 0.000 | 0.899 |
| 55 | 0.017 | 0.118 | 0.000 | 1.962 | |
| 65 | 0.013 | 0.100 | −0.001 | 1.991 |
| D Time (min) | Serotype | |||
|---|---|---|---|---|
| S. Enteritidis | S. Derby | S. Typhimurium | S. Agona | |
| D45 °C | >6 | 7.4 | 2.8 | >7.2 |
| D55 °C | 3.3 | 5.9 | 2.8 | 3.1 |
| D65 °C | 2.4 | 4.65 | 1.95 | 2.35 |
| Serotype | S. Enteritidis | S. Derbi | S. Typhimurium | S. Agona | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAA concentration (%) | 1 | 2 | 5 | 1 | 2 | 5 | 1 | 2 | 5 | 1 | 2 | 5 |
| d | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| a | 1.203 ± 0.005 | 0.000 ± 0.000 | 0.000 ± 0.000 | 2.600 ± 0.004 | 1.450 ± 0.000 | 0.000 ± 0.000 | 2.160 ± 0.001 | 1.700 ± 0.002 | 0.000 ± 0.000 | 3.413 ± 0.005 | 2.250 ± 0.009 | 0.000 ± 0.000 |
| c | 1.249 ± 0.011 | 1.000 ± 0.003 | 1.000 ± 0.002 | 1.000 ± 0.006 | 2.988 ± 0.010 | 1.000 ± 0.019 | 1.000 ± 0.025 | 3.307 ± 0.042 | 1.000 ± 0.028 | 1.167 ± 0.009 | 2.842 ± 0.0210 | 1.000 ± 0.0290 |
| b | 7.158 ± 0.019 | 7.146 ± 0.007 | 7.146 ± 0.014 | 36.178 ± 0.048 | 17.393 ± 0.163 | 7.146 ± 0.082 | 24.153 ± 0.083 | 15.800 ± 0.048 | 7.146 ± 0.071 | 2.33 ± 0.010 | 10.966 ± 0.042 | 7.146 ± 0.017 |
| Serotype | PAA (%) | χ2 | RMSE | MBE | MPE |
|---|---|---|---|---|---|
| S. Enteritidis | 1 | 0.000 | 0.001 | −0.001 | 0.030 |
| 2 | 0.000 | 0.000 | 0.000 | 0.000 | |
| 5 | 0.000 | 0.000 | 0.000 | 0.000 | |
| S. Derbi | 1 | 0.017 | 0.112 | −0.025 | 5.471 |
| 2 | 0.015 | 0.106 | 0.000 | 5.232 | |
| 5 | 0.000 | 0.000 | 0.000 | 0.000 | |
| S. Typhimurium | 1 | 0.003 | 0.045 | −0.010 | 2.455 |
| 2 | 0.060 | 0.212 | 0.000 | 9.120 | |
| 5 | 0.000 | 0.000 | 0.000 | 0.000 | |
| S. Agona | 1 | 0.005 | 0.063 | −0.014 | 3.888 |
| 2 | 0.015 | 0.106 | −0.002 | 3.386 | |
| 5 | 0.000 | 0.000 | 0.000 | 0.000 |
| D Time (min) | Serotype | |||
|---|---|---|---|---|
| S. Enteritidis | S. Derby | S. Typhimurium | S. Agona | |
| D1% | 0.7 | 1.5 | 1.45 | 0.95 |
| D2% | 0.1 | 0.95 | 1.15 | 0.8 |
| D5% | 0.1 | 0.1 | 0.1 | 0.1 |
| Box–Behnken Design | Surviving Cell Concentration(log CFU/mL) | Cell Damage Rate(%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PAA Concentration (%) | Temperature (°C) | Contact Time (min) | S. Enteritidis | S. Typhimurium | S. Derby | S. Agona | S. Enteritidis | S. Typhimurium | S. Derby | S. Agona |
| 1 | 55 | 3 | 2.2 | 1.87 | 2.48 | 1.84 | 27 | 22 | 30 | 21 |
| 2 | 55 | 3 | 0.9 | 1.4 | 1.26 | 0.7 | 25 | 20 | 50 | 0 |
| 1 | 65 | 3 | 1.23 | 1.175 | 1.84 | 1.12 | 59 | 33 | 26 | 23 |
| 2 | 65 | 3 | 0 | 0 | 0.4 | 0 | 0 | 0 | 0 | 0 |
| 1 | 60 | 1 | 1.56 | 1.57 | 2.21 | 1.21 | 19 | 30.5 | 28 | 19 |
| 2 | 60 | 1 | 0.85 | 0.91 | 1.84 | 0.78 | 28.5 | 25 | 19 | 30 |
| 1 | 60 | 5 | 0 | 1.71 | 1.91 | 1.3 | 0 | 6 | 29 | 16 |
| 2 | 60 | 5 | 0 | 0 | 0.3 | 0 | 0 | 0 | 0 | 0 |
| 1.5 | 55 | 1 | 2.05 | 1.98 | 2.5 | 1.86 | 37 | 45 | 41 | 8 |
| 1.5 | 65 | 1 | 2.01 | 1.96 | 2.43 | 1.63 | 22 | 26 | 15.5 | 5 |
| 1.5 | 55 | 5 | 1.93 | 1.77 | 2.33 | 1.4 | 54 | 51 | 17 | 20 |
| 1.5 | 65 | 5 | 0.47 | 0.3 | 1.68 | 0 | 50 | 0 | 29 | 0 |
| 1.5 | 60 | 3 | 0.7 | 1.08 | 1.26 | 1.01 | 20 | 17 | 17 | 0 |
| 1.5 | 60 | 3 | 0.7 | 1.0 | 1.26 | 1.0 | 20 | 10 | 17 | 0 |
| 1.5 | 60 | 3 | 0.7 | 1.08 | 1.26 | 1.0 | 20 | 17 | 17 | 0 |
| Factor | df | SSE | SST | SSD | SSA | DSE | DST | DSD | DSA |
|---|---|---|---|---|---|---|---|---|---|
| PAA concentration | 1 | 1.31 * | 2.02 ** | 2.69 ** | 1.99 ** | 331.53 | 270.28 | 242.00 | 300.13 |
| Temperature | 1 | 1.42 * | 1.61 ** | 0.62 * | 1.16 ** | 18.00 | 780.13 | 569.53 * | 55.13 |
| Time | 1 | 2.07 ** | 0.87 ** | 0.95 ** | 0.97 ** | 0.78 | 603.78 | 101.53 | 84.50 |
| PAA concentration × PAA concentration | 1 | 0.37 | 0.15 | 0.17 | 0.22 | 411.94 | 112.54 | 7.63 | 333.23 |
| Temperature × Temperature | 1 | 1.80 ** | 0.24 | 0.76 * | 0.09 | 1238.21 | 340.58 | 240.01 | 8.31 |
| Time × Time | 1 | 0.17 | 0.14 | 1.01 ** | 0.01 | 21.94 | 143.27 | 1.17 | 168.23 |
| PAA concentration × Temperature | 1 | 0.00 | 0.12 | 0.01 | 0.00 | 812.25 | 240.25 | 529.00 * | 1.00 |
| PAA concentration × Time | 1 | 0.13 | 0.28 | 0.38 * | 0.19 | 22.56 | 0.06 | 100.00 | 182.25 |
| Temperature × Time | 1 | 0.50 | 0.53 * | 0.08 | 0.34 | 30.25 | 256.00 | 351.56 | 72.25 |
| Error | 5 | 0.50 | 0.27 | 0.24 | 0.29 | 1775.31 | 675.60 | 340.06 | 468.25 |
| r2 | 0.940 | 0.957 | 0.965 | 0.946 | 0.628 | 0.804 | 0.863 | 0.713 |
| Box–Behnken Design | Surviving Cells Concentration(log CFU/mL) | Cell Damage Rate(%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PAA Concentration (%) | Temperature (°C) | Contact Time (min) | S. Enteritidis | S. Typhimurium | S. Derby | S. Agona | S. Enteritidis | S. Typhimurium | S. Derby | S. Agona |
| 1 | 55 | 3 | 2.43 | 2.31 | 3.42 | 2.24 | 41 | 35 | 61 | 43 |
| 2 | 55 | 3 | 2.21 | 1.91 | 2.95 | 1.91 | 17 | 15 | 12 | 21 |
| 1 | 65 | 3 | 2.32 | 2.12 | 2.80 | 1.68 | 9 | 9 | 6 | 23 |
| 2 | 65 | 3 | 0.60 | 0.30 | 2.02 | 0.00 | 0 | 0 | 12 | 0 |
| 1 | 60 | 1 | 1.88 | 2.00 | 2.65 | 1.45 | 16 | 7 | 5 | 11 |
| 2 | 60 | 1 | 0.00 | 0.00 | 0.48 | 0.00 | 0 | 0 | 33 | 0 |
| 1 | 60 | 5 | 1.00 | 1.00 | 2.23 | 0.90 | 30 | 40 | 12 | 13 |
| 2 | 60 | 5 | 0.00 | 1.00 | 0.40 | 0.90 | 0 | 0 | 40 | 0 |
| 1.5 | 55 | 1 | 1.48 | 1.41 | 2.21 | 1.34 | 13 | 27 | 9 | 14 |
| 1.5 | 65 | 1 | 0.84 | 1.30 | 1.51 | 1.21 | 0 | 15 | 34 | 0 |
| 1.5 | 55 | 5 | 2.62 | 2.24 | 2.98 | 2.21 | 28 | 47 | 29 | 51 |
| 1.5 | 65 | 5 | 1.48 | 1.41 | 2.21 | 1.34 | 23 | 51 | 13 | 23 |
| 1.5 | 60 | 3 | 2.05 | 1.84 | 1.49 | 1.74 | 43 | 30 | 78 | 25 |
| 1.5 | 60 | 3 | 2.05 | 1.84 | 1.49 | 1.74 | 43 | 30 | 78 | 24 |
| 1.5 | 60 | 3 | 2.05 | 1.84 | 1.49 | 1.74 | 43 | 30 | 78 | 25 |
| Factor | df | SSE | SST | SSD | SSA | DSE | DST | DSD | DSA |
|---|---|---|---|---|---|---|---|---|---|
| PAA concentration | 1 | 2.90 * | 2.23 ** | 3.45 * | 1.50 ** | 793.33 ** | 722.58 * | 17.82 | 575.20 |
| Temperature | 1 | 1.01 | 0.55 * | 0.25 | 0.92 * | 874.80 ** | 1396.76 ** | 2681.51 * | 346.14 |
| Time | 1 | 1.54 * | 0.94 ** | 1.14 | 1.50 ** | 555.68 * | 297.12 | 267.94 | 858.44 * |
| PAA concentration × PAA concentration | 1 | 0.48 | 0.16 | 4.04 * | 0.17 | 418.02 * | 51.50 | 2929.56 * | 152.80 |
| Temperature × Temperature | 1 | 0.10 | 0.11 | 0.12 | 0.23 | 341.56 * | 966.27 ** | 21.85 | 487.74 |
| Time × Time | 1 | 2.41 * | 0.77 ** | 0.35 | 0.68 * | 937.32 ** | 2.64 | 2940.71 * | 325.61 |
| PAA concentration × Temperature | 1 | 0.56 | 0.51 * | 0.02 | 0.46 | 59.99 | 30.20 | 753.87 | 0.43 |
| PAA concentration × Time | 1 | 0.19 | 1.00 ** | 0.03 | 0.53 * | 50.27 | 272.25 | 0.24 | 0.99 |
| Temperature × Time | 1 | 0.06 | 0.13 | 0.00 | 0.13 | 18.90 | 63.05 | 409.94 | 57.62 |
| Error | 5 | 1.00 | 0.21 | 1.43 | 0.36 | 206.38 | 230.70 | 1779.53 | 487.68 |
| r2 | 0.903 | 0.968 | 0.869 | 0.944 | 0.948 | 0.944 | 0.833 | 0.853 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šovljanski, O.; Ranitović, A.; Tomić, A.; Ćetković, N.; Miljković, A.; Saveljić, A.; Cvetković, D. Synergistic Strategies of Heat and Peroxyacetic Acid Disinfection Treatments for Salmonella Control. Pathogens 2023, 12, 1336. https://doi.org/10.3390/pathogens12111336
Šovljanski O, Ranitović A, Tomić A, Ćetković N, Miljković A, Saveljić A, Cvetković D. Synergistic Strategies of Heat and Peroxyacetic Acid Disinfection Treatments for Salmonella Control. Pathogens. 2023; 12(11):1336. https://doi.org/10.3390/pathogens12111336
Chicago/Turabian StyleŠovljanski, Olja, Aleksandra Ranitović, Ana Tomić, Nenad Ćetković, Ana Miljković, Anja Saveljić, and Dragoljub Cvetković. 2023. "Synergistic Strategies of Heat and Peroxyacetic Acid Disinfection Treatments for Salmonella Control" Pathogens 12, no. 11: 1336. https://doi.org/10.3390/pathogens12111336
APA StyleŠovljanski, O., Ranitović, A., Tomić, A., Ćetković, N., Miljković, A., Saveljić, A., & Cvetković, D. (2023). Synergistic Strategies of Heat and Peroxyacetic Acid Disinfection Treatments for Salmonella Control. Pathogens, 12(11), 1336. https://doi.org/10.3390/pathogens12111336







