Why the SAFE—S Strategy for Trachoma? Are Musca sorbens or Scatophaga stercoraria Really the Culprit?—A Brief Historical Review from an Italian Point of View
Abstract
:1. History and Italian Background
2. Biology and Pathogenesis
3. Laboratory
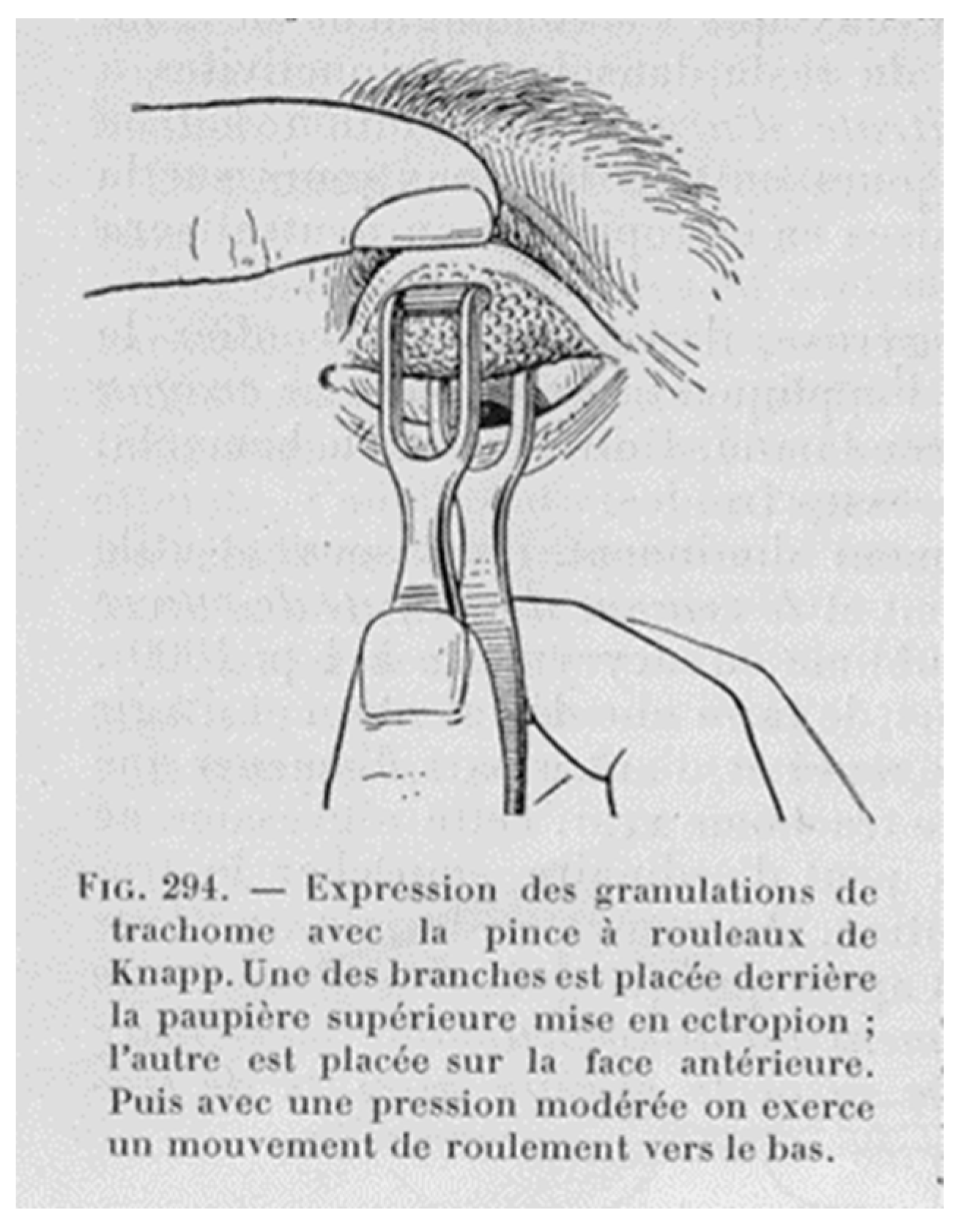
4. Contagion and Clinic, The SAFE-S Strategy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Oriel, J.D.; Ridgway, G.L. Genital Infection by Chlamydia Trachomatis; Elsevier Biomedical USA: Gainesville, FL, USA, 1982. [Google Scholar]
- Mackenzie, W. Traité Pratique des Maladies De l’Oeil; In Trad Franç, 4th ed.; Masson: Paris, France, 1856. [Google Scholar]
- Gallenga, P.E. Storia della cataratta nel secondo dopoguerra. In Evoluzione Della Chirurgia Della Cataratta in Italia; Storia, Buratto, L., Eds.; Fabiano: Moasca, Italy, 2019; pp. 1–40. [Google Scholar]
- Gallenga, C. Sul trattamento operativo delle cicatrici corneali. Gazz. Clin. 1885, 21, 1–9. [Google Scholar]
- Gallenga, C. Seconda osservazione di concrezione calcarea delle palpebre. Gazz. Clin. 1886, 3, 1–2. [Google Scholar]
- von Prowazek, S.; Halberstädter, L. Zur Aetiologie des Trachoms. Dtsch. Med. Wochenschr. 1907, 33, 1285–1287. [Google Scholar]
- Gallenga, C. Della Specificità Dei Corpuscoli Di Prowazeck E Halberstaedter Nella Congiuntivite Granulomatosa; Atti Società Italiana Patologia, VI riunione; Società Tipografica Modenese; Antica Tipografia Soliani: Modena, Italy, 1909. [Google Scholar]
- Gallenga, C. Zur Färbung der Prowazeck Halberstädterischen Trachom-Körperchen. Klin. Augenheilkd. 1910, 1, 195s. [Google Scholar]
- Axenfeld, T. Traité d’Ophtalmologie; Steinheil: Paris, France, 1914. [Google Scholar]
- Dark, A.J. Inclusion bodies in trachoma. Brit. J. Ophth. 1955, 39, 751. [Google Scholar] [CrossRef] [PubMed]
- Gallenga, C.E.; Del Boccio, M.; Del Boccio, G.; Perri, P.; Contini, C. Who is afraid of a bug mantled like Zorro? EC Microbiol. 2019, 15, 511–513. Available online: www.researchgate.net/publication/334251061 (accessed on 20 January 2023).
- Bietti, A. Trattato di Oftalmoiatria; Istituto Editoriale Scientifico: Milan, Italy, 1925. [Google Scholar]
- Gallenga, R. Plasmoma Della Congiuntiva Bulbare Superiore; Atti Congr Soc It Oftalmol: Roma, Italy, 1931; L’Universale Tipografia Poliglotta: Roma, Italy, 1932. [Google Scholar]
- Gallenga, R. The Ophthalmology course, duplicated lecture notes (Gagna E. typographer. 5th year Medical School University of Turin). 1966; and OMC&O Turin conference, in the University Eye Clinic 1967. [Google Scholar]
- Werner, G.H.; Latte, B.; Contini, A. Trachoma. Sci. Am. 1964, 210, 79–86. [Google Scholar] [CrossRef]
- Contini, A. Un Sassarese in Un Tempio Della Scienza; EDES: Sassari, Italy; ISBN 9788860252081.
- Scuderi, G. Istopatologia Del Trachoma; Minerva Medica: Turin, Italy, 1957. [Google Scholar]
- Bedson, S. The laboratory diagnosis of virus infections of man: A review. J. Clin. Path. 1947, 1, 2–9. [Google Scholar] [CrossRef]
- Dautry-Varsat, A.; Balanà, M.E.; Wyplosz, B. Chlamydia—Host Cell Interactions: Recent Advances on Bacterial Entry and Intracellular Development. Trafic 2004, 5, 561–570. [Google Scholar] [CrossRef]
- Bietti, G.B. I risultati della terapia penicillinica nel tracoma. Boll. Ocul. 1947, 26, 209. [Google Scholar]
- Cavara, V.; Bietti, G.B. Manifestazioni Oculari Delle Malattie Da Virus e Rickettsie; Cappelli, Ed.; Tip. Arte della Stampa: Roma, Italy; Bologna, Italy, 1952; ISBN 1952 10049. [Google Scholar]
- Page, L.A. Proposal for the recognition of two species in the genus Chlamydia. Jones, Rake, and Stearns, 1945. Int. J. Syst. Bacteriol. 1968, 18, 51–66. [Google Scholar] [CrossRef]
- Allan, I.; Pearce, J.H. Host modification of Chlamydiae: Presence of an egg antigen on the surface of chlamydiae grown in the chick embryo. J. Gen. Microbiol. 1979, 112, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Schachter, J.; Wyrick, P.B. Culture and isolation of Chlamydia trachomatis. Met. Enzymol. 1994, 236, 377–390. [Google Scholar]
- Black, C.M. (Ed.) Chlamydial Infection: A Clinical and Public Health Perspective; Karger: Basel, Switzerland, 2013; Volume 7, pp. 1–8. [Google Scholar] [CrossRef]
- Villareal, C.; Whittum-Hudson, J.A.; Hudson, A.P. Persistent Chlamydiae and chronic arthritis. Arthritis Res. 2002, 4, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Faal, N.; Bailey, R.L.; Sarr, I.; Joof, H.; Mabey, D.C.W.; Holland, M.J. Temporal cytokine gene expression patterns in subjects with trachoma identify distinct conjunctival responses associated with infection. Clin. Exp. Immunol. 2005, 142, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Skwor, T.A.; Atik, B.; Kandel, R.P.; Adhikari, H.K.; Sharma, B.; Dean, D. Role of secreted conjunctival mucosal cytokine and chemokine proteins in different stages of trachomatous disease. PLoS Negl. Trop. Dis. 2008, 2, e264. [Google Scholar] [CrossRef]
- Burton, M.J.; Ramadhani, A.; Weiss, H.A.; Hu, V.; Massae, P.; Burr, S.E.; Shangali, W.; Holland, M.J.; Mabey, D.C.; Bailey, R.L. Active trachoma is associated with increased conjunctival expression of IL-17A and profibrotic cytokines. Infect. Immun. 2011, 79, 4977–4983. [Google Scholar] [CrossRef]
- Contini, C.; Seraceni, S.; Maritati, M.; Cavazzini, F.; Perri, P. Role of Chlamydia in the development of ocular adnexal lymphoma. J. Cancer Ther. 2013, 4, 662–677. [Google Scholar] [CrossRef]
- Del Boccio, M.; Lobefalo, L.; Pennelli, A.; Toniato, E.; Martinotti, S.; Tenaglia, R.; Neri, G.; Del Boccio, G.; Gallenga, P.E. Can latent synergism of intestinal pathogens be responsible for inflammaging process causing Reiter’s syndrome in a young patient HLA-B27 infected by atypical pathogens? A holistic view and clinical biochemical reinterpretation. J. Biol. Regul. Homeost. Agents 2012, 26, 741–755. [Google Scholar]
- Gallenga, P.E.; Del Boccio, M.; Lobefalo, L.; Rapinese, M.; Mastropasqua, L.; Del Boccio, G. Molecular diagnosis of Chlamydia trachomatis conjunctivitis is improved by polymerase chain reaction. Rev. Int. Trac. Paris. 2004, 81, 69–84. [Google Scholar]
- Mei, C. Confronto di Metodiche Analitiche, Immunocromatografiche e Biomolecolari per la Determinazione di Chlamydia Trachomatis in Campioni Biologici. Ph.D. Thesis, Università C. Bo, Urbino, Italy, 2017. [Google Scholar]
- Gallenga, P.E.; Del Boccio, M.; Rapinese, L.; Lobefalo, M.; Pennelli, A.; Martinotti, S. Ocular clinical pictures disclosed by PCR molecular diagnosis of Chlamydia trachomatis infection performed following the appropriate sampling modality in ocular eco system. Int. J. Immunopathol. Pharmacol. 2012, 25, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Gallenga, P.E.; Del Boccio, M.; Gallenga, C.E.; Neri, G.; Pennelli, A.; Toniato, E.; Lobefalo, L.; Maritati, M.; Perri, P.; Contini, C. Diagnosis of a neonatal ophthalmic discharge, Ophtalmia Neonatorum, in the ‘Molecular age’: Investigation for a correct therapy. J. Biol. Regul. Homeost. Agents 2018, 32, 177–184. [Google Scholar] [PubMed]
- Astrauskiene, D.; Griskevicius, A.; Luksiene, R.; Panaviene, V.; Venaliene, J. Chlamydia trachomatis, Ureaplasma urealyticum, and Mycoplasma hominis in sexually intact girls with arthritides. Scand. J. Rheumatol. 2012, 41, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Neri, G.; Pennelli, A.; Del Boccio, M.; Neri, L.; Gallenga, C.E.; Toniato, E.; Tenaglia, R. Molecular Evidences of Chlamydia Trachomatis and Urogenital Mycoplasmas Infections from Birth Evolving into Multisite Infections. EC Microbiol. 2019, 15, 385–394. Available online: https://ecronicon.net/assets/ecmi/pdf/ECMI-15-00639.pdf (accessed on 20 January 2023).
- Frezzotti, R.; Guerra, R. Oftalmologia Essenziale, 2nd ed.; CEA: Milano, Italy, 2006. [Google Scholar]
- Schachter, J.; Causse, G.; Tarizzo, M.L. Chlamydiae as agents of sexually transmitted disease. WHO Bull. 1976, 54, 245–254. [Google Scholar]
- Delle Noci, N. George Bartisch. L’Ophthalmodouleia e l’Oculistica Rinascimentale; Théa special ed; Centro Grafico srl: Foggia, Italy, 2019. [Google Scholar]
- Akinsolu, F.T.; Nemieboka, P.O.; Njuguna, D.W.; Ahadji, M.N.; Dezso, D.; Varga, O. Emerging resistance of neglected tropical diseases: A scoping review of the literature. Int. J. Environ. Res. Public Health. 2019, 16, 1925. [Google Scholar] [CrossRef]
- Lewies, A.; Wentzel, J.F.; Jacobs, G.; Du Plessis, L.H. The potential use of natural and structural Antimicrobial Peptides in the fight against neglected Tropical Diseases. Molecules 2015, 20, 15392–15433. [Google Scholar] [CrossRef]
- Quin, J. Gene and peptide in the treatment of chlamydia trachomatis infections: Beginning of an era? Theoretical. Nat. Sci. 2023, 4, 18–25. [Google Scholar] [CrossRef]
- Bailey, R.; Lietman, T. The SAFE strategy for the elimination of trachoma by 2020: Will work? Bull. World Health Organ. 2001, 79, 233–236. [Google Scholar]
- WHO. Alliance for the Global Elimination of Trachoma by 2020: Progress report, 2019. WER 2020, 30, 349–360. [Google Scholar]
- WHO. Ending the Neglect to Attain the Sustainable Development Goals: A Rationale for Continued Investment in Tackling Neglected Tropical Diseases 2021–2030; WHO: Geneva, Switzerland, 2022; ISBN 9789240052932. [Google Scholar]
- Robinson, A.; Bristow, J.; Holl, M.V.; Makalo, P.; Alemayehu, W.; Bailey, R.L.; Macleod, D.; Birkett, M.A.; Caulfield, J.C.; Sarah, V. Responses of the putative trachoma vector, Musca sorbens, to volatile semiochemicals from human faeces. PLoS Negl. Trop. Dis. 2020, 14, e0007719. [Google Scholar] [CrossRef] [PubMed]
- Hirschberger, P.; Degro, H.N. Oviposition of the dung beetle Aphodius ater in relation to the abundance of yellow dung fly larvae (Scatophaga stercoraria). Ecol. Entomol. 1996, 21, 352–357. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U.; Pemberton, A.J.; Bussière, L.F.; Roembke, J.; Floate, K.D. A Review of the Natural History and Laboratory Culture Methods for the Yellow Dung Fly, Scathophaga stercoraria. J. Insect Sci. 2010, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Gallenga, P.E.; Del Boccio, M.; Neri, G.; Tenaglia, R.; Martinotti, S. Conjunctival chronic pain by Chlamydia trachomatis solved after asymptomatic chronic prostatitis treatment with echoguided drugs infiltration: A case report. Int. J. Med. Clin. Res. 2011, 2, 93–97. [Google Scholar]
- Neri, G.; Del Boccio, M.; Pennelli, A.; Martinotti, S.; Tenaglia, R.; Pugliese, M.; Toniato, E.; Croce, A.; Gallenga, P.E. Jugulodigastric lymph node inflammation derived from chronic atypical oropharyngeal phlogosis recurring annually after flu virus vaccination: A holistic vision of a clinical case solved after chlamydicidal antibiotic therapy. Int. J. Immunopathol. Pharmacol. 2012, 25, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Neri, G.; Pugliese, M.; Castriotta, A.; Mastronardi, V.; Pasqualini, P.; Colasante, A.; Cazzato, F.; Talamonti, R.; Del Boccio, G. White-line: A new finding in laryngopharyngeal reflux objective evaluation. Med. Hypotheses 2013, 80, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Di Bonaventura, G.; Del Boccio, M.; Pennelli, A.; Rapinese, M.; Tenaglia, R.; Gallenga, P.E.; Neri, G.; Boccio, G.D. Unusual Oropharyngeal Asymptomatic Manifestations Caused by Atypical Pathogens Detected by PCR into Altered Ecosystems of an Infertile Couple. Microbiol. Res. J. Int. 2014, 5, 447–458. [Google Scholar] [CrossRef]
- Contini, C.; Seraceni, S.; Carradori, S.; Cultrera, R.; Perri, P.; Lanza, F. Identification of Chlamydia trachomatis in a patient with ocular lymphoma. Am. J. Hematol. 2009, 84, 597–599. [Google Scholar] [CrossRef]
- Robman, L.; Mahdi, O.; McCarty, C.; Dimitrov, P.; Tikellis, G.; McNeil, J.; Byrne, G.; Taylor, H.; Guymer, R. Exposure to Chlamydia pneumoniae infection and progression of age-related macular degeneration. Am. J. Epidemiol. 2005, 161, 1013–1019. [Google Scholar] [CrossRef]
- Gallenga, C.E.; Parmeggiani, F.; Costagliola, C.; Sebastiani, A.; Gallenga, P.E. Inflammaging: Should this term be suitable for age related macular degeneration too? Inflamm. Res. 2014, 63, 105–107. [Google Scholar] [CrossRef]
- Breyer, B.N.; Huang, W.H.; Rabkin, C.S.; Alderete, J.F.; Pakpahan, R.; Beason, T.S.; Kenfield, S.A.; Mabie, J.; Ragard, L.; Wolin, K.Y.; et al. Sexually transmitted infections, benign prostatic hyperplasia and lower urinary tract symptom-related outcomes: Results from the Prostate, Lung, Colorectal and Ovarian Screening Trial. Brit. J. Urol. 2016, 117, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Mabey, D.C.; Solomon, A.W.; Foster, A. Trachoma. Lancet 2003, 362, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.R.; Turner, A.; Taylor, H.R. Trachoma. Lancet 2008, 371, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Stary, G.; Olive, A.; Radovic-Moreno, A.F.; Gondek, D.; Alvarez, D.; Basto, P.A.; Perro, M.; Vrbanac, V.D.; Tager, A.M.; Shi, J.; et al. Vaccines. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 2015, 348, 8205. [Google Scholar] [CrossRef]
- Gottlieb, S.L.; Johnston, C. Future prospects for new vaccines against sexually transmitted infections. Curr. Opin. Infect Dis. 2017, 30, 77–86. [Google Scholar] [CrossRef]





| a-IT and ATB | 1st Week | 2nd Week | 3rd Week | 4th Week |
| I cycle | CAF/HC ointment 3 times/8 h/day | AZT drop 3 times/8 h/day | N/D gel 3 times/8 h/day | 1stcultural check |
| a-IT and ATB | 5th week | 6th week | 7th week | 8th week |
| II cycle | TC/SMT ointment 3 times/8 h/day | ERYsyrup 2 times/day | CAF/TC ointment 3 times/8 h/day | TC drop 3 times/8 h/day |
| a-IT and ATB | 9th week | 10th week | 11th week | 12th week |
| III cycle | AZT drop 3 times/8 h/day | CAF/HC ointment 3 times/8 h/day | TC drop 3 times/8 h/day | N/D gel 3 times/8 h/day |
| a-IT and ATB | 13th week | 14th week | 15th week | 16th week |
| Cultural and molecular check | No therapy | No therapy | No therapy | 2nd control visit, cultural and molecular check |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallenga, C.E.; Maritati, M.; Del Boccio, M.; D’Aloisio, R.; Conti, P.; Mura, M.; Contini, C.; Gallenga, P.E. Why the SAFE—S Strategy for Trachoma? Are Musca sorbens or Scatophaga stercoraria Really the Culprit?—A Brief Historical Review from an Italian Point of View. Pathogens 2023, 12, 1419. https://doi.org/10.3390/pathogens12121419
Gallenga CE, Maritati M, Del Boccio M, D’Aloisio R, Conti P, Mura M, Contini C, Gallenga PE. Why the SAFE—S Strategy for Trachoma? Are Musca sorbens or Scatophaga stercoraria Really the Culprit?—A Brief Historical Review from an Italian Point of View. Pathogens. 2023; 12(12):1419. https://doi.org/10.3390/pathogens12121419
Chicago/Turabian StyleGallenga, Carla Enrica, Martina Maritati, Marco Del Boccio, Rossella D’Aloisio, Pio Conti, Marco Mura, Carlo Contini, and Pier Enrico Gallenga. 2023. "Why the SAFE—S Strategy for Trachoma? Are Musca sorbens or Scatophaga stercoraria Really the Culprit?—A Brief Historical Review from an Italian Point of View" Pathogens 12, no. 12: 1419. https://doi.org/10.3390/pathogens12121419






