Tick-Borne Co-Infections: Challenges in Molecular and Serologic Diagnoses
Abstract
1. Introduction
2. Ixodes scapularis (the Blacklegged Tick)
| Tick Vector | Agent | Disease | Prevalence in Ticks (%) | References |
|---|---|---|---|---|
| Ixodes scapularis | Borrelia burgdorferi | Lyme disease | N: 10–25%, A: 40–70% | [19,20,26,27,28,43] |
| Borrelia miyamotoi | Borrelia miyamotoi disease | N: 0.5–3%, A: <5% | [19,21,38,39,43] | |
| Borrelia mayonii | Lyme disease | N: 0.5–4%, A: <6% | [38,39] | |
| Anaplasma phagocytophilum | Human granulocytic anaplasmosis | N: 1–9%, A: 5–25% | [19,20,33,34,35,36,37,38,39,43] | |
| Ehrlichia muris eauclairensis | Ehrlichiosis | N: 0.5–2%, A: <3% | [38,44] | |
| Babesia microti | Babesiosis | N: 3–11%, A: 5–25% | [19,20,33,35,36,37,38,39,43] | |
| Powassan virus | Powassan encephalitis | N: <2%, A: <2% | [19,21,38,43] | |
| Amblyomma americanum | Ehrlichia chaffeensis | Human monocytic ehrlichiosis | N: 1–3%, A: 5–12% | [19,45,46,47,48] |
| Ehrlichia ewingii | Human ewingii ehrlichiosis | N: 1–3%, A: 3–8% | [19,45,46,47,48] | |
| Panola Mountain Ehrlichia | Not confirmed | N: <1%, A: <2% | [19,45] | |
| Rickettsia amblyommatis | Not confirmed | N: 15–55%, A: 40–85% | [19,47,48,49,50,51,52,53] | |
| Borrelia lonestari | Not confirmed | N: <1%, A: 1–5% | [19,45,46,47,48,54] | |
| Francisella tularensis | Tularemia | N: <0.05%, A: <0.05% | [55] | |
| Heartland virus | Heartland virus disease | N: <2%, A: <2% | [56,57] | |
| Bourbon virus | Bourbon virus disease | N: <1%, A: <1% | [56,58] |
3. Amblyomma americanum (the Lone Star Tick)
4. Other Clinically Relevant Tick Species
5. Co-Infections in Patients with TBDs
6. Laboratory Diagnosis
6.1. Culture and Microscopy
6.2. Molecular Methods
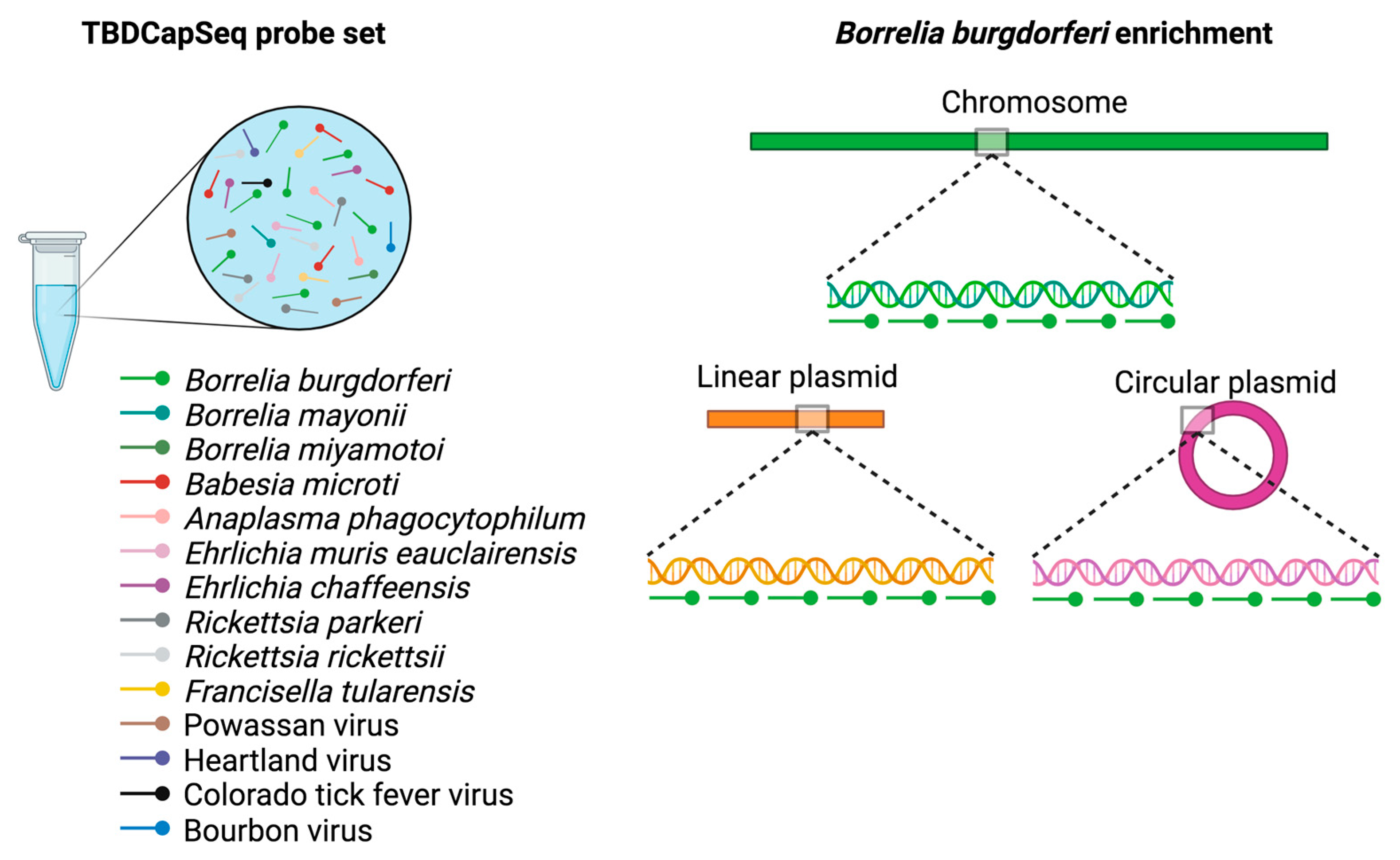
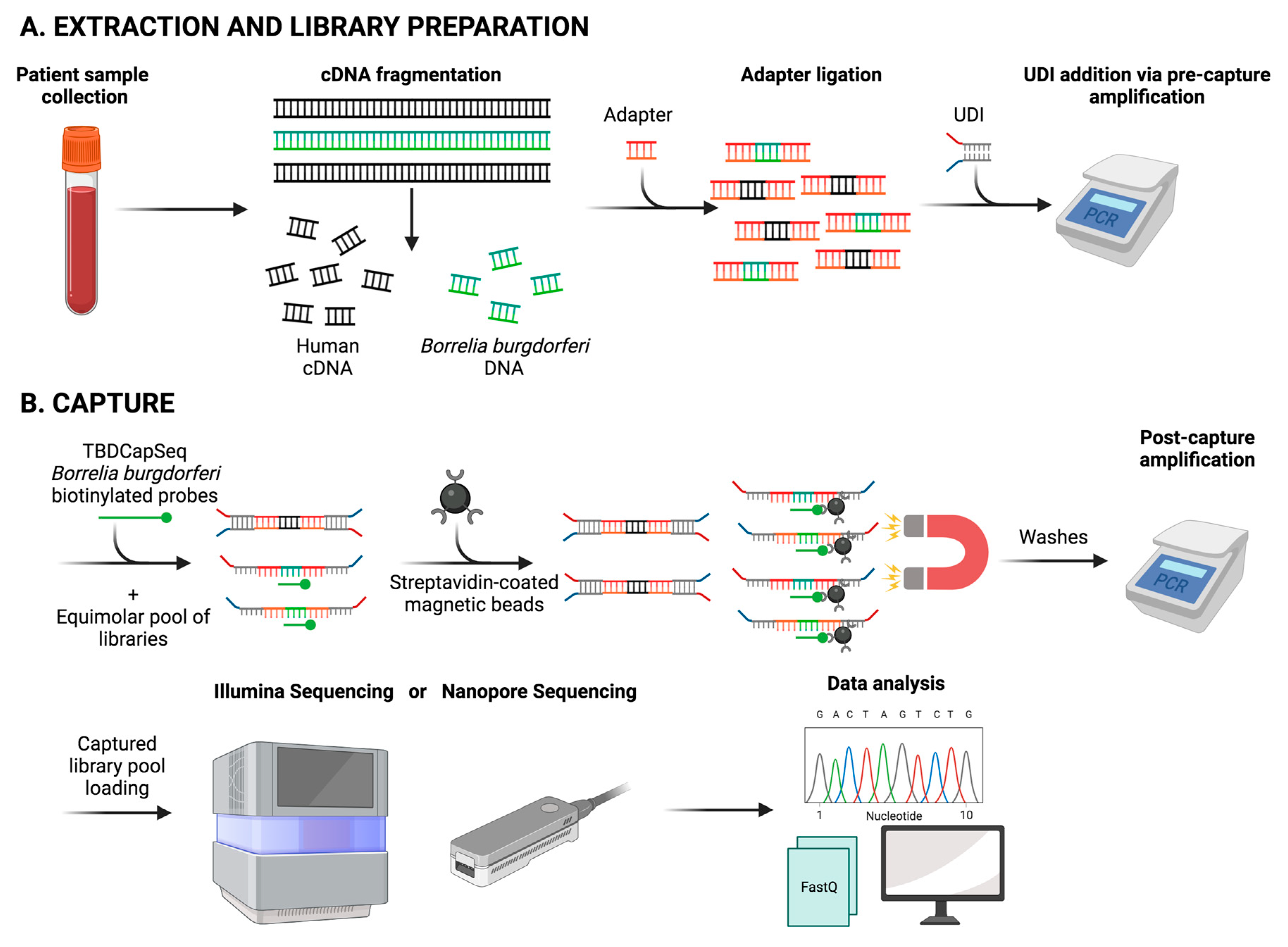
6.3. Next-Generation Sequencing (NGS)
6.4. Serology
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindgren, E.; Talleklint, L.; Polfeldt, T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 2000, 108, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S.; Dautel, H.; Estrada-Pena, A.; Kahl, O.; Lindgren, E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 593232. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Maarouf, A.; Barker, I.K.; Bigras-Poulin, M.; Lindsay, L.R.; Morshed, M.G.; O’Callaghan, C.J.; Ramay, F.; Waltner-Toews, D.; Charron, D.F. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. Int. J. Parasitol. 2006, 36, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.; Jaenson, D.G.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Sagurova, I.; Ludwig, A.; Ogden, N.H.; Pelcat, Y.; Dueymes, G.; Gachon, P. Predicted Northward Expansion of the Geographic Range of the Tick Vector Amblyomma americanum in North America under Future Climate Conditions. Env. Health Perspect. 2019, 127, 107014. [Google Scholar] [CrossRef]
- Raghavan, R.K.; Peterson, A.T.; Cobos, M.E.; Ganta, R.; Foley, D. Current and Future Distribution of the Lone Star Tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS ONE 2019, 14, e0209082. [Google Scholar] [CrossRef]
- Springer, Y.P.; Jarnevich, C.S.; Barnett, D.T.; Monaghan, A.J.; Eisen, R.J. Modeling the Present and Future Geographic Distribution of the Lone Star Tick, Amblyomma americanum (Ixodida: Ixodidae), in the Continental United States. Am. J. Trop. Med. Hyg. 2015, 93, 875–890. [Google Scholar] [CrossRef]
- Boorgula, G.D.Y.; Peterson, A.T.; Foley, D.H.; Ganta, R.R.; Raghavan, R.K. Assessing the current and future potential geographic distribution of the American dog tick, Dermacentor variabilis (Say) (Acari: Ixodidae) in North America. PLoS ONE 2020, 15, e0237191. [Google Scholar] [CrossRef]
- Bouchard, C.; Dibernardo, A.; Koffi, J.; Wood, H.; Leighton, P.A.; Lindsay, L.R. Increased risk of tick-borne diseases with climate and environmental changes. Can. Commun. Dis. Rep. 2019, 45, 83–89. [Google Scholar] [CrossRef]
- Eisen, R.J.; Paddock, C.D. Tick and Tickborne Pathogen Surveillance as a Public Health Tool in the United States. J. Med. Entomol. 2021, 58, 1490–1502. [Google Scholar] [CrossRef]
- Holden, K.; Hodzic, E.; Feng, S.; Freet, K.J.; Lefebvre, R.B.; Barthold, S.W. Coinfection with Anaplasma phagocytophilum alters Borrelia burgdorferi population distribution in C3H/HeN mice. Infect. Immun. 2005, 73, 3440–3444. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Anguita, J.; Barthold, S.W.; Fikrig, E. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis alters murine immune responses, pathogen burden, and severity of Lyme arthritis. Infect. Immun. 2001, 69, 3359–3371. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Telford, S.R.; Spielman, A.; Sikand, V.; Ryan, R.; Christianson, D.; Burke, G.; Brassard, P.; Pollack, R.; Peck, J.; et al. Concurrent lyme disease and babesiosis—Evidence for increased severity and duration of illness. JAMA-J. Am. Med. Assoc. 1996, 275, 1657–1660. [Google Scholar] [CrossRef]
- Cutler, S.J.; Vayssier-Taussat, M.; Estrada-Pena, A.; Potkonjak, A.; Mihalca, A.D.; Zeller, H. Tick-borne diseases and co-infection: Current considerations. Ticks Tick Borne Dis. 2021, 12, 101607. [Google Scholar] [CrossRef] [PubMed]
- Benach, J.L.; Coleman, J.L.; Habicht, G.S.; MacDonald, A.; Grunwaldt, E.; Giron, J.A. Serological evidence for simultaneous occurrences of Lyme disease and babesiosis. J. Infect. Dis. 1985, 152, 473–477. [Google Scholar] [CrossRef]
- Curcio, S.R.; Tria, L.P.; Gucwa, A.L. Seroprevalence of Babesia microti in Individuals with Lyme Disease. Vector Borne Zoonotic Dis. 2016, 16, 737–743. [Google Scholar] [CrossRef]
- Horowitz, H.W.; Aguero-Rosenfeld, M.E.; Holmgren, D.; McKenna, D.; Schwartz, I.; Cox, M.E.; Wormser, G.P. Lyme disease and human granulocytic anaplasmosis coinfection: Impact of case definition on coinfection rates and illness severity. Clin. Infect. Dis. 2013, 56, 93–99. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L. The Blacklegged Tick, Ixodes scapularis: An Increasing Public Health Concern. Trends Parasitol. 2018, 34, 295–309. [Google Scholar] [CrossRef]
- Sanchez-Vicente, S.; Tagliafierro, T.; Coleman, J.L.; Benach, J.L.; Tokarz, R. Polymicrobial Nature of Tick-Borne Diseases. mBio 2019, 10, e02055-19. [Google Scholar] [CrossRef]
- Tokarz, R.; Jain, K.; Bennett, A.; Briese, T.; Lipkin, W.I. Assessment of polymicrobial infections in ticks in New York state. Vector Borne Zoonotic Dis. 2010, 10, 217–221. [Google Scholar] [CrossRef]
- Tokarz, R.; Tagliafierro, T.; Cucura, D.M.; Rochlin, I.; Sameroff, S.; Lipkin, W.I. Detection of Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi, Borrelia miyamotoi, and Powassan Virus in Ticks by a Multiplex Real-Time Reverse Transcription-PCR Assay. mSphere 2017, 2, e00151-17. [Google Scholar] [CrossRef] [PubMed]
- Burgdorfer, W.; Barbour, A.G.; Hayes, S.F.; Benach, J.L.; Grunwaldt, E.; Davis, J.P. Lyme disease-a tick-borne spirochetosis? Science 1982, 216, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital Signs: Trends in Reported Vectorborne Disease Cases—United States and Territories, 2004–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef]
- Kugeler, K.J.; Schwartz, A.M.; Delorey, M.J.; Mead, P.S.; Hinckley, A.F. Estimating the Frequency of Lyme Disease Diagnoses, United States, 2010–2018. Emerg Infect. Dis. 2021, 27, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.A.; Saha, S.; Kugeler, K.J.; Delorey, M.J.; Shankar, M.B.; Hinckley, A.F.; Mead, P.S. Incidence of Clinician-Diagnosed Lyme Disease, United States, 2005–2010. Emerg Infect. Dis. 2015, 21, 1625–1631. [Google Scholar] [CrossRef]
- Steiner, F.E.; Pinger, R.R.; Vann, C.N.; Grindle, N.; Civitello, D.; Clay, K.; Fuqua, C. Infection and co-infection rates of Anaplasma phagocytophilum variants, Babesia spp., Borrelia burgdorferi, and the rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania, and Wisconsin. J. Med. Entomol. 2008, 45, 289–297. [Google Scholar] [CrossRef]
- Aliota, M.T.; Dupuis, A.P., 2nd; Wilczek, M.P.; Peters, R.J.; Ostfeld, R.S.; Kramer, L.D. The prevalence of zoonotic tick-borne pathogens in Ixodes scapularis collected in the Hudson Valley, New York State. Vector Borne Zoonotic Dis. 2014, 14, 245–250. [Google Scholar] [CrossRef]
- Tokarz, R.; Tagliafierro, T.; Sameroff, S.; Cucura, D.M.; Oleynik, A.; Che, X.; Jain, K.; Lipkin, W.I. Microbiome analysis of Ixodes scapularis ticks from New York and Connecticut. Ticks Tick Borne Dis. 2019, 10, 894–900. [Google Scholar] [CrossRef]
- Telford, S.R., 3rd; Dawson, J.E.; Katavolos, P.; Warner, C.K.; Kolbert, C.P.; Persing, D.H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. USA 1996, 93, 6209–6214. [Google Scholar] [CrossRef]
- Spielman, A.; Clifford, C.M.; Piesman, J.; Corwin, M.D. Human Babesiosis on Nantucket-Island, USA—Description of the Vector, Ixodes (Ixodes) Dammini, N-Sp (Acarina, Ixodidae). J. Med. Entomol. 1979, 15, 218–234. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Parasites-Babesiosis-Data & Statistics. Available online: https://www.cdc.gov/parasites/babesiosis/data-statistics/index.html (accessed on 10 June 2023).
- Centers for Disease Control and Prevention. Epidemiology and Statistics of Anaplasmosis in the United States, 2000–2019. Available online: https://www.cdc.gov/anaplasmosis/stats/index.html (accessed on 28 October 2023).
- Feldman, K.A.; Connally, N.P.; Hojgaard, A.; Jones, E.H.; White, J.L.; Hinckley, A.F. Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. J. Vector Ecol. 2015, 40, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.; Coyle, D.R.; Johnson, D.K.; Murphy, M.W.; McGeehin, M.A.; Murphy, R.J.; Raffa, K.F.; Paskewitz, S.M. Prevalence of Borrelia burgdorferi and Anaplasma phagocytophilum in Ixodes scapularis (Acari: Ixodidae) nymphs collected in managed red pine forests in Wisconsin. J. Med. Entomol. 2014, 51, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Graham, C.B.; Boegler, K.A.; Cherry, C.C.; Maes, S.E.; Pilgard, M.A.; Hojgaard, A.; Buttke, D.E.; Eisen, R.J. Prevalence and Diversity of Tick-Borne Pathogens in Nymphal Ixodes scapularis (Acari: Ixodidae) in Eastern National Parks. J. Med. Entomol. 2017, 54, 742–751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Edwards, M.J.; Russell, J.C.; Davidson, E.N.; Yanushefski, T.J.; Fleischman, B.L.; Heist, R.O.; Leep-Lazar, J.G.; Stuppi, S.L.; Esposito, R.A.; Suppan, L.M. A 4-Yr Survey of the Range of Ticks and Tick-Borne Pathogens in the Lehigh Valley Region of Eastern Pennsylvania. J. Med. Entomol. 2019, 56, 1122–1134. [Google Scholar] [CrossRef]
- Prusinski, M.A.; Kokas, J.E.; Hukey, K.T.; Kogut, S.J.; Lee, J.; Backenson, P.B. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands in the Hudson Valley Region, New York State. J. Med. Entomol. 2014, 51, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Graham, C.B.; Maes, S.E.; Hojgaard, A.; Fleshman, A.; Boegler, K.A.; Delory, M.J.; Slater, K.S.; Karpathy, S.E.; Bjork, J.K.; et al. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs in Minnesota, USA. Ticks Tick Borne Dis. 2018, 9, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Lehane, A.; Maes, S.E.; Graham, C.B.; Jones, E.; Delorey, M.; Eisen, R.J. Prevalence of single and coinfections of human pathogens in Ixodes ticks from five geographical regions in the United States, 2013–2019. Ticks Tick Borne Dis. 2021, 12, 101637. [Google Scholar] [CrossRef]
- Oliver, J.D.; Bennett, S.W.; Beati, L.; Bartholomay, L.C. Range Expansion and Increasing Borrelia burgdorferi Infection of the Tick Ixodes scapularis (Acari: Ixodidae) in Iowa, 1990–2013. J. Med. Entomol. 2017, 54, 1727–1734. [Google Scholar] [CrossRef]
- Schulze, T.L.; Jordan, R.A.; Healy, S.P.; Roegner, V.E. Detection of Babesia microti and Borrelia burgdorferi in host-seeking Ixodes scapularis (Acari: Ixodidae) in Monmouth County, New Jersey. J. Med. Entomol. 2013, 50, 379–383. [Google Scholar] [CrossRef]
- Hersh, M.H.; Ostfeld, R.S.; McHenry, D.J.; Tibbetts, M.; Brunner, J.L.; Killilea, M.E.; LoGiudice, K.; Schmidt, K.A.; Keesing, F. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE 2014, 9, e99348. [Google Scholar] [CrossRef]
- Narvaez, Z.E.; Rainey, T.; Puelle, R.; Khan, A.; Jordan, R.A.; Egizi, A.M.; Price, D.C. Detection of multiple tick-borne pathogens in Ixodes scapularis from Hunterdon County, NJ, USA. Curr. Res. Parasitol. Vector-Borne Dis. 2023, 4, 100140. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Foster, E.; Ribbe, F.; Hojgaard, A.; Eisen, R.J.; Paull, S.; Rich, S.M. Detection of Ehrlichia muris eauclairensis in Blacklegged Ticks (Ixodes scapularis) and White-Footed Mice (Peromyscus leucopus) in Massachusetts. Vector Borne Zoonotic Dis. 2023, 23, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Castellaw, A.H.; Showers, J.; Goddard, J.; Chenney, E.F.; Varela-Stokes, A.S. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J. Med. Entomol. 2010, 47, 473–476. [Google Scholar] [CrossRef]
- Schulze, T.L.; Jordan, R.A.; Schulze, C.J.; Mixson, T.; Papero, M. Relative encounter frequencies and prevalence of selected Borrelia, Ehrlichia, and Anaplasma infections in Amblyomma americanum and Ixodes scapularis (Acari: Ixodidae) ticks from central New Jersey. J. Med. Entomol. 2005, 42, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A.; White, J.C.; Roegner, V.E.; Healy, S.P. Geographical distribution and prevalence of selected Borrelia, Ehrlichia, and Rickettsia infections in Amblyomma americanum (Acari: Ixodidae) in New Jersey. J. Am. Mosq. Control Assoc. 2011, 27, 236–244. [Google Scholar] [CrossRef]
- Mixson, T.R.; Campbell, S.R.; Gill, J.S.; Ginsberg, H.S.; Reichard, M.V.; Schulze, T.L.; Dasch, G.A. Prevalence of Ehrlichia, Borrelia, and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J. Med. Entomol. 2006, 43, 1261–1268. [Google Scholar] [CrossRef]
- Egizi, A.; Gable, S.; Jordan, R.A. Rickettsia spp. Infecting Lone Star Ticks (Amblyomma americanum) (Acari: Ixodidae) in Monmouth County, New Jersey. J. Med. Entomol. 2020, 57, 974–978. [Google Scholar] [CrossRef]
- Karpathy, S.E.; Slater, K.S.; Goldsmith, C.S.; Nicholson, W.L.; Paddock, C.D. Rickettsia amblyommatis sp. nov., a spotted fever group Rickettsia associated with multiple species of Amblyomma ticks in North, Central and South America. Int. J. Syst. Evol. Microbiol. 2016, 66, 5236–5243. [Google Scholar] [CrossRef]
- Occi, J.; Egizi, A.M.; Goncalves, A.; Fonseca, D.M. New Jersey-Wide Survey of Spotted Fever Group Rickettsia (Proteobacteria: Rickettsiaceae) in Dermacentor variabilis and Amblyomma americanum (Acari: Ixodida: Ixodidae). Am. J. Trop. Med. Hyg. 2020, 103, 1009–1016. [Google Scholar] [CrossRef]
- Small, M.; Brennan, R.E. Detection of Rickettsia amblyommatis and Ehrlichia chaffeensis in Amblyomma americanum Inhabiting Two Urban Parks in Oklahoma. Vector Borne Zoonotic Dis. 2021, 21, 385–387. [Google Scholar] [CrossRef]
- Sayler, K.A.; Loftis, A.D.; Beatty, S.K.; Boyce, C.L.; Garrison, E.; Clemons, B.; Cunningham, M.; Alleman, A.R.; Barbet, A.F. Prevalence of Tick-Borne Pathogens in Host-Seeking Amblyomma americanum (Acari: Ixodidae) and Odocoileus virginianus (Artiodactyla: Cervidae) in Florida. J. Med. Entomol. 2016, 53, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Stegall-Faulk, T.; Clark, D.C.; Wright, S.M. Detection of Borrelia lonestari in Amblyomma americanum (Acari: Ixodidae) from Tennessee. J. Med. Entomol. 2003, 40, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, E.L.; Alford, H.I. Incidence of Tularemia and Rocky Mountain Spotted Fever among Common Ticks of Arkansas. Am. J. Trop. Med. Hyg. 1955, 4, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Savage, H.M.; Godsey, M.S., Jr.; Panella, N.A.; Burkhalter, K.L.; Manford, J.; Trevino-Garrison, I.C.; Straily, A.; Wilson, S.; Bowen, J.; Raghavan, R.K. Surveillance for Tick-Borne Viruses Near the Location of a Fatal Human Case of Bourbon Virus (Family Orthomyxoviridae: Genus Thogotovirus) in Eastern Kansas, 2015. J. Med. Entomol. 2018, 55, 701–705. [Google Scholar] [CrossRef]
- Tuten, H.C.; Burkhalter, K.L.; Noel, K.R.; Hernandez, E.J.; Yates, S.; Wojnowski, K.; Hartleb, J.; Debosik, S.; Holmes, A.; Stone, C.M. Heartland Virus in Humans and Ticks, Illinois, USA, 2018–2019. Emerg. Infect. Dis. 2020, 26, 1548–1552. [Google Scholar] [CrossRef]
- Savage, H.M.; Burkhalter, K.L.; Godsey, M.S., Jr.; Panella, N.A.; Ashley, D.C.; Nicholson, W.L.; Lambert, A.J. Bourbon Virus in Field-Collected Ticks, Missouri, USA. Emerg. Infect. Dis. 2017, 23, 2017–2022. [Google Scholar] [CrossRef]
- Gugliotta, J.L.; Goethert, H.K.; Berardi, V.P.; Telford, S.R., 3rd. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N. Engl. J. Med. 2013, 368, 240–245. [Google Scholar] [CrossRef]
- Telford, S.R., 3rd; Armstrong, P.M.; Katavolos, P.; Foppa, I.; Garcia, A.S.; Wilson, M.L.; Spielman, A. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg. Infect. Dis. 1997, 3, 165–170. [Google Scholar] [CrossRef]
- Pritt, B.S.; Allerdice, M.E.J.; Sloan, L.M.; Paddock, C.D.; Munderloh, U.G.; Rikihisa, Y.; Tajima, T.; Paskewitz, S.M.; Neitzel, D.F.; Hoang Johnson, D.K.; et al. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans. Int. J. Syst. Evol. Microbiol. 2017, 67, 2121–2126. [Google Scholar] [CrossRef]
- Pritt, B.S.; Mead, P.S.; Johnson, D.K.H.; Neitzel, D.F.; Respicio-Kingry, L.B.; Davis, J.P.; Schiffman, E.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: A descriptive study. Lancet Infect. Dis. 2016, 16, 556–564. [Google Scholar] [CrossRef]
- Pritt, B.S.; Respicio-Kingry, L.B.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; Bjork, J.; Liu, G.; Kingry, L.C.; Mead, P.S.; Neitzel, D.F.; et al. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int. J. Syst. Evol. Microbiol. 2016, 66, 4878–4880. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Narasimhan, S.; Wormser, G.P.; Barbour, A.G.; Platonov, A.E.; Brancato, J.; Lepore, T.; Dardick, K.; Mamula, M.; Rollend, L.; et al. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg. Infect. Dis. 2014, 20, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Powassan Virus Human Disease Cases By year of Illness Onset, 2004–2022. Available online: https://www.cdc.gov/powassan/statistics-data/historic-data.html (accessed on 30 October 2023).
- Centers for Disease Control and Prevention. Tickborne Diseases of the United States. Ehrlichiosis. Available online: https://www.cdc.gov/ticks/tickbornediseases/ehrlichiosis.html (accessed on 30 October 2023).
- Levine, J.F.; Wilson, M.L.; Spielman, A. Mice as Reservoirs of the Lyme-Disease Spirochete. Am. J. Trop. Med. Hyg. 1985, 34, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Telford, S.R.; Spielman, A. Reservoir Competence of White-Footed Mice for Babesia-Microti. J. Med. Entomol. 1993, 30, 223–227. [Google Scholar] [CrossRef]
- Ebel, G.D.; Campbell, E.N.; Goethert, H.K.; Spielman, A.; Telford, S.R., 3rd. Enzootic transmission of deer tick virus in New England and Wisconsin sites. Am. J. Trop. Med. Hyg. 2000, 63, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G.; Bunikis, J.; Travinsky, B.; Hoen, A.G.; Diuk-Wasser, M.A.; Fish, D.; Tsao, J.I. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg. 2009, 81, 1120–1131. [Google Scholar] [CrossRef]
- Dunn, J.M.; Krause, P.J.; Davis, S.; Vannier, E.G.; Fitzpatrick, M.C.; Rollend, L.; Belperron, A.A.; States, S.L.; Stacey, A.; Bockenstedt, L.K.; et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS ONE 2014, 9, e115494. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.J. Changes in the geographic distribution of the blacklegged tick, Ixodes scapularis, in the United States. Ticks Tick Borne Dis. 2023, 14, 102233. [Google Scholar] [CrossRef]
- Foster, E.; Maes, S.A.; Holcomb, K.M.; Eisen, R.J. Prevalence of five human pathogens in host-seeking Ixodes scapularis and Ixodes pacificus by region, state, and county in the contiguous United States generated through national tick surveillance. Ticks Tick-Borne Dis. 2023, 14, 102250. [Google Scholar] [CrossRef]
- Fleshman, A.C.; Foster, E.; Maes, S.E.; Eisen, R.J. Reported County-Level Distribution of Seven Human Pathogens Detected in Host-Seeking Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Contiguous United States. J. Med. Entomol. 2022, 59, 1328–1335. [Google Scholar] [CrossRef]
- Monzon, J.D.; Atkinson, E.G.; Henn, B.M.; Benach, J.L. Population and Evolutionary Genomics of Amblyomma americanum, an Expanding Arthropod Disease Vector. Genome Biol. Evol. 2016, 8, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Molaei, G.; Little, E.A.H.; Williams, S.C.; Stafford, K.C. Bracing for the Worst—Range Expansion of the Lone Star Tick in the Northeastern United States. N. Engl. J. Med. 2019, 381, 2189–2192. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.E.; Sims, K.G.; Olson, J.G.; Childs, J.E.; Piesman, J.F.; Happ, C.M.; Maupin, G.O.; Johnson, B.J. Amblyomma americanum: A potential vector of human ehrlichiosis. Am. J. Trop. Med. Hyg. 1993, 49, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Loftis, A.D.; Mixson, T.R.; Stromdahl, E.Y.; Yabsley, M.J.; Garrison, L.E.; Williamson, P.C.; Fitak, R.R.; Fuerst, P.A.; Kelly, D.J.; Blount, K.W. Geographic distribution and genetic diversity of the Ehrlichia sp. from Panola Mountain in Amblyomma americanum. BMC Infect. Dis. 2008, 8, 54. [Google Scholar] [CrossRef]
- Kosoy, O.I.; Lambert, A.J.; Hawkinson, D.J.; Pastula, D.M.; Goldsmith, C.S.; Hunt, D.C.; Staples, J.E. Novel thogotovirus associated with febrile illness and death, United States, 2014. Emerg. Infect. Dis. 2015, 21, 760–764. [Google Scholar] [CrossRef]
- Liu, S.C.; Kannan, S.; Meeks, M.; Sanchez, S.; Girone, K.W.; Broyhill, J.C.; Martines, R.B.; Bernick, J.; Flammia, L.; Murphy, J.; et al. Fatal Case of Heartland Virus Disease Acquired in the Mid-Atlantic Region, United States. Emerg. Infect. Dis. 2023, 29, 992–996. [Google Scholar] [CrossRef]
- Godsey, M.S.; Savage, H.M.; Burkhalter, K.L.; Bosco-Lauth, A.M.; Delorey, M.J. Transmission of Heartland Virus (Bunyaviridae: Phlebovirus) by Experimentally Infected Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 2016, 53, 1226–1233. [Google Scholar] [CrossRef]
- Dupuis, A.P., 2nd; Prusinski, M.A.; O’Connor, C.; Maffei, J.G.; Koetzner, C.A.; Zembsch, T.E.; Zink, S.D.; White, A.L.; Santoriello, M.P.; Romano, C.L.; et al. Bourbon Virus Transmission, New York, USA. Emerg. Infect. Dis. 2023, 29, 145–148. [Google Scholar] [CrossRef]
- Dupuis, A.P., 2nd; Prusinski, M.A.; O’Connor, C.; Maffei, J.G.; Ngo, K.A.; Koetzner, C.A.; Santoriello, M.P.; Romano, C.L.; Xu, G.; Ribbe, F.; et al. Heartland Virus Transmission, Suffolk County, New York, USA. Emerg. Infect. Dis. 2021, 27, 3128–3132. [Google Scholar] [CrossRef]
- Egizi, A.; Wagner, N.E.; Jordan, R.A.; Price, D.C. Lone star ticks (Acari: Ixodidae) infected with Bourbon virus in New Jersey, USA. J. Med. Entomol. 2023, 60, 842–846. [Google Scholar] [CrossRef]
- Masters, E.J.; Grigery, C.N.; Masters, R.W. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect. Dis. Clin. N. Am. 2008, 22, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Paul, W.S.; Schriefer, M.E.; Craven, R.B.; Robbins, K.E.; Dennis, D.T. Epidemiologic and diagnostic studies of patients with suspected early Lyme disease, Missouri, 1990–1993. J. Infect. Dis. 1995, 172, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Varela, A.S.; Moore, V.A.; Little, S.E. Disease agents in Amblyomma americanum from northeastern Georgia. J. Med. Entomol. 2004, 41, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.P.; Ponnusamy, L.; Jiang, J.; Ayyash, L.A.; Richards, A.L.; Apperson, C.S. Bacterial pathogens in ixodid ticks from a Piedmont County in North Carolina: Prevalence of rickettsial organisms. Vector Borne Zoonotic Dis. 2010, 10, 939–952. [Google Scholar] [CrossRef]
- Mendell, N.L.; Reynolds, E.S.; Blanton, L.S.; Hermance, M.E.; Londoño, A.F.; Hart, C.E.; Quade, B.R.; Esterly, A.T.; Hendrix, C.B.; Teel, P.D.; et al. Detection of Rickettsiae, Borreliae, and Ehrlichiae in Ticks Collected from Walker County, Texas, 2017–2018. Insects 2019, 10, 315. [Google Scholar] [CrossRef]
- Vazquez Guillamet, L.J.; Marx, G.E.; Benjamin, W.; Pappas, P.; Lieberman, N.A.P.; Bachiashvili, K.; Leal, S.; Lieberman, J.A. Relapsing Fever Caused by Borrelia lonestari after Tick Bite in Alabama, USA. Emerg. Infect. Dis. 2023, 29, 441–444. [Google Scholar] [CrossRef]
- Dahlgren, F.S.; Paddock, C.D.; Springer, Y.P.; Eisen, R.J.; Behravesh, C.B. Expanding Range of Amblyomma americanum and Simultaneous Changes in the Epidemiology of Spotted Fever Group Rickettsiosis in the United States. Am. J. Trop. Med. Hyg. 2016, 94, 35–42. [Google Scholar] [CrossRef]
- Ponnusamy, L.; Gonzalez, A.; Van Treuren, W.; Weiss, S.; Parobek, C.M.; Juliano, J.J.; Knight, R.; Roe, R.M.; Apperson, C.S.; Meshnick, S.R. Diversity of Rickettsiales in the microbiome of the lone star tick, Amblyomma americanum. Appl. Env. Microbiol. 2014, 80, 354–359. [Google Scholar] [CrossRef]
- Vaughn, M.F.; Delisle, J.; Johnson, J.; Daves, G.; Williams, C.; Reber, J.; Mendell, N.L.; Bouyer, D.H.; Nicholson, W.L.; Moncayo, A.C.; et al. Seroepidemiologic study of human infections with spotted fever group Rickettsiae in North Carolina. J. Clin. Microbiol. 2014, 52, 3960–3966. [Google Scholar] [CrossRef]
- Delisle, J.; Mendell, N.L.; Stull-Lane, A.; Bloch, K.C.; Bouyer, D.H.; Moncayo, A.C. Human Infections by Multiple Spotted Fever Group Rickettsiae in Tennessee. Am. J. Trop. Med. Hyg. 2016, 94, 1212–1217. [Google Scholar] [CrossRef]
- Apperson, C.S.; Engber, B.; Nicholson, W.L.; Mead, D.G.; Engel, J.; Yabsley, M.J.; Dail, K.; Johnson, J.; Watson, D.W. Tick-borne diseases in North Carolina: Is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008, 8, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Sajib, M.I.; Lamba, P.; Spitzer, E.D.; Marcos, L.A. False-Positive Serology for Rocky Mountain Spotted Fever in Long Island, New York, during 2011–2021. Pathogens 2023, 12, 503. [Google Scholar] [CrossRef] [PubMed]
- Yen, W.Y.; Stern, K.; Mishra, S.; Helminiak, L.; Sanchez-Vicente, S.; Kim, H.K. Virulence potential of Rickettsia amblyommatis for spotted fever pathogenesis in mice. Pathog. Dis. 2021, 79, ftab024. [Google Scholar] [CrossRef] [PubMed]
- Rivas, J.J.; Moreira-Soto, A.; Alvarado, G.; Taylor, L.; Calderon-Arguedas, O.; Hun, L.; Corrales-Aguilar, E.; Morales, J.A.; Troyo, A. Pathogenic potential of a Costa Rican strain of ‘Candidatus Rickettsia amblyommii’ in guinea pigs (Cavia porcellus) and protective immunity against Rickettsia rickettsii. Ticks Tick Borne Dis. 2015, 6, 805–811. [Google Scholar] [CrossRef]
- Snellgrove, A.N.; Krapiunaya, I.; Scott, P.; Levin, M.L. Assessment of the Pathogenicity of Rickettsia amblyommatis, Rickettsia bellii, and Rickettsia montanensis in a Guinea Pig Model. Vector Borne Zoonotic Dis. 2021, 21, 232–241. [Google Scholar] [CrossRef]
- Mani, R.J.; Metcalf, J.A.; Clinkenbeard, K.D. Amblyomma americanum as a bridging vector for human infection with Francisella tularensis. PLoS ONE 2015, 10, e0130513. [Google Scholar] [CrossRef]
- Hopla, C.E. The transmission of tularemia organisms by ticks in the southern states. South. Med. J. 1960, 53, 92–97. [Google Scholar] [CrossRef]
- Hopla, C.E. Experimental studies on tick transmission of tularemia organisms. Am. J. Hyg. 1953, 58, 101–118. [Google Scholar] [CrossRef]
- Whitlow, A.M.; Cumbie, A.N.; Eastwood, G. Pathogen prevalence in Amblyomma americanum and Ixodes scapularis ticks from central Appalachian Virginia, USA. J. Vector Ecol. 2022, 47, 51–60. [Google Scholar] [CrossRef]
- Fritzen, C.M.; Huang, J.; Westby, K.; Freye, J.D.; Dunlap, B.; Yabsley, M.J.; Schardein, M.; Dunn, J.R.; Jones, T.F.; Moncayo, A.C. Infection prevalences of common tick-borne pathogens in adult lone star ticks (Amblyomma americanum) and American dog ticks (Dermacentor variabilis) in Kentucky. Am. J. Trop. Med. Hyg. 2011, 85, 718–723. [Google Scholar] [CrossRef]
- Killmaster, L.F.; Loftis, A.D.; Zemtsova, G.E.; Levin, M.L. Detection of bacterial agents in Amblyomma americanum (Acari: Ixodidae) from Georgia, USA, and the use of a multiplex assay to differentiate Ehrlichia chaffeensis and Ehrlichia ewingii. J. Med. Entomol. 2014, 51, 868–872. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hecht, J.A.; Allerdice, M.E.J.; Dykstra, E.A.; Mastel, L.; Eisen, R.J.; Johnson, T.L.; Gaff, H.D.; Varela-Stokes, A.S.; Goddard, J.; Pagac, B.B.; et al. Multistate Survey of American Dog Ticks (Dermacentor variabilis) for Rickettsia Species. Vector Borne Zoonotic Dis. 2019, 19, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, J.J.; Swerdlow, D.L.; Krebs, J.W.; Holman, R.C.; Mandel, E.; Harvey, A.; Haberling, D.; Massung, R.F.; McQuiston, J.H. Rocky mountain spotted fever in the United States, 2000–2007: Interpreting contemporary increases in incidence. Am. J. Trop. Med. Hyg. 2010, 83, 174–182. [Google Scholar] [CrossRef]
- Drexler, N.A.; Dahlgren, F.S.; Heitman, K.N.; Massung, R.F.; Paddock, C.D.; Behravesh, C.B. National Surveillance of Spotted Fever Group Rickettsioses in the United States, 2008–2012. Am. J. Trop. Med. Hyg. 2016, 94, 26–34. [Google Scholar] [CrossRef]
- Moncayo, A.C.; Cohen, S.B.; Fritzen, C.M.; Huang, E.; Yabsley, M.J.; Freye, J.D.; Dunlap, B.G.; Huang, J.; Mead, D.G.; Jones, T.F.; et al. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am. J. Trop. Med. Hyg. 2010, 83, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Kugeler, K.J.; Eisen, L.; Beard, C.B.; Paddock, C.D. Tick-Borne Zoonoses in the United States: Persistent and Emerging Threats to Human Health. ILAR J. 2017, 58, 319–335. [Google Scholar] [CrossRef]
- Reese, S.M.; Petersen, J.M.; Sheldon, S.W.; Dolan, M.C.; Dietrich, G.; Piesman, J.; Eisen, R.J. Transmission efficiency of Francisella tularensis by adult american dog ticks (Acari: Ixodidae). J. Med. Entomol. 2011, 48, 884–890. [Google Scholar] [CrossRef]
- Goethert, H.K.; Telford, S.R., 3rd. Nonrandom distribution of vector ticks (Dermacentor variabilis) infected by Francisella tularensis. PLoS Pathog. 2009, 5, e1000319. [Google Scholar] [CrossRef]
- Whitten, T.; Demontigny, C.; Bjork, J.; Foss, M.; Peterson, M.; Scheftel, J.; Neitzel, D.; Sullivan, M.; Smith, K. Prevalence of Francisella tularensis in Dermacentor variabilis Ticks, Minnesota, 2017. Vector Borne Zoonotic Dis 2019, 19, 596–603. [Google Scholar] [CrossRef]
- Goethert, H.K.; Shani, I.; Telford, S.R., 3rd. Genotypic diversity of Francisella tularensis infecting Dermacentor variabilis ticks on Martha’s Vineyard, Massachusetts. J. Clin. Microbiol. 2004, 42, 4968–4973. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Rocky Mountain spotted fever. Lancet Infect. Dis. 2007, 7, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, W.I.; Tsynman, L.; Egizi, A.M.; Tokarz, R.; Maestas, L.P.; Fonseca, D.M. The Gulf Coast Tick, Amblyomma maculatum (Ixodida: Ixodidae), and Spotted Fever Group Rickettsia in the Highly Urbanized Northeastern United States. J. Med. Entomol. 2022, 59, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Maestas, L.P.; Reeser, S.R.; McGay, P.J.; Buoni, M.H. Surveillance for Amblyomma maculatum (Acari: Ixodidae) and Rickettsia parkeri (Rickettsiales: Rickettsiaceae) in the State of Delaware, and Their Public Health Implications. J. Med. Entomol. 2020, 57, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Molaei, G.; Little, E.A.H.; Khalil, N.; Ayres, B.N.; Nicholson, W.L.; Paddock, C.D. Established Population of the Gulf Coast Tick, Amblyomma maculatum (Acari: Ixodidae), Infected with Rickettsia parkeri (Rickettsiales: Rickettsiaceae), in Connecticut. J. Med. Entomol. 2021, 58, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garofalo, J.R.; Curley, S.R.; Field, C.E.; Hart, C.E.; Thangamani, S. Established Populations of Rickettsia parkeri-Infected Amblyomma maculatum Ticks in New York City, New York, USA. Vector Borne Zoonotic Dis. 2022, 22, 184–187. [Google Scholar] [CrossRef]
- Paddock, C.D.; Goddard, J. The Evolving Medical and Veterinary Importance of the Gulf Coast tick (Acari: Ixodidae). J. Med. Entomol. 2015, 52, 230–252. [Google Scholar] [CrossRef]
- Boyer, P.H.; Lenormand, C.; Jaulhac, B.; Talagrand-Reboul, E. Human Co-Infections between Borrelia burgdorferi s.l. and Other Ixodes-Borne Microorganisms: A Systematic Review. Pathogens 2022, 11, 282. [Google Scholar] [CrossRef]
- Ebel, G.D.; Kramer, L.D. Short report: Duration of tick attachment required for transmission of powassan virus by deer ticks. Am. J. Trop. Med. Hyg. 2004, 71, 268–271. [Google Scholar] [CrossRef]
- Breuner, N.E.; Dolan, M.C.; Replogle, A.J.; Sexton, C.; Hojgaard, A.; Boegler, K.A.; Clark, R.J.; Eisen, L. Transmission of Borrelia miyamotoi sensu lato relapsing fever group spirochetes in relation to duration of attachment by Ixodes scapularis nymphs. Ticks Tick Borne Dis. 2017, 8, 677–681. [Google Scholar] [CrossRef]
- Eisen, L. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick Borne Dis. 2018, 9, 535–542. [Google Scholar] [CrossRef]
- Wormser, G.P.; McKenna, D.; Scavarda, C.; Cooper, D.; El Khoury, M.Y.; Nowakowski, J.; Sudhindra, P.; Ladenheim, A.; Wang, G.; Karmen, C.L.; et al. Co-infections in Persons with Early Lyme Disease, New York, USA. Emerg. Infect. Dis. 2019, 25, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; McHugh, G.; Suarez, C.; Hoitt, J.; Damle, N.; Sikand, V.K. Prospective study of coinfection in patients with erythema migrans. Clin. Infect. Dis. 2003, 36, 1078–1081. [Google Scholar] [CrossRef]
- Belongia, E.A. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis. 2002, 2, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; McKay, K.; Thompson, C.A.; Sikand, V.K.; Lentz, R.; Lepore, T.; Closter, L.; Christianson, D.; Telford, S.R.; Persing, D.; et al. Disease-specific diagnosis of coinfecting tickborne zoonoses: Babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin. Infect. Dis. 2002, 34, 1184–1191. [Google Scholar] [CrossRef]
- Wang, T.J.; Liang, M.H.; Sangha, O.; Phillips, C.B.; Lew, R.A.; Wright, E.A.; Berardi, V.; Fossel, A.H.; Shadick, N.A. Coexposure to Borrelia burgdorferi and Babesia microti does not worsen the long-term outcome of lyme disease. Clin. Infect. Dis. 2000, 31, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Reed, K.D.; Mitchell, P.D.; Chyou, P.H.; Mueller-Rizner, N.; Finkel, M.F.; Schriefer, M.E. Clinical and epidemiological features of early Lyme disease and human granulocytic ehrlichiosis in Wisconsin. Clin. Infect. Dis. 1999, 29, 1472–1477. [Google Scholar] [CrossRef]
- Coleman, J.L.; LeVine, D.; Thill, C.; Kuhlow, C.; Benach, J.L. Babesia microti and Borrelia burgdorferi follow independent courses of infection in mice. J. Infect. Dis. 2005, 192, 1634–1641. [Google Scholar] [CrossRef]
- Moro, M.H.; Zegarra-Moro, O.L.; Bjornsson, J.; Hofmeister, E.K.; Bruinsma, E.; Germer, J.J.; Persing, D.H. Increased arthritis severity in mice coinfected with Borrelia burgdorferi and Babesia microti. J. Infect. Dis. 2002, 186, 428–431. [Google Scholar] [CrossRef]
- Djokic, V.; Akoolo, L.; Primus, S.; Schlachter, S.; Kelly, K.; Bhanot, P.; Parveen, N. Protozoan Parasite Babesia microti Subverts Adaptive Immunity and Enhances Lyme Disease Severity. Front. Microbiol. 2019, 10, 1596. [Google Scholar] [CrossRef]
- Marques, A.R. Laboratory Diagnosis of Lyme Disease Advances and Challenges. Infect. Dis. Clin. N. Am. 2015, 29, 295–307. [Google Scholar] [CrossRef]
- Thomas, R.J.; Dumler, J.S.; Carlyon, J.A. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert. Rev. Anti Infect. Ther. 2009, 7, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Labruna, M.B.; Raoult, D. Tick-borne rickettsioses in America: Unanswered questions and emerging diseases. Curr. Infect. Dis. Rep. 2009, 11, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Bittker, S.; Cooper, D.; Nowakowski, J.; Nadelman, R.B.; Pavia, C. Yield of large-volume blood cultures in patients with early Lyme disease. J. Infect. Dis. 2001, 184, 1070–1072. [Google Scholar] [CrossRef]
- Nowakowski, J.; Schwartz, I.; Liveris, D.; Wang, G.; Aguero-Rosenfeld, M.E.; Girao, G.; McKenna, D.; Nadelman, R.B.; Cavaliere, L.F.; Wormser, G.P.; et al. Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: A comparison of different techniques. Clin. Infect. Dis. 2001, 33, 2023–2027. [Google Scholar] [CrossRef]
- Dumler, J.S.; Madigan, J.E.; Pusterla, N.; Bakken, J.S. Ehrlichioses in humans: Epidemiology, clinical presentation, diagnosis, and treatment. Clin. Infect. Dis. 2007, 45 (Suppl. 1), S45–S51. [Google Scholar] [CrossRef] [PubMed]
- Killmaster, L.F.; Levin, M.L. Isolation and Short-Term Persistence of Ehrlichia ewingii in Cell Culture. Vector Borne Zoonotic Dis. 2016, 16, 445–448. [Google Scholar] [CrossRef]
- Schuster, F.L. Cultivation of Babesia and Babesia-like blood parasites: Agents of an emerging zoonotic disease. Clin. Microbiol. Rev. 2002, 15, 365–373. [Google Scholar] [CrossRef]
- Benach, J.L.; Bosler, E.M.; Hanrahan, J.P.; Coleman, J.L.; Habicht, G.S.; Bast, T.F.; Cameron, D.J.; Ziegler, J.L.; Barbour, A.G.; Burgdorfer, W.; et al. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 1983, 308, 740–742. [Google Scholar] [CrossRef]
- Sanchez, E.; Vannier, E.; Wormser, G.P.; Hu, L.T. Diagnosis, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis A Review. JAMA-J. Am. Med. Assoc. 2016, 315, 1767–1777. [Google Scholar] [CrossRef]
- Buller, R.S.; Arens, M.; Hmiel, S.P.; Paddock, C.D.; Sumner, J.W.; Rikhisa, Y.; Unver, A.; Gaudreault-Keener, M.; Manian, F.A.; Liddell, A.M.; et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 1999, 341, 148–155. [Google Scholar] [CrossRef]
- Wang, G.Q.; Wormser, G.P.; Zhuge, J.; Villafuerte, P.; Ip, D.; Zeren, C.; Fallon, J.T. Utilization of a real-time PCR assay for diagnosis of Babesia microti infection in clinical practice. Ticks Tick-Borne Dis. 2015, 6, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Teal, A.E.; Habura, A.; Ennis, J.; Keithly, J.S.; Madison-Antenucci, S. A New Real-Time PCR Assay for Improved Detection of the Parasite Babesia microti. J. Clin. Microbiol. 2012, 50, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Guzman, N.; Yarrarapu, S.N.S.; Beidas, S.O. Anaplasma phagocytophilum; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Vannier, E.; Krause, P.J. Human babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, J.; Baron, J.; Branda, J.; Lee-Lewandrowski, E. Laboratory Testing for Tick-Borne Infections in a Large Northeastern Academic Medical Center An 11-Year Experience. Am. J. Clin. Pathol. 2018, 150, 415–420. [Google Scholar] [CrossRef]
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L.; et al. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis—United States. Mmwr Recomm. Rep. 2016, 65, 1–44. [Google Scholar] [CrossRef]
- Brettschneider, S.; Bruckbauer, H.; Klugbauer, N.; Hofmann, H. Diagnostic value of PCR for detection of Borrelia burgdorferi in skin biopsy and urine samples from patients with skin borreliosis. J. Clin. Microbiol. 1998, 36, 2658–2665. [Google Scholar] [CrossRef]
- Brouqui, P.; Bacellar, F.; Baranton, G.; Birtles, R.J.; Bjoersdorff, A.; Blanco, J.R.; Caruso, G.; Cinco, M.; Fournier, P.E.; Francavilla, E.; et al. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin. Microbiol. Infect. 2004, 10, 1108–1132. [Google Scholar] [CrossRef]
- Wormser, G.P.; Aguero-Rosenfeld, M.E.; Cox, M.E.; Nowakowski, J.; Nadelman, R.B.; Holmgren, D.; McKenna, D.; Bittker, S.; Zentmaier, L.; Cooper, D.; et al. Differences and Similarities between Culture-Confirmed Human Granulocytic Anaplasmosis and Early Lyme Disease. J. Clin. Microbiol. 2013, 51, 954–958. [Google Scholar] [CrossRef]
- Olano, J.P.; Masters, E.; Hogrefe, W.; Walker, D.H. Human monocytotropic ehrlichiosis, Missouri. Emerg. Infect. Dis. 2003, 9, 1579–1586. [Google Scholar] [CrossRef]
- Everett, E.D.; Evans, K.A.; Henry, R.B.; McDonald, G. Human ehrlichiosis in adults after tick exposure. Diagnosis using polymerase chain reaction. Ann. Intern. Med. 1994, 120, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Standaert, S.M.; Yu, T.; Scott, M.A.; Childs, J.E.; Paddock, C.D.; Nicholson, W.L.; Singleton, J., Jr.; Blaser, M.J. Primary isolation of Ehrlichia chaffeensis from patients with febrile illnesses: Clinical and molecular characteristics. J. Infect. Dis. 2000, 181, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.H.; Robinson, L.K.; Stewart-Juba, J.J.; Dasch, G.A.; Kato, C.Y. Analytically sensitive Rickettsia species detection for laboratory diagnosis. Am. J. Trop. Med. Hyg. 2022, 106, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Kakumanu, M.L.; Ponnusamy, L.; Sutton, H.T.; Meshnick, S.R.; Nicholson, W.L.; Apperson, C.S. Development and Validation of an Improved PCR Method Using the 23S-5S Intergenic Spacer for Detection of Rickettsiae in Dermacentor variabilis Ticks and Tissue Samples from Humans and Laboratory Animals. J. Clin. Microbiol. 2016, 54, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.Y.; Chung, I.H.; Robinson, L.K.; Austin, A.L.; Dasch, G.A.; Massung, R.F. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J. Clin. Microbiol. 2013, 51, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Aguero-Rosenfeld, M.E.; Wang, G.; Schwartz, I.; Wormser, G.P. Diagnosis of lyme borreliosis. Clin. Microbiol. Rev. 2005, 18, 484–509. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; McHugh, G.A.; Damle, N.; Sikand, V.K.; Glickstein, L.; Steere, A.C. Burden and Viability of Borrelia burgdorferi in Skin and Joints of Patients with Erythema Migrans or Lyme Arthritis. Arthritis Rheum. 2011, 63, 2238–2247. [Google Scholar] [CrossRef]
- Nocton, J.J.; Dressler, F.; Rutledge, B.J.; Rys, P.N.; Persing, D.H.; Steere, A.C. Detection of Borrelia-Burgdorferi DNA by Polymerase Chain-Reaction in Synovial-Fluid from Patients with Lyme Arthritis. N. Engl. J. Med. 1994, 330, 229–234. [Google Scholar] [CrossRef]
- Hermance, M.E.; Thangamani, S. Powassan Virus: An Emerging Arbovirus of Public Health Concern in North America. Vector Borne Zoonotic Dis. 2017, 17, 453–462. [Google Scholar] [CrossRef]
- Staples, J.E.; Pastula, D.M.; Panella, A.J.; Rabe, I.B.; Kosoy, O.I.; Walker, W.L.; Velez, J.O.; Lambert, A.J.; Fischer, M. Investigation of Heartland Virus Disease Throughout the United States, 2013–2017. Open Forum Infect. Dis. 2020, 7, ofaa125. [Google Scholar] [CrossRef]
- Buchan, B.W.; Jobe, D.A.; Mashock, M.; Gerstbrein, D.; Faron, M.L.; Ledeboer, N.A.; Callister, S.M. Evaluation of a Novel Multiplex High-Definition PCR Assay for Detection of Tick-Borne Pathogens in Whole-Blood Specimens. J. Clin. Microbiol. 2019, 57, e00513-19. [Google Scholar] [CrossRef] [PubMed]
- Shakir, S.M.; Mansfield, C.R.; Hays, E.D.; Couturier, M.R.; Hillyard, D.R. Evaluation of a Novel High-Definition PCR Multiplex Assay for Simultaneous Detection of Tick-Borne Pathogens in Human Clinical Specimens. J. Clin. Microbiol. 2020, 58, e01655-19. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Marras, S.A.E.; Parveen, N. Sensitive multiplex PCR assay to differentiate Lyme spirochetes and emerging pathogens Anaplasma phagocytophilum and Babesia microti. BMC Microbiol. 2013, 13, 295. [Google Scholar] [CrossRef]
- Nigrovic, L.E.; Neville, D.N.; Chapman, L.; Balamuth, F.; Levas, M.N.; Thompson, A.D.; Kharbanda, A.B.; Gerstbrein, D.; Branda, J.A.; Buchan, B.W.; et al. Multiplex High-Definition Polymerase Chain Reaction Assay for the Diagnosis of Tick-borne Infections in Children. Open Forum Infect. Dis. 2023, 10, ofad121. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Pearson, P.; Rich, S.M. Ehrlichia muris in Ixodes cookei Ticks, Northeastern United States, 2016–2017. Emerg. Infect. Dis. 2018, 24, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- Pritt, B.S.; Sloan, L.M.; Johnson, D.K.; Munderloh, U.G.; Paskewitz, S.M.; McElroy, K.M.; McFadden, J.D.; Binnicker, M.J.; Neitzel, D.F.; Liu, G.; et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N. Engl. J. Med. 2011, 365, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Naccache, S.N.; Samayoa, E.; Messacar, K.; Arevalo, S.; Federman, S.; Stryke, D.; Pham, E.; Fung, B.; Bolosky, W.J.; et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res. 2019, 29, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu. Rev. Pathol-Mech. 2019, 14, 319–338. [Google Scholar] [CrossRef]
- Blauwkamp, T.A.; Thair, S.; Rosen, M.J.; Blair, L.; Lindner, M.S.; Vilfan, I.D.; Kawli, T.; Christians, F.C.; Venkatasubrahmanyam, S.; Wall, G.D.; et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat. Microbiol. 2019, 4, 663–674. [Google Scholar] [CrossRef]
- Hong, D.K.; Blauwkamp, T.A.; Kertesz, M.; Bercovici, S.; Truong, C.; Banaei, N. Liquid biopsy for infectious diseases: Sequencing of cell-free plasma to detect pathogen DNA in patients with invasive fungal disease. Diagn. Microbiol. Infect. Dis. 2018, 92, 210–213. [Google Scholar] [CrossRef]
- Schlaberg, R.; Chiu, C.Y.; Miller, S.; Procop, G.W.; Weinstock, G.; Comm, P.P.; Microbiology, A.S.; Pathologists, C.A. Validation of Metagenomic Next-Generation Sequencing Tests for Universal Pathogen Detection. Arch. Pathol. Lab. Med. 2017, 141, 776–786. [Google Scholar] [CrossRef]
- Pjevac, P.; Hausmann, B.; Schwarz, J.; Kohl, G.; Herbold, C.W.; Loy, A.; Berry, D. An Economical and Flexible Dual Barcoding, Two-Step PCR Approach for Highly Multiplexed Amplicon Sequencing. Front. Microbiol. 2021, 12, 669776. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.H.; Wu, H.C.; Liao, Y.C.; Yang Lauderdale, T.L.; Huang, I.W.; Chen, F.J. nanoMLST: Accurate multilocus sequence typing using Oxford Nanopore Technologies MinION with a dual-barcode approach to multiplex large numbers of samples. Microb. Genom. 2020, 6, e000336. [Google Scholar] [CrossRef] [PubMed]
- Rynkiewicz, E.C.; Hemmerich, C.; Rusch, D.B.; Fuqua, C.; Clay, K. Concordance of bacterial communities of two tick species and blood of their shared rodent host. Mol. Ecol. 2015, 24, 2566–2579. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Zhang, Y.; Allen, M.S. Bacterial microbiomes of Ixodes scapularis ticks collected from Massachusetts and Texas, USA. BMC Microbiol. 2019, 19, 138. [Google Scholar] [CrossRef]
- Tufts, D.M.; Sameroff, S.; Tagliafierro, T.; Jain, K.; Oleynik, A.; VanAcker, M.C.; Diuk-Wasser, M.A.; Lipkin, W.I.; Tokarz, R. A metagenomic examination of the pathobiome of the invasive tick species, Haemaphysalis longicornis, collected from a New York City borough, USA. Ticks Tick Borne Dis. 2020, 11, 101516. [Google Scholar] [CrossRef]
- Cross, S.T.; Kapuscinski, M.L.; Perino, J.; Maertens, B.L.; Weger-Lucarelli, J.; Ebel, G.D.; Stenglein, M.D. Co-Infection Patterns in Individual Ixodes scapularis Ticks Reveal Associations between Viral, Eukaryotic and Bacterial Microorganisms. Viruses 2018, 10, 388. [Google Scholar] [CrossRef]
- Tokarz, R.; Sameroff, S.; Tagliafierro, T.; Jain, K.; Williams, S.H.; Cucura, D.M.; Rochlin, I.; Monzon, J.; Carpi, G.; Tufts, D.; et al. Identification of Novel Viruses in Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis Ticks. mSphere 2018, 3, e00614-17. [Google Scholar] [CrossRef]
- Tokarz, R.; Williams, S.H.; Sameroff, S.; Leon, M.S.; Jain, K.; Lipkin, W.I. Virome Analysis of Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis Ticks Reveals Novel Highly Divergent Vertebrate and Invertebrate Viruses. J. Virol. 2014, 88, 11480–11492. [Google Scholar] [CrossRef]
- Briese, T.; Kapoor, A.; Mishra, N.; Jain, K.; Kumar, A.; Jabado, O.J.; Lipkin, W.I. Virome Capture Sequencing Enables Sensitive Viral Diagnosis and Comprehensive Virome Analysis. Mbio 2015, 6, e01491-15. [Google Scholar] [CrossRef]
- Allicock, O.M.; Guo, C.; Uhlemann, A.C.; Whittier, S.; Chauhan, L.V.; Garcia, J.; Price, A.; Morse, S.S.; Mishra, N.; Briese, T.; et al. BacCapSeq: A Platform for Diagnosis and Characterization of Bacterial Infections. Mbio 2018, 9, e02007-18. [Google Scholar] [CrossRef]
- Jain, K.; Tagliafierro, T.; Marques, A.; Sanchez-Vicente, S.; Gokden, A.; Fallon, B.; Mishra, N.; Briese, T.; Kapoor, V.; Sameroff, S.; et al. Development of a capture sequencing assay for enhanced detection and genotyping of tick-borne pathogens. Sci. Rep. 2021, 11, 12384. [Google Scholar] [CrossRef]
- Sanchez-Vicente, S.; Jain, K.; Tagliafierro, T.; Gokden, A.; Kapoor, V.; Guo, C.; Horn, E.J.; Lipkin, W.I.; Tokarz, R. Capture Sequencing Enables Sensitive Detection of Tick-Borne Agents in Human Blood. Front. Microbiol. 2022, 13, 837621. [Google Scholar] [CrossRef]
- Sheka, D.; Alabi, N.; Gordon, P.M.K. Oxford nanopore sequencing in clinical microbiology and infection diagnostics. Brief. Bioinform. 2021, 22, bbaa403. [Google Scholar] [CrossRef]
- Schutzer, S.E.; Body, B.A.; Boyle, J.; Branson, B.M.; Dattwyler, R.J.; Fikrig, E.; Gerald, N.J.; Gomes-Solecki, M.; Kintrup, M.; Ledizet, M.; et al. Direct Diagnostic Tests for Lyme Disease. Clin. Infect. Dis. 2019, 68, 1052–1057. [Google Scholar] [CrossRef]
- Stewart, A.G.; Stewart, A.G.A. An Update on the Laboratory Diagnosis of Rickettsia spp. Infection. Pathogens 2021, 10, 1319. [Google Scholar] [CrossRef]
- Bamunuarachchi, G.; Harastani, H.; Rothlauf, P.W.; Dai, Y.N.; Ellebedy, A.; Fremont, D.; Whelan, S.P.J.; Wang, D.; Boon, A.C.M. Detection of Bourbon Virus-Specific Serum Neutralizing Antibodies in Human Serum in Missouri, USA. mSphere 2022, 7, e0016422. [Google Scholar] [CrossRef]
- Kemenesi, G.; Banyai, K. Tick-Borne Flaviviruses, with a Focus on Powassan Virus. Clin. Microbiol. Rev. 2019, 32, e00106-17. [Google Scholar] [CrossRef]
- Mead, P.; Petersen, J.; Hinckley, A. Updated CDC Recommendation for Serologic Diagnosis of Lyme Disease. Morb. Mortal. Wkly. Rep. 2019, 68, 703. [Google Scholar] [CrossRef]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primers 2016, 2, 16090. [Google Scholar] [CrossRef]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef] [PubMed]
- Arnaboldi, P.M.; Seedarnee, R.; Sambir, M.; Callister, S.M.; Imparato, J.A.; Dattwyler, R.J. Outer surface protein C peptide derived from Borrelia burgdorferi sensu stricto as a target for serodiagnosis of early lyme disease. Clin. Vaccine Immunol. 2013, 20, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Lahey, L.J.; Panas, M.W.; Mao, R.; Delanoy, M.; Flanagan, J.J.; Binder, S.R.; Rebman, A.W.; Montoya, J.G.; Soloski, M.J.; Steere, A.C.; et al. Development of a Multiantigen Panel for Improved Detection of Borrelia burgdorferi Infection in Early Lyme Disease. J. Clin. Microbiol. 2015, 53, 3834–3841. [Google Scholar] [CrossRef] [PubMed]
- Signorino, G.; Arnaboldi, P.M.; Petzke, M.M.; Dattwyler, R.J. Identification of OppA2 linear epitopes as serodiagnostic markers for Lyme disease. Clin. Vaccine Immunol. 2014, 21, 704–711. [Google Scholar] [CrossRef]
- Toumanios, C.; Prisco, L.; Dattwyler, R.J.; Arnaboldi, P.M. Linear B Cell Epitopes Derived from the Multifunctional Surface Lipoprotein BBK32 as Targets for the Serodiagnosis of Lyme Disease. mSphere 2019, 4, e00111-19. [Google Scholar] [CrossRef]
- Tokarz, R.; Mishra, N.; Tagliafierro, T.; Sameroff, S.; Caciula, A.; Chauhan, L.; Patel, J.; Sullivan, E.; Gucwa, A.; Fallon, B.; et al. A multiplex serologic platform for diagnosis of tick-borne diseases. Sci. Rep. 2018, 8, 3158. [Google Scholar] [CrossRef]
- Tokarz, R.; Tagliafierro, T.; Caciula, A.; Mishra, N.; Thakkar, R.; Chauhan, L.V.; Sameroff, S.; Delaney, S.; Wormser, G.P.; Marques, A.; et al. Identification of immunoreactive linear epitopes of Borrelia miyamotoi. Ticks Tick Borne Dis. 2020, 11, 101314. [Google Scholar] [CrossRef]
- Tagliafierro, T.; Joshi, S.; Sameroff, S.; Marques, A.; Dumler, J.S.; Mishra, N.; Sanchez-Vicente, S.; Wormser, G.P.; Marcos, L.A.; Lipkin, W.I.; et al. Detection of antibodies to Anaplasma phagocytophilum and Babesia microti using linear peptides. Ticks Tick Borne Dis. 2022, 13, 101999. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Vicente, S.; Tokarz, R. Tick-Borne Co-Infections: Challenges in Molecular and Serologic Diagnoses. Pathogens 2023, 12, 1371. https://doi.org/10.3390/pathogens12111371
Sanchez-Vicente S, Tokarz R. Tick-Borne Co-Infections: Challenges in Molecular and Serologic Diagnoses. Pathogens. 2023; 12(11):1371. https://doi.org/10.3390/pathogens12111371
Chicago/Turabian StyleSanchez-Vicente, Santiago, and Rafal Tokarz. 2023. "Tick-Borne Co-Infections: Challenges in Molecular and Serologic Diagnoses" Pathogens 12, no. 11: 1371. https://doi.org/10.3390/pathogens12111371
APA StyleSanchez-Vicente, S., & Tokarz, R. (2023). Tick-Borne Co-Infections: Challenges in Molecular and Serologic Diagnoses. Pathogens, 12(11), 1371. https://doi.org/10.3390/pathogens12111371




