Coxiella burnetii Infection in Cats
Abstract
1. Introduction
2. Etiology
3. Epidemiology
3.1. Q Fever Human Cases Related to Infected Cats
3.2. Epidemiological Surveys in Cats
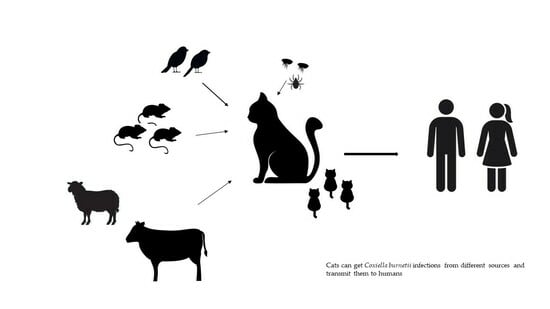
4. Sources of Infection
4.1. Arthropods
4.2. Pet Food
4.3. Milk
4.4. Preys
5. Pathogenesis and Clinical Forms
6. Diagnosis
7. Treatment and Prophylaxis
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oyston, P.C.F.; Davies, C. Q Fever: The Neglected Biothreat Agent. J. Med. Microbiol. 2011, 60, 9–21. [Google Scholar] [CrossRef]
- Pires, H.; Cardoso, L.; Lopes, A.P.; Fontes, M.D.C.; Matos, M.; Pintado, C.; Figueira, L.; Mesquita, J.R.; Matos, A.C.; Coelho, A.C. Seropositivity for Coxiella burnetii in Wild Boar (Sus scrofa) and Red Deer (Cervus elaphus) in Portugal. Pathogens 2023, 12, 421. [Google Scholar] [CrossRef]
- Greene, C.E. Q fever (Chapter 46). In Infectious Diseases of the Dogs and Cats, 4th ed.; Greene, C.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; Volume 2, pp. 482–484. [Google Scholar]
- Vitale, K.R.; Behnke, A.C.; Udell, M.A.R. Attachment bonds between domestic cats and humans. Curr. Biol. 2019, 29, R864–R865. [Google Scholar] [CrossRef]
- Brown, R.R.; Elston, T.H.; Evans, L.; Glaser, C.; Gulledge, M.L.; Jarboe, L.; Lappin, M.R.; Marcus, L.C.; American Association of Feline Practitioners. Feline zoonoses guidelines from the American Association of Feline Practitioners. J. Feline Med. Surg. 2005, 7, 243–274, Erratum in J. Feline Med. Surg. 2005, 7, 375. [Google Scholar] [CrossRef]
- Gerhold, R.W.; Jessup, D.A. Zoonotic diseases associated with free-roaming cats. Zoonoses Public Health 2013, 60, 189–195. [Google Scholar] [CrossRef]
- Maurin, M.; Raoult, D. Q fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef]
- Louisiana Office of Public Health—Infectious Disease Epidemiology Section—Q Fever. 2018. Available online: https://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/EpiManual/QFeverManual.pdf (accessed on 3 September 2023).
- Scott, G.H.; Williams, J.C. Susceptibility of Coxiella burnetii to chemical disinfectants. Ann. N. Y. Acad. Sci. 1990, 590, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ben Amara, A.; Ghigo, E.; Le Priol, Y.; Lépolard, C.; Salcedo, S.P.; Lemichez, E.; Bretelle, F.; Capo, C.; Mege, J.-L. Coxiella burnetii, the Agent of Q Fever, Replicates within Trophoblasts and Induces a Unique Transcriptional Response. PLoS ONE 2010, 5, e15315. [Google Scholar] [CrossRef] [PubMed]
- Amano, K.; Williams, J.C. Chemical and immunological characterization of lipopolysaccharides from phase I and phase II Coxiella burnetii. J. Bacteriol. 1984, 160, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Beare, P.A.; Jeffrey, B.M.; Long, C.M.; Martens, C.M.; Heinzen, R.A. Genetic mechanisms of Coxiella burnetii lipopolysaccharide phase variation. PLoS Pathog. 2018, 14, e1006922. [Google Scholar] [CrossRef]
- Celina, S.S.; Cerný, J. Coxiella burnetii in ticks, livestock, pets and wildlife: A mini-review. Front. Veter. Sci. 2022, 9, 1068129. [Google Scholar] [CrossRef]
- Kosatsky, T. Household outbreak of Q-fever pneumonia related to a parturient cat. Lancet 1984, 2, 1447–1449. [Google Scholar] [CrossRef]
- Marrie, T.J.; Durant, H.; Williams, J.C.; Mintz, E.; Waag, D.M. Exposure to parturient cats: A risk factor for acquisition of Q fever in Maritime Canada. J. Infect. Dis. 1988, 158, 101–108. [Google Scholar] [CrossRef]
- Marrie, T.J.; MacDonald, A.; Durant, H.; Yates, L.; McCormick, L. An outbreak of Q fever probably due to contact with a parturient cat. Chest 1988, 93, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.M.; Marrie, T.J.; Covert, A.; Waag, D.M.; Williams, J.C. Poker players’ pneumonia. An urban outbreak of Q fever following exposure to a parturient cat. N. Engl. J. Med. 1988, 319, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Marrie, T.J.; Langille, D.; Papukna, V.; Yates, L. Truckin’ pneumonia--an outbreak of Q fever in a truck repair plant probably due to aerosols from clothing contaminated by contact with newborn kittens. Epidemiol. Infect. 1989, 102, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, J.W.; Gordon, D.A.; Meis, A.; Parker, R.R. Q fever in laundry workers presumably transmitted from contaminated clothing. Am. J. Hyg. 1949, 49, 76–82. [Google Scholar] [PubMed]
- Marmion, B.P.; Stoker, M.G. The varying epidemiology of Q fever in the southeast region of Great Britain II. In two rural areas. J. Hyg. 1956, 54, 547–561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnson, D.W. Epidemiology of Q fever in Queensland: A seven year survey. Med. J. Aust. 1966, 1, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, R.L.; Fishbein, D.B.; Greene, C.R.; Gensheimer, K.F. An outbreak of cat-associated Q fever in the United States. J. Infect. Dis. 1991, 164, 202–204. [Google Scholar] [CrossRef]
- Kopecny, L.; Bosward, K.L.; Shapiro, A.; Norris, J.M. Investigating Coxiella burnetii infection in a breeding cattery at the centre of a Q fever outbreak. J. Feline Med. Surg. 2013, 15, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Malo, J.A.; Colbran, C.; Young, M.; Vasant, B.; Jarvinen, K.; Viney, K.; Lambert, S.B. An outbreak of Q fever associated with parturient cat exposure at an animal refuge and veterinary clinic in southeast Queensland Aust. N. Z. J. Public Health 2018, 42, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, A.S.; Jolley, W.B.; Dietrich, W.H.; Hunter, C.C. Coxiellosis in pound cats. Feline Pr. 1974, 4, 37–38. [Google Scholar]
- Willeberg, P.; Ruppanner, R.; Behymer, D.E.; Haghighi, S.; Kaneko, J.J.; Franti, C.E. Environmental exposure to Coxiella burnetii seroepidemiologic survey among domestic animals. Am. J. Epidemiol. 1980, 111, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Cairns, K.; Brewer, M.; Lappin, M.R. Prevalence of Coxiella burnetii DNA in vaginal and uterine samples from healthy cats of north-central Colorado. J. Feline Med. Surg. 2007, 9, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Marrie, T.J.; Van Buren, J.; Fraser, J.; Haldane, E.V.; Faulkner, R.S.; Williams, J.C.; Kwan, C. Seroepidemiology of Q fever among domestic animals in Nova Scotia. Am. J. Public Health 1985, 75, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.; Marrie, T.J. Seroepidemiology of Q-fever among cats in New Brunswick and Prince Edward Island. Ann. N. Y. Acad. Sci. 1990, 590, 271–274. [Google Scholar] [CrossRef]
- Cyr, J.; Turcotte, M.È.; Desrosiers, A.; Bélanger, D.; Harel, J.; Tremblay, D.; Leboeuf, A.; Gagnon, C.A.; Côté, J.C.; Arsenault, J. Prevalence of Coxiella burnetii seropositivity and shedding in farm, pet and feral cats and associated risk factors in farm cats in Quebec, Canada. Epidemiol. Infect. 2021, 149, e57. [Google Scholar] [CrossRef]
- Morita, C.; Katsuyama, J.; Yanase, T.; Ueno, H.; Muramatsu, Y.; Hohdatsu, T.; Koyama, H. Seroepidemiological survey of Coxiella burnetii in domestic cats in Japan. Microbiol. Immunol. 1994, 38, 1001–1003. [Google Scholar] [CrossRef]
- Komiya, T.; Sadamasu, K.; Kang, M.I.; Tsuboshima, S.; Fukushi, H.; Hirai, K. Seroprevalence of Coxiella burnetii infections among cats in different living environments. J. Vet. Med. Sci. 2003, 65, 1047–1048. [Google Scholar] [CrossRef]
- Mousapour, M.; Oveisi, A.; Key, Y.A.; Mikaeili, E.; Rahimi, F.; Shademan, B.; Bedoustani, A.B.; Fattahi, S.; Fasaei, M.S.; Abbasnezhad, A.D.; et al. First Serological & Molecular Study of Coxiella burnetii in Stray, Domestic Cats, and Their Owners in Iran. Top Companion Anim. Med. 2020, 41, 100471. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, S.; Komiya, T.; Celebi, B.; Aydin, N.; Saito, J.; Toriniwa, H.; Karatepe, B.; Babur, C. Seroprevalence of Coxiella burnetii in Stray Cats in Central Anatolia, Turk. J. Vet. Anim. Sci. 2008, 32. Available online: https://journals.tubitak.gov.tr/veterinary/vol32/iss6/13 (accessed on 7 September 2023).
- Saengsawang, P.; Pangjai, D.; Kaewmongkol, G.; Inpankaew, T. Detection of antibodies against three zoonotic Bartonella spp. and cross-reactivity among species and Coxiella burnetii in dogs and cats from Central Thailand. Comp. Immunol. Microbiol. Infect. Dis. 2022, 81, 101743. [Google Scholar] [CrossRef] [PubMed]
- Matthewman, L.; Kelly, P.; Hayter, D.; Downie, S.; Wray, K.; Bryson, N.; Rycroft, A.; Raoult, D. Exposure of cats in southern Africa to Coxiella burnetii, the agent of Q fever. Eur. J. Epidemiol. 1997, 13, 477–479. [Google Scholar] [CrossRef]
- Abdel-Moein, K.A.; Zaher, H.M. Parturient Cat As a Potential Reservoir for Coxiella burnetii: A Hidden Threat to Pet Owners. Vector Borne Zoonotic Dis. 2021, 21, 264–268. [Google Scholar] [CrossRef]
- Meredith, A.L.; Cleaveland, S.C.; Denwood, M.J.; Brown, J.K.; Shaw, D.J. Coxiella burnetii (Q-Fever) Seroprevalence in Prey and Predators in the United Kingdom: Evaluation of Infection in Wild Rodents, Foxes and Domestic Cats Using a Modified ELISA. Transbound. Emerg. Dis. 2015, 62, 639–649. [Google Scholar] [CrossRef]
- Candela, M.G.; Caballol, A.; Atance, P.M. Wide exposure to Coxiella burnetii in ruminant and feline species living in a natural environment: Zoonoses in a human-livestock-wildlife interface. Epidemiol. Infect. 2017, 145, 478–481. [Google Scholar] [CrossRef]
- Candela, M.G.; Fanelli, A.; Carvalho, J.; Serrano, E.; Domenech, G.; Alonso, F.; Martínez-Carrasco, C. Urban landscape and infection risk in free-roaming cats. Zoonoses Public Health 2022, 69, 295–311. [Google Scholar] [CrossRef]
- Anastácio, S.; Anjos, S.; Neves, S.; Neves, T.; Esteves, P.; Craveiro, H.; Madeira, B.; Pires, M.D.A.; Sousa, S.; da Silva, G.; et al. Coxiella burnetii in Dogs and Cats from Portugal: Serological and Molecular Analysis. Pathogens 2022, 11, 1525. [Google Scholar] [CrossRef]
- Ebani, V.V.; Guardone, L.; Marra, F.; Altomonte, I.; Nardoni, S.; Mancianti, F. Arthropod-Borne Pathogens in Stray Cats from Northern Italy: A Serological and Molecular Survey. Animals 2020, 10, 2334. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Bosward, K.L.; Heller, J.; Norris, J.M. Seroprevalence of Coxiella burnetii in domesticated and feral cats in eastern Australia. Veter. Microbiol. 2015, 177, 154–161. [Google Scholar] [CrossRef]
- Ma, G.C.; Norris, J.M.; Mathews, K.O.; Chandra, S.; Šlapeta, J.; Bosward, K.L.; Ward, M.P. New insights on the epidemiology of Coxiella burnetii in pet dogs and cats from New South Wales, Australia. Acta Trop. 2020, 205, 105416. [Google Scholar] [CrossRef]
- Lang, G.H. Q fever: An emerging public health concern in Canada. Can. J. Veter. Res. 1989, 53, 1. [Google Scholar]
- Philip, C.B. Observations on experimental Q fever. J. Parasitol. 1948, 34, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, H.; Hussein, H.A.; El-Razik, K.A.; Barakat, A.M.; Soliman, Y.A. Q fever: A neglected disease of camels in Giza and Cairo Provinces, Egypt. Veter-World. 2019, 12, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Kazar, J. Coxiella burnetii infection. Ann. N. Y. Acad. Sci. 2005, 1063, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bitam, I.; Dittmar, K.; Parola, P.; Whiting, M.F.; Raoult, D. Fleas and flea-borne diseases. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2010, 14, e667–e676. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Gage, K.L. Transmission of flea-borne zoonotic agents. Annu. Rev. Entomol. 2012, 57, 61–82. [Google Scholar] [CrossRef]
- Kamani, J.; Baneth, G.; Gutiérrez, R.; Nachum-Biala, Y.; Salant, H.; Mumcuoglu, K.Y.; Harrus, S. Molecular screening of Ctenocephalides felis fleas collected from stray cats in the Jerusalem District, Israel, for Bartonella spp., Rickettsia spp. and Coxiella burnetii. Veter. Parasitol Reg. Stud. Reports 2015, 1–2, 59–64. [Google Scholar] [CrossRef]
- Huang, H.H.H.; Power, R.I.; Mathews, K.O.; Ma, G.C.; Bosward, K.L.; Šlapeta, J. Cat fleas (Ctenocephalides felis clade ‘Sydney’) are dominant fleas on dogs and cats in New South Wales, Australia: Presence of flea-borne Rickettsia felis, Bartonella spp. but absence of Coxiella burnetii DNA. Curr. Res. Parasitol. Vector. Borne Dis. 2021, 1, 100045. [Google Scholar] [CrossRef]
- Wu, Y.L.; Hu, S.F.; Zhang, X.L.; Wang, H.M.; Pan, H.Y.; Liu, G.H.; Deng, Y.P. Complete bacterial profile and potential pathogens of cat fleas Ctenocephalides felis. Acta Trop. 2023, 243, 106923. [Google Scholar] [CrossRef]
- Loftis, A.D.; Reeves, W.K.; Szumlas, D.E.; Abbassy, M.M.; Helmy, I.M.; Moriarity, J.R.; Dasch, G.A. Surveillance of Egyptian fleas for agents of public health significance: Anaplasma, Bartonella, Coxiella, Ehrlichia, Rickettsia, and Yersinia pestis. Am. J. Trop. Med. Hyg. 2006, 75, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Psaroulaki, A.; Chochlakis, D.; Ioannou, I.; Angelakis, E.; Tselentis, Y. Presence of Coxiella burnetii in Fleas in Cyprus. Vector-Borne Zoonotic Dis. 2014, 14, 685–687. [Google Scholar] [CrossRef]
- Reusken, C.; van der Plaats, R.; Opsteegh, M.; de Bruin, A.; Swart, A. Coxiella burnetii (Q fever) in Rattus norvegicus and Rattus rattus at livestock farms and urban locations in the Netherlands; could Rattus spp. represent reservoirs for (re)introduction? Prev. Veter. Med. 2011, 101, 124–130. [Google Scholar] [CrossRef]
- Shapiro, A.; Bosward, K.; Mathews, K.; Vincent, G.; Stenos, J.; Tadepalli, M.; Norris, J. Molecular detection of Coxiella burnetii in raw meat intended for pet consumption. Zoonoses Public Health 2020, 67, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.S.; Banazis, M.J.; Yang, R.; Reid, S.A.; Fenwick, S.G. Prevalence of Coxiella burnetii in western grey kangaroos (Macropus fuliginosus) in Western Australia. J. Wildl. Dis. 2011, 47, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Barnes, T.; Potter, A.; Ketheesan, N.; Govan, B. Determination of Coxiella burnetii seroprevalence in macropods in Australia. Veter Microbiol. 2012, 155, 317–323. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Gateway to Dairy Production and Products. 2019. Available online: https://www.fao.org/dairy-production-products/products/types-and-characteristics/en/ (accessed on 7 November 2023).
- Meerburg, B.G.; Reusken, C.B.E.M. The role of wild rodents in spread and transmission of Coxiella burnetii needs further elucidation. Wildl. Res. 2011, 38, 617–625. [Google Scholar] [CrossRef]
- Buczek, A.M.; Buczek, W.; Buczek, A.; Bartosik, K. The Potential Role of Migratory Birds in the Rapid Spread of Ticks and Tick-Borne Pathogens in the Changing Climatic and Environmental Conditions in Europe. Int. J. Environ. Res. Public Health 2020, 17, 2117. [Google Scholar] [CrossRef]
- Ebani, V.V.; Mancianti, F. Potential Role of Birds in the Epidemiology of Coxiella burnetii, Coxiella-like Agents and Hepatozoon spp. Pathogens 2022, 11, 298. [Google Scholar] [CrossRef]
- Babudieri, B.; Moscovici, C. Experimental and natural infection of birds by Coxiella burnetii. Nature 1952, 169, 95–96. [Google Scholar] [CrossRef]
- Stein, A.; Raoult, D. Pigeon pneumonia in Provence: A bird-borne Q fever outbreak. Clin. Infect. Dis. 1999, 29, 617–620. [Google Scholar] [CrossRef]
- Ebani, V.V.; Bertelloni, F.; Mani, P. Molecular survey on zoonotic tick-borne bacteria and chlamydiae in feral pigeons (Columba livia domestica) Asian Pac. J. Trop. Med. 2016, 9, 324–327. [Google Scholar] [CrossRef]
- Tokarevich, N.K.; Panferova, Y.A.; Freylikhman, O.A.; Blinova, O.V.; Medvedev, S.G.; Mironov, S.V.; Grigoryeva, L.A.; Tretyakov, K.A.; Dimova, T.; Zaharieva, M.M.; et al. Coxiella burnetii in ticks and wild birds. Ticks Tick-Borne Dis. 2019, 10, 377–385. [Google Scholar] [CrossRef]
- Yadav, M.P.; Sethi, M.S. Poikilotherms as reservoirs of Q-fever (Coxiella burnetii) in Uttar Pradesh. J. Wildl. Dis. 1979, 15, 15–17. [Google Scholar] [CrossRef]
- Gillepsie, J.H.; Baker, J.A. Experimental Q fever in cats. Am. J. Veter. Res. 1952, 13, 91–94. [Google Scholar]
- Babudieri, B. Q fever: A zoonosis. Adv. Veter. Sci. 1959, 5, 81–181. [Google Scholar]
- Nagaoka, H.; Sugieda, M.; Akiyama, M.; Nishina, T.; Akahane, S.; Fujiwara, K. Isolation of Coxiella burnetii from the vagina of feline clients at veterinary clinics. J. Veter. Med. Sci. 1998, 60, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Berri, M.; Souriau, A.; Crosby, M.; Crochet, D.; Lechopier, P.; Rodolakis, A. Relationships between the shedding of Coxiella burnetii, clinical signs and serological responses of 34 sheep. Veter. Rec. 2001, 148, 502–505. [Google Scholar] [CrossRef]
- Rodolakis, A.; Berri, M.; Hechard, C.; Caudron, C.; Souriau, A.; Bodier, C.C.; Blanchard, B.; Camuset, P.; Devillechaise, P.; Natorp, J.C.; et al. Comparison of Coxiella burnetii shedding in milk of dairy bovine, caprine, and ovine herds. J. Dairy Sci. 2007, 90, 5352–5360. [Google Scholar] [CrossRef]
- Boden, K.; Brueckmann, A.; Wagner-Wiening, C.; Hermann, B.; Henning, K.; Junghanss, T.; Seidel, T.; Baier, M.; Straube, E.; Theegarten, D. Maternofetal consequences of Coxiella burnetii infection in pregnancy: A case series of two outbreaks. BMC Infect. Dis. 2012, 12, 359. [Google Scholar] [CrossRef]
- Colville, J.L.; Berryhill, D.L. Q Fever; Colville, J.L., Berryhill, D.L., Eds.; Handbook of Zoonoses: St. Louis, MO, USA, 2007; pp. 139–141. [Google Scholar] [CrossRef]
- Rousset, E.; Duquesne, V.; Russo, P.; Aubert, M.F. Q fever. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 6th ed.; Office International des Epizooties: Paris, France, 2010; pp. 292–303. [Google Scholar]
- Herr, S.; Huchzermeyer, H.F.; Te Brugge, L.A.; Williamson, C.C.; Roos, J.A.; Schiele, G.J. The use of a single complement fixation test technique in bovine brucellosis, Johne’s disease, dourine, equine piroplasmosis and Q fever serology. Onderstepoort. J. Veter. Res. 1985, 52, 279–282. [Google Scholar]
- Peter, O.; Flepp, M.; Bestetti, G.; Nicolet, J.; Luthy, R.; Dupuis, G. Q fever endocarditis: Diagnostic approaches and monitoring of therapeutic effects. Clin. Investig. 1992, 70, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.E.; Marrie, T.J.; Raoult, D. Diagnosis of Q fever. J. Clin. Microbiol. 1998, 36, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M.; Williams, J.C.; Marrie, T.J. The immune response to phase I and phase II Coxiella burnetii antigens as measured by Western immunoblotting. Ann. N. Y. Acad. Sci. 1990, 590, 187–202. [Google Scholar] [CrossRef]
- Tissot-Dupont, H.; Thirion, X.; Raoult, D. Q fever serology: Cutoff determination for microimmunofluorescence. Clin. Diagn. Lab Immunol. 1994, 1, 189–196. [Google Scholar] [CrossRef]
- Jensen, T.K.; Montgomery, D.L.; Jaeger, P.T.; Lindhardt, T.; Agerholm, J.S.; Bille-Hansen, V.; Boye, M. Application of fluorescent in situ hybridisation for demonstration of Coxiella burnetii in placentas from ruminant abortions. APMIS 2007, 115, 347–353. [Google Scholar] [CrossRef]
- Cichon, R.; Platt-Samoraj, A.; Uradziński, J. Benjamin Caballero. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Zoonoses, Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 6284–6289. ISBN 9780122270550. [Google Scholar] [CrossRef]
- ESCCAP—European Scientific Counsel Companion Animal Parasites. Control of Ectoparasites in Dogs and Cats. Available online: https://www.esccap.org/page/GL3+Control+of+Ectoparasites+in+Dogs+and+Cats/27/ (accessed on 25 September 2023).
- NSW Government. Q Fever. NSW Control Guidelines for Public Health Units. 2019. Available online: https://www.health.nsw.gov.au/Infectious/controlguideline/Documents/qfever.pdf (accessed on 7 November 2023).
| Country | Examined Population | Test | Antigen | Prevalence (n°Positive/n°Examined) | References |
|---|---|---|---|---|---|
| California (USA) | Pound cats | Capillary agglutination | phase I | 19.8% | [25] |
| California (USA) | Stray cats | Microagglutination | phase II | 9% (7/80) | [26] |
| Colorado (USA) | Pet cats Shelter cats | PCR | - | 8.5% (4/47) 0% (0/50) | [27] |
| Canada | Domestic cats | IFA IFA | phase I phase II | 24.1% (52/216) 6% (13/216) | [28] |
| Canada | Domestic cats | IFA | phase I and II | 6.2% (6/97) 19.2% (20/104) | [29] |
| Canada | Pet cats Feral cats Farm cats | ELISA | phase I and II | 0% (0/73) 0% (0/52) 3.4% (2/59) | [30] |
| Japan | Domestic cats | IFA | phase II | 16% (16/100) | [31] |
| Japan | Stray cats Pet cats | IFA | nr | 41.7% (15/36) 14.2% (44/310) | [32] |
| Korea | Pet cats | IFA | nr | 8.6% (10/116) | [32] |
| Iran | Stray cats Pet cats | ELISA | phase I and II | 22.35% (19/85) 11.53% (9/78) | [33] |
| Turkey | Stray cats | IFA | phase II | 4.9% (7/143) | [34] |
| Thailand | Domestic cats | IFA | phase I and II | 0.51% (2/390) | [35] |
| South Africa | nr | IFA | phase II | 2% (1/52) | [36] |
| Zimbabwe | nr | IFA | phase II | 13% (15/19) | [36] |
| Egypt | Pet cats | PCR | - | 7.5% (3/40) | [37] |
| United Kingdom | Domestic cats | ELISA | phase I and II | 61.5% (16/26) | [38] |
| Spain | Wildcats | ELISA | phase I and II | 33.3% (3/9) | [39] |
| Spain | Free-roaming cats | ELISA | phase I and II | 37% (108/291) | [40] |
| Portugal | Domestic cats | ELISA | phase I and II | 17.2% (5/29) 2012 0% (0/47) 2021 | [41] |
| Portugal | Domestic cats | PCR | - | 0% (0/107) | [41] |
| Italy | Stray cats | PCR | - | 29.4% (25/85) | [42] |
| Australia | Cattery cats Pet cats Feral cats Shelter cats | IFA and ELISA | phase I and II | 9.3% (35/376) 1% (2/198) 0% (0/50) 0% (0/98) | [43] |
| Australia | Domestic cats | IFA | phase I and II | 13.1% (19/145) | [44] |
| Australia | Domestic cats | PCR | - | 0% (0/145) | [44] |
| Host | Clinical Signs | References |
|---|---|---|
| Cats | Abortion premature delivery stillbirth and perinatal mortality fever anorexia lethargy respiratory disorders splenomegaly | [3,71] |
| Humans | fever sore throat chills headache lethargy myalgia nausea, vomiting diarrhea, abdominal pains chest pains pneumonia osteomyelitis, osteoarthritis hepatitis cholecystitis endocarditis, myocarditis, pericarditis encephalitis acute lymphadenitis exanthema placentitis, premature delivery spontaneous abortions | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebani, V.V. Coxiella burnetii Infection in Cats. Pathogens 2023, 12, 1415. https://doi.org/10.3390/pathogens12121415
Ebani VV. Coxiella burnetii Infection in Cats. Pathogens. 2023; 12(12):1415. https://doi.org/10.3390/pathogens12121415
Chicago/Turabian StyleEbani, Valentina Virginia. 2023. "Coxiella burnetii Infection in Cats" Pathogens 12, no. 12: 1415. https://doi.org/10.3390/pathogens12121415
APA StyleEbani, V. V. (2023). Coxiella burnetii Infection in Cats. Pathogens, 12(12), 1415. https://doi.org/10.3390/pathogens12121415