Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome—An Extempore Game of Misfiring with Defense Arsenals
Abstract
:1. Introduction
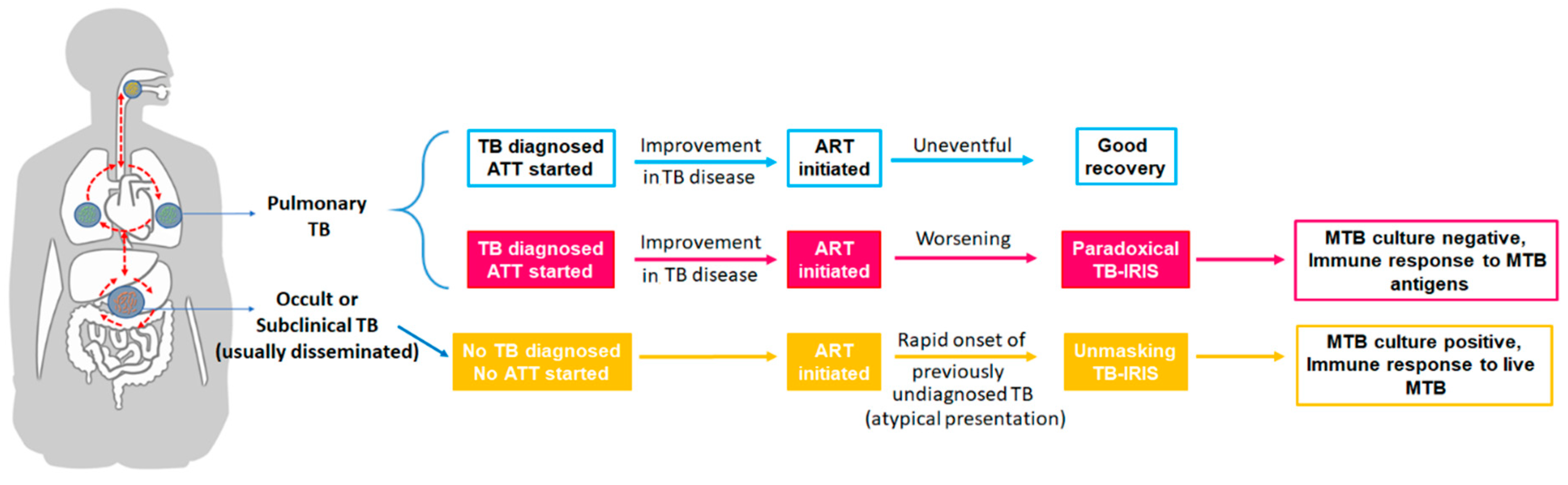
2. TB-IRIS: Types and Definitions
3. Incidence and Risk Factors
4. Challenges in Predicting and/or Diagnosing TB-IRIS
5. Newer Host Genetic Markers to Predict TB-IRIS
6. Immunopathogenesis of Paradoxical TB-IRIS
6.1. Deranged Functional Restoration of Cellular Immune Responses
6.2. Role of Innate Immunity
6.3. Role of Adaptive Immune Activation
7. Unmasking TB-IRIS
8. Prevention and Management of TB-IRIS
9. COVID-19 and TB-IRIS
10. Future Directions and Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Global Tuberculosis Report 2021. Available online: https://www.who.int/publications-detail-redirect/9789240037021 (accessed on 19 November 2022).
- Shankar, E.M.; Vignesh, R.; Ellegård, R.; Barathan, M.; Chong, Y.K.; Bador, M.K.; Rukumani, D.V.; Sabet, N.S.; Kamarulzaman, A.; Velu, V.; et al. HIV-Mycobacterium Tuberculosis Co-Infection: A “danger-Couple Model” of Disease Pathogenesis. Pathog. Dis. 2014, 70, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevaal, P.M.; Bekker, L.-G.; Hermans, S. TB-IRIS Pathogenesis and New Strategies for Intervention: Insights from Related Inflammatory Disorders. Tuberculosis 2019, 118, 101863. [Google Scholar] [CrossRef] [PubMed]
- Kwan, C.K.; Ernst, J.D. HIV and Tuberculosis: A Deadly Human Syndemic. Clin. Microbiol. Rev. 2011, 24, 351–376. [Google Scholar] [CrossRef] [Green Version]
- Waters, R.; Ndengane, M.; Abrahams, M.-R.; Diedrich, C.R.; Wilkinson, R.J.; Coussens, A.K. The Mtb-HIV Syndemic Interaction: Why Treating M. Tuberculosis Infection May Be Crucial for HIV-1 Eradication. Future Virol. 2020, 15, 101–125. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.; Nguyen, J.; Blair, L.; Banjanin, M.; Grewal, B.; Bowman, S.; Boyd, H.; Gerstner, G.; Cho, H.J.; Panfilov, D.; et al. Pathogenesis of Human Immunodeficiency Virus-Mycobacterium Tuberculosis Co-Infection. J. Clin. Med. 2020, 9, 3575. [Google Scholar] [CrossRef]
- Tuberculosis (TB). Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 19 November 2022).
- Sharan, R.; Bucşan, A.N.; Ganatra, S.; Paiardini, M.; Mohan, M.; Mehra, S.; Khader, S.; Kaushal, D. Chronic Immune Activation in TB/HIV Co-Infection. Trends Microbiol. 2020, 28, 619–632. [Google Scholar] [CrossRef]
- Dupont, M.; Souriant, S.; Balboa, L.; Vu Manh, T.-P.; Pingris, K.; Rousset, S.; Cougoule, C.; Rombouts, Y.; Poincloux, R.; Ben Neji, M.; et al. Tuberculosis-Associated IFN-I Induces Siglec-1 on Tunneling Nanotubes and Favors HIV-1 Spread in Macrophages. Elife 2020, 9, e52535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suthar, A.B.; Lawn, S.D.; del Amo, J.; Getahun, H.; Dye, C.; Sculier, D.; Sterling, T.R.; Chaisson, R.E.; Williams, B.G.; Harries, A.D.; et al. Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis. PLoS Med. 2012, 9, e1001270. [Google Scholar] [CrossRef]
- Walker, N.F.; Stek, C.; Wasserman, S.; Wilkinson, R.J.; Meintjes, G. The Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome: Recent Advances in Clinical and Pathogenesis Research. Curr. Opin. HIV AIDS 2018, 13, 512–521. [Google Scholar] [CrossRef]
- Quinn, C.M.; Poplin, V.; Kasibante, J.; Yuquimpo, K.; Gakuru, J.; Cresswell, F.V.; Bahr, N.C. Tuberculosis IRIS: Pathogenesis, Presentation, and Management across the Spectrum of Disease. Life 2020, 10, 262. [Google Scholar] [CrossRef]
- Shankar, E.M.; Vignesh, R.; Murugavel, K.G.; Balakrishnan, P.; Sekar, R.; Lloyd, C.A.; Solomon, S.; Kumarasamy, N. Immune Reconstitution Inflammatory Syndrome in Association with HIV/AIDS and Tuberculosis: Views over Hidden Possibilities. AIDS Res. Ther. 2007, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignesh, R.; Swathirajan, C.R.; Solomon, S.S.; Shankar, E.M.; Murugavel, K.G. Risk Factors and Frequency of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome among HIV/Tuberculosis Co-Infected Patients in Southern India. Indian J. Med. Microbiol. 2017, 35, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, R.; Kumarasamy, N.; Lim, A.; Solomon, S.; Murugavel, K.G.; Balakrishnan, P.; Solomon, S.S.; Mayer, K.H.; Swathirajan, C.R.; Chandrasekaran, E.; et al. TB-IRIS after Initiation of Antiretroviral Therapy Is Associated with Expansion of Preexistent Th1 Responses against Mycobacterium Tuberculosis Antigens. JAIDS J. Acquir. Immune Defic. Syndr. 2013, 64, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravimohan, S.; Tamuhla, N.; Steenhoff, A.P.; Letlhogile, R.; Nfanyana, K.; Bellamy, S.L.; MacGregor, R.R.; Gross, R.; Weissman, D.; Bisson, G.P. Immunological Profiling of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome and Non-Immune Reconstitution Inflammatory Syndrome Death in HIV-Infected Adults with Pulmonary Tuberculosis Starting Antiretroviral Therapy: A Prospective Observational Cohort Study. Lancet Infect. Dis. 2015, 15, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Gopalan, N.; Santhanakrishnan, R.K.; Palaniappan, A.N.; Menon, P.A.; Lakshman, S.; Chandrasekaran, P.; Sivaramakrishnan, G.N.; Reddy, D.; Kannabiran, B.P.; Agiboth, H.K.K.; et al. Daily vs Intermittent Antituberculosis Therapy for Pulmonary Tuberculosis in Patients With HIV: A Randomized Clinical Trial. JAMA Intern. Med. 2018, 178, 485–493. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Chai, C.; Liu, T.; Li, P.; Qu, M.; Zhao, H. Association of CD4 T Cell Count and Optimal Timing of Antiretroviral Therapy Initiation with Immune Reconstitution Inflammatory Syndrome and All-Cause Mortality for HIV-Infected Adults with Newly Diagnosed Pulmonary Tuberculosis: A Systematic Review and Meta-Analysis. Int. J. Clin. Exp. Pathol. 2021, 14, 670–679. [Google Scholar]
- Tan, D.B.A.; Yong, Y.K.; Tan, H.Y.; Kamarulzaman, A.; Tan, L.H.; Lim, A.; James, I.; French, M.; Price, P. Immunological Profiles of Immune Restoration Disease Presenting as Mycobacterial Lymphadenitis and Cryptococcal Meningitis. HIV Med. 2008, 9, 307–316. [Google Scholar] [CrossRef]
- Tan, H.Y.; Yong, Y.K.; Lim, S.H.; Ponnampalavanar, S.; Omar, S.F.S.; Pang, Y.K.; Kamarulzaman, A.; Price, P.; Crowe, S.M.; French, M.A. Tuberculosis (TB)-Associated Immune Reconstitution Inflammatory Syndrome in TB-HIV Co-Infected Patients in Malaysia: Prevalence, Risk Factors, and Treatment Outcomes. Sex Health 2014, 11, 532–539. [Google Scholar] [CrossRef]
- Yapa, H.M.; Kim, H.-Y.; Petoumenos, K.; Post, F.A.; Jiamsakul, A.; De Neve, J.-W.; Tanser, F.; Iwuji, C.; Baisley, K.; Shahmanesh, M.; et al. CD4+ T-Cell Count at Antiretroviral Therapy Initiation in the “Treat-All” Era in Rural South Africa: An Interrupted Time Series Analysis. Clin. Infect. Dis. 2021, 74, 1350–1359. [Google Scholar] [CrossRef]
- Ismail, S.D.; Pankrac, J.; Ndashimye, E.; Prodger, J.L.; Abrahams, M.-R.; Mann, J.F.S.; Redd, A.D.; Arts, E.J. Addressing an HIV Cure in LMIC. Retrovirology 2021, 18, 21. [Google Scholar] [CrossRef]
- Carmona, S.; Bor, J.; Nattey, C.; Maughan-Brown, B.; Maskew, M.; Fox, M.P.; Glencross, D.K.; Ford, N.; MacLeod, W.B. Persistent High Burden of Advanced HIV Disease Among Patients Seeking Care in South Africa’s National HIV Program: Data From a Nationwide Laboratory Cohort. Clin. Infect. Dis. 2018, 66, S111–S117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, P.; Fatti, G.; Ford, N.; Jennings, K.; Kruger, J.; Gunst, C.; Louis, F.; Grobbelaar, N.; Shanaube, K.; Floyd, S.; et al. Attrition When Providing Antiretroviral Treatment at CD4 Counts >500 cells/ΜL at Three Government Clinics Included in the HPTN 071 (PopART) Trial in South Africa. PLoS ONE 2018, 13, e0195127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebelonyane, R.; Bachanas, P.; Block, L.; Ussery, F.; Abrams, W.; Roland, M.; Theu, J.; Kapanda, M.; Matambo, S.; Lockman, S.; et al. Rapid Antiretroviral Therapy Initiation in the Botswana Combination Prevention Project: A Quasi-Experimental before and after Study. Lancet HIV 2020, 7, e545–e553. [Google Scholar] [CrossRef]
- Colocci, I.; Perlo, J.; Rajagopal, S.S.; Betancourt, T.S.; Pradeep, A.; Mayer, K.H.; Kumarasamy, N.; O’Cleirigh, C.; Katz, I.T.; Chan, B.T. Economic Vulnerability and Non-Initiation of Antiretroviral Therapy in India: A Qualitative Study. AIDS Care 2021, 33, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Ravimohan, S.; Tamuhla, N.; Kung, S.-J.; Nfanyana, K.; Steenhoff, A.P.; Gross, R.; Weissman, D.; Bisson, G.P. Matrix Metalloproteinases in Tuberculosis-Immune Reconstitution Inflammatory Syndrome and Impaired Lung Function Among Advanced HIV/TB Co-Infected Patients Initiating Antiretroviral Therapy. EBioMedicine 2016, 3, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Narendran, G.; Jyotheeswaran, K.; Senguttuvan, T.; Vinhaes, C.L.; Santhanakrishnan, R.K.; Manoharan, T.; Selvaraj, A.; Chandrasekaran, P.; Menon, P.A.; Bhavani, K.P.; et al. Characteristics of Paradoxical Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome and Its Influence on Tuberculosis Treatment Outcomes in Persons Living with HIV. Int. J. Infect. Dis. 2020, 98, 261–267. [Google Scholar] [CrossRef]
- Sharma, S.K.; Soneja, M. HIV & Immune Reconstitution Inflammatory Syndrome (IRIS). Indian J. Med. Res. 2011, 134, 866–877. [Google Scholar] [CrossRef]
- Manzardo, C.; Guardo, A.C.; Letang, E.; Plana, M.; Gatell, J.M.; Miro, J.M. Opportunistic Infections and Immune Reconstitution Inflammatory Syndrome in HIV-1-Infected Adults in the Combined Antiretroviral Therapy Era: A Comprehensive Review. Expert Rev. Anti. Infect. Ther. 2015, 13, 751–767. [Google Scholar] [CrossRef]
- Nelson, A.M.; Manabe, Y.C.; Lucas, S.B. Immune Reconstitution Inflammatory Syndrome (IRIS): What Pathologists Should Know. Semin. Diagn. Pathol. 2017, 34, 340–351. [Google Scholar] [CrossRef]
- Rewari, B.B.; Kumar, A.; Mandal, P.P.; Puri, A.K. HIV TB Coinfection—Perspectives from India. Expert. Rev. Respir. Med. 2021, 15, 911–930. [Google Scholar] [CrossRef]
- Manosuthi, W.; Van Tieu, H.; Mankatitham, W.; Lueangniyomkul, A.; Ananworanich, J.; Avihingsanon, A.; Siangphoe, U.; Klongugkara, S.; Likanonsakul, S.; Thawornwan, U.; et al. Clinical Case Definition and Manifestations of Paradoxical Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. AIDS 2009, 23, 2467–2471. [Google Scholar] [CrossRef]
- Namale, P.E.; Abdullahi, L.H.; Fine, S.; Kamkuemah, M.; Wilkinson, R.J.; Meintjes, G. Paradoxical TB-IRIS in HIV-Infected Adults: A Systematic Review and Meta-Analysis. Future Microbiol. 2015, 10, 1077–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hohlfeld, A.; Namale, P.; Meintjes, G.; Maartens, G.; Engel, M.E. Risk of Immune Reconstitution Inflammatory Syndrome with Integrase Inhibitors versus Other Classes of Antiretrovirals: A Systematic Review and Meta-Analysis of Randomised Trials. J. Acquir. Immune Defic. Syndr. 2022, 90, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Manosuthi, W.; Wiboonchutikul, S.; Sungkanuparph, S. Integrated Therapy for HIV and Tuberculosis. AIDS Res. Ther. 2016, 13, 22. [Google Scholar] [CrossRef] [Green Version]
- Meintjes, G.; Lawn, S.D.; Scano, F.; Maartens, G.; French, M.A.; Worodria, W.; Elliott, J.H.; Murdoch, D.; Wilkinson, R.J.; Seyler, C.; et al. Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome: Case Definitions for Use in Resource-Limited Settings. Lancet Infect. Dis. 2008, 8, 516–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, L.C.K.; Breen, R.; Miller, R.F.; Noursadeghi, M.; Lipman, M. Paradoxical Reactions and Immune Reconstitution Inflammatory Syndrome in Tuberculosis. Int. J. Infect. Dis. 2015, 32, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshun-Wilson, I.; Havers, F.; Nachega, J.B.; Prozesky, H.W.; Taljaard, J.J.; Zeier, M.D.; Cotton, M.; Simon, G.; Soentjens, P. Evaluation of Paradoxical TB-Associated IRIS with the Use of Standardized Case Definitions for Resource-Limited Settings. J. Int. Assoc. Physicians AIDS Care (Chic) 2010, 9, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Haddow, L.J.; Moosa, M.-Y.S.; Easterbrook, P.J. Validation of a Published Case Definition for Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. AIDS 2010, 24, 103–108. [Google Scholar] [CrossRef]
- Stek, C.; Buyze, J.; Menten, J.; Schutz, C.; Thienemann, F.; Blumenthal, L.; Maartens, G.; Boyles, T.; Wilkinson, R.J.; Meintjes, G.; et al. Diagnostic Accuracy of the INSHI Consensus Case Definition for the Diagnosis of Paradoxical Tuberculosis-IRIS. J. Acquir. Immune. Defic. Syndr. 2021, 86, 587–592. [Google Scholar] [CrossRef]
- Van Rie, A.; Sawry, S.; Link-Gelles, R.; Madhi, S.; Fairlie, L.; Verwey, C.; Mahomed, N.; Murdoch, D.; Moultrie, H. Paradoxical Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome in Children. Pediatr. Pulmonol. 2016, 51, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Luetkemeyer, A.F.; Kendall, M.A.; Nyirenda, M.; Wu, X.; Ive, P.; Benson, C.A.; Andersen, J.W.; Swindells, S.; Sanne, I.M.; Havlir, D.V.; et al. Tuberculosis Immune Reconstitution Inflammatory Syndrome in A5221 STRIDE: Timing, Severity, and Implications for HIV-TB Programs. J. Acquir. Immune. Defic. Syndr. 2014, 65, 423–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amogne, W.; Aderaye, G.; Habtewold, A.; Yimer, G.; Makonnen, E.; Worku, A.; Sonnerborg, A.; Aklillu, E.; Lindquist, L. Efficacy and Safety of Antiretroviral Therapy Initiated One Week after Tuberculosis Therapy in Patients with CD4 Counts < 200 Cells/ΜL: TB-HAART Study, a Randomized Clinical Trial. PLoS ONE 2015, 10, e0122587. [Google Scholar] [CrossRef] [Green Version]
- Haddow, L.J.; Moosa, M.-Y.S.; Mosam, A.; Moodley, P.; Parboosing, R.; Easterbrook, P.J. Incidence, Clinical Spectrum, Risk Factors and Impact of HIV-Associated Immune Reconstitution Inflammatory Syndrome in South Africa. PLoS ONE 2012, 7, e40623. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, C.J.; Harrington, R.D.; Dhanireddy, S.; Crane, H.M.; Casper, C.; Kitahata, M.M. Paradoxical Immune Reconstitution Inflammatory Syndrome in HIV-Infected Patients Treated With Combination Antiretroviral Therapy After AIDS-Defining Opportunistic Infection. Clin. Infect. Dis. 2012, 54, 424–433. [Google Scholar] [CrossRef]
- Limmahakhun, S.; Chaiwarith, R.; Nuntachit, N.; Sirisanthana, T.; Supparatpinyo, K. Treatment Outcomes of Patients Co-Infected with Tuberculosis and HIV at Chiang Mai University Hospital, Thailand. Int. J. STD AIDS 2012, 23, 414–418. [Google Scholar] [CrossRef]
- Worodria, W.; Menten, J.; Massinga-Loembe, M.; Mazakpwe, D.; Bagenda, D.; Koole, O.; Mayanja-Kizza, H.; Kestens, L.; Mugerwa, R.; Reiss, P.; et al. Clinical Spectrum, Risk Factors and Outcome of Immune Reconstitution Inflammatory Syndrome in Patients with Tuberculosis-HIV Coinfection. Antivir. Ther. 2012, 17, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Ali, K.; Klotz, S.A. The Immune Reconstitution Inflammatory Syndrome with Tuberculosis: A Common Problem in Ethiopian HIV-Infected Patients Beginning Antiretroviral Therapy. J. Int. Assoc. Physicians AIDS Care (Chic) 2012, 11, 198–202. [Google Scholar] [CrossRef] [Green Version]
- Narendran, G.; Andrade, B.B.; Porter, B.O.; Chandrasekhar, C.; Venkatesan, P.; Menon, P.A.; Subramanian, S.; Anbalagan, S.; Bhavani, K.P.; Sekar, S.; et al. Paradoxical Tuberculosis Immune Reconstitution Inflammatory Syndrome (TB-IRIS) in HIV Patients with Culture Confirmed Pulmonary Tuberculosis in India and the Potential Role of IL-6 in Prediction. PLoS ONE 2013, 8, e63541. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Xie, R.; Pang, Y.; Yan, S.; Du, Y.; Guan, C.; Chen, B. Prevalence and Risk Factors of Paradoxical Tuberculosis Associated Immune Reconstitution Inflammatory Syndrome among HIV-Infected Patients in Beijing, China. BMC Infect. Dis. 2020, 20, 554. [Google Scholar] [CrossRef]
- Worodria, W.; Massinga-Loembe, M.; Mayanja-Kizza, H.; Namaganda, J.; Kambugu, A.; Manabe, Y.C.; Kestens, L.; Colebunders, R. Antiretroviral Treatment-Associated Tuberculosis in a Prospective Cohort of HIV-Infected Patients Starting ART. Clin. Dev. Immunol. 2011, 2011, 758350. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Samperio, P. Diagnosis of Tuberculosis in HIV Co-Infected Individuals: Current Status, Challenges and Opportunities for the Future. Scand. J. Immunol. 2017, 86, 76–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathmanathan, I.; Dokubo, E.K.; Shiraishi, R.W.; Agolory, S.G.; Auld, A.F.; Onotu, D.; Odafe, S.; Dalhatu, I.; Abiri, O.; Debem, H.C.; et al. Incidence and Predictors of Tuberculosis among HIV-Infected Adults after Initiation of Antiretroviral Therapy in Nigeria, 2004–2012. PLoS ONE 2017, 12, e0173309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulterys, M.A.; Wagner, B.; Redard-Jacot, M.; Suresh, A.; Pollock, N.R.; Moreau, E.; Denkinger, C.M.; Drain, P.K.; Broger, T. Point-Of-Care Urine LAM Tests for Tuberculosis Diagnosis: A Status Update. J. Clin. Med. 2019, 9, E111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, N.F.; Wilkinson, K.A.; Meintjes, G.; Tezera, L.B.; Goliath, R.; Peyper, J.M.; Tadokera, R.; Opondo, C.; Coussens, A.K.; Wilkinson, R.J.; et al. Matrix Degradation in Human Immunodeficiency Virus Type 1–Associated Tuberculosis and Tuberculosis Immune Reconstitution Inflammatory Syndrome: A Prospective Observational Study. Clin. Infect. Dis. 2017, 65, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.Y.; Yong, Y.K.; Andrade, B.B.; Shankar, E.M.; Ponnampalavanar, S.; Omar, S.F.S.; Narendran, G.; Kamarulzaman, A.; Swaminathan, S.; Sereti, I.; et al. Plasma Interleukin-18 Levels Are a Biomarker of Innate Immune Responses That Predict and Characterize Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. AIDS 2015, 29, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Musselwhite, L.W.; Andrade, B.B.; Ellenberg, S.S.; Tierney, A.; Belaunzaran-Zamudio, P.F.; Rupert, A.; Lederman, M.M.; Sanne, I.; Sierra Madero, J.G.; Sereti, I. Vitamin D, D-Dimer, Interferon γ, and SCD14 Levels Are Independently Associated with Immune Reconstitution Inflammatory Syndrome: A Prospective, International Study. EBioMedicine 2016, 4, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, B.G.; Elliott, J.H.; Price, P.; Phillips, M.; Saphonn, V.; Vun, M.C.; Kaldor, J.M.; Cooper, D.A.; French, M.A. Mediators of Innate and Adaptive Immune Responses Differentially Affect Immune Restoration Disease Associated with Mycobacterium Tuberculosis in HIV Patients Beginning Antiretroviral Therapy. J. Infect. Dis. 2010, 202, 1728–1737. [Google Scholar] [CrossRef] [Green Version]
- de Sá, N.B.R.; de Souza, N.C.S.; Neira-Goulart, M.; Ribeiro-Alves, M.; Da Silva, T.P.; Pilotto, J.H.; Rolla, V.C.; Giacoia-Gripp, C.B.W.; de Oliveira Pinto, L.M.; Scott-Algara, D.; et al. Inflammasome Genetic Variants Are Associated with Tuberculosis, HIV-1 Infection, and TB/HIV-Immune Reconstitution Inflammatory Syndrome Outcomes. Front. Cell Infect. Microbiol. 2022, 12, 962059. [Google Scholar] [CrossRef]
- Dirix, V.; Schepers, K.; Massinga-Loembe, M.; Worodria, W.; Colebunders, R.; Singh, M.; Locht, C.; Kestens, L.; Mascart, F. TB-IRIS study group Added Value of Long-Term Cytokine Release Assays to Detect Mycobacterium Tuberculosis Infection in HIV-Infected Subjects in Uganda. J. Acquir. Immune. Defic. Syndr. 2016, 72, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Tibúrcio, R.; Barreto-Duarte, B.; Naredren, G.; Queiroz, A.T.L.; Anbalagan, S.; Nayak, K.; Ravichandran, N.; Subramani, R.; Antonelli, L.R.V.; Satagopan, K.; et al. Dynamics of T-Lymphocyte Activation Related to Paradoxical Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome in Persons With Advanced HIV. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Ellis, J.; Cresswell, F.V.; Rhein, J.; Ssebambulidde, K.; Boulware, D.R. Cryptococcal Meningitis and Tuberculous Meningitis Co-Infection in HIV-Infected Ugandan Adults. Open Forum. Infect. Dis. 2018, 5, ofy193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conradie, F.; Foulkes, A.S.; Ive, P.; Yin, X.; Roussos, K.; Glencross, D.K.; Lawrie, D.; Stevens, W.; Montaner, L.J.; Sanne, I.; et al. Natural Killer Cell Activation Distinguishes M. Tuberculosis-Mediated Immune Reconstitution Syndrome (IRIS) from Chronic HIV and HIV-MTB Co-Infection. J. Acquir. Immune. Defic. Syndr. 2011, 58, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Affandi, J.S.; Kumar, M.; Agarwal, U.; Singh, S.; Price, P. The Search for a Genetic Factor Associating with Immune Restoration Disease in HIV Patients Co-Infected with Mycobacterium Tuberculosis. Dis. Markers 2013, 34, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Narendran, G.; Kavitha, D.; Karunaianantham, R.; Gil-Santana, L.; Almeida-Junior, J.L.; Reddy, S.D.; Kumar, M.M.; Hemalatha, H.; Jayanthi, N.N.; Ravichandran, N.; et al. Role of LTA4H Polymorphism in Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome Occurrence and Clinical Severity in Patients Infected with HIV. PLoS ONE 2016, 11, e0163298. [Google Scholar] [CrossRef] [Green Version]
- de Sá, N.B.R.; Ribeiro-Alves, M.; da Silva, T.P.; Pilotto, J.H.; Rolla, V.C.; Giacoia-Gripp, C.B.W.; Scott-Algara, D.; Morgado, M.G.; Teixeira, S.L.M. Clinical and Genetic Markers Associated with Tuberculosis, HIV-1 Infection, and TB/HIV-Immune Reconstitution Inflammatory Syndrome Outcomes. BMC Infect. Dis. 2020, 20, 59. [Google Scholar] [CrossRef] [Green Version]
- Mbandi, S.K.; Painter, H.; Penn-Nicholson, A.; Toefy, A.; Erasmus, M.; Hanekom, W.A.; Scriba, T.J.; Lai, R.P.J.; Marais, S.; Fletcher, H.A.; et al. Host Transcriptomic Signatures of Tuberculosis Can Predict Immune Reconstitution Inflammatory Syndrome in HIV Patients. Eur. J. Immunol. 2022, 52, 1112–1119. [Google Scholar] [CrossRef]
- Silva, C.A.M.; Graham, B.; Webb, K.; Ashton, L.V.; Harton, M.; Luetkemeyer, A.F.; Bokatzian, S.; Almubarak, R.; Mahapatra, S.; Hovind, L.; et al. A Pilot Metabolomics Study of Tuberculosis Immune Reconstitution Inflammatory Syndrome. Int. J. Infect. Dis. 2019, 84, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ANRS. Emerging Infectious Diseases. Micro RNA as Prediction and/or Prognostic Markers of IRIS in TB-HIV Co-Infected Patients; NIH US National Library of Medicine: Bethesda, MD, USA, 2020. Available online: clinicaltrials.gov (accessed on 21 January 2023).
- Sabir, N.; Hussain, T.; Shah, S.Z.A.; Peramo, A.; Zhao, D.; Zhou, X. MiRNAs in Tuberculosis: New Avenues for Diagnosis and Host-Directed Therapy. Front. Microbiol. 2018, 9, 602. [Google Scholar] [CrossRef] [Green Version]
- Vinhaes, C.L.; Sheikh, V.; Oliveira-de-Souza, D.; Wang, J.; Rupert, A.; Roby, G.; Arriaga, M.B.; Fukutani, K.F.; Sawe, F.; Shaffer, D.; et al. An Inflammatory Composite Score Predicts Mycobacterial Immune Reconstitution Inflammatory Syndrome in People with Advanced HIV: A Prospective International Cohort Study. J. Infect. Dis. 2021, 223, 1275–1283. [Google Scholar] [CrossRef]
- Antonelli, L.R.V.; Mahnke, Y.; Hodge, J.N.; Porter, B.O.; Barber, D.L.; DerSimonian, R.; Greenwald, J.H.; Roby, G.; Mican, J.; Sher, A.; et al. Elevated Frequencies of Highly Activated CD4+ T Cells in HIV+ Patients Developing Immune Reconstitution Inflammatory Syndrome. Blood 2010, 116, 3818–3827. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, E.; Romero-Rodríguez, D.P.; Cantoral-Díaz, M.-T.; Reyes-Terán, G. Transient Expansion of Activated CD8(+) T Cells Characterizes Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome in Patients with HIV: A Case Control Study. J. Inflamm. (Lond) 2013, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Haridas, V.; Pean, P.; Jasenosky, L.D.; Madec, Y.; Laureillard, D.; Sok, T.; Sath, S.; Borand, L.; Marcy, O.; Chan, S.; et al. TB-IRIS, T-Cell Activation, and Remodeling of the T-Cell Compartment in Highly Immunosuppressed HIV-Infected Patients with TB. AIDS 2015, 29, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Walker, N.F.; Meintjes, G.; Wilkinson, R.J. HIV-1 and the Immune Response to TB. Future Virol. 2013, 8, 57–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbins, G.K.; Spritzler, J.G.; Chan, E.S.; Asmuth, D.M.; Gandhi, R.T.; Rodriguez, B.; Skowron, G.; Skolnik, P.R.; Shafer, R.W.; Pollard, R.B.; et al. Incomplete Reconstitution of T Cell Subsets on Combination Antiretroviral Therapy in the AIDS Clinical Trials Group Protocol 384. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 48, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Ravimohan, S.; Tamuhla, N.; Nfanyana, K.; Steenhoff, A.P.; Letlhogile, R.; Frank, I.; MacGregor, R.R.; Gross, R.; Weissman, D.; Bisson, G.P. Robust Reconstitution of Tuberculosis-Specific Polyfunctional CD4+ T-Cell Responses and Rising Systemic Interleukin 6 in Paradoxical Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. Clin. Infect. Dis. 2016, 62, 795–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravimohan, S.; Tamuhla, N.; Steenhoff, A.P.; Letlhogile, R.; Makutu, D.K.; Nfanyana, K.; Rantleru, T.; Tierney, A.; Nkakana, K.; Schwartz, A.B.; et al. Early Immunologic Failure Is Associated with Early Mortality among Advanced HIV-Infected Adults Initiating Antiretroviral Therapy with Active Tuberculosis. J. Infect. Dis. 2013, 208, 1784–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, H.T.T.; Van den Bergh, R.; Vu, T.N.; Laukens, K.; Worodria, W.; Loembé, M.M.; Colebunders, R.; Kestens, L.; De Baetselier, P.; Raes, G.; et al. The Role of Monocytes in the Development of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. Immunobiology 2014, 219, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.T.; Van den Bergh, R.; Loembé, M.M.; Worodria, W.; Mayanja-Kizza, H.; Colebunders, R.; Mascart, F.; Stordeur, P.; Kestens, L.; De Baetselier, P.; et al. Modulation of the Complement System in Monocytes Contributes to Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. AIDS 2013, 27, 1725–1734. [Google Scholar] [CrossRef]
- Andrade, B.B.; Singh, A.; Narendran, G.; Schechter, M.E.; Nayak, K.; Subramanian, S.; Anbalagan, S.; Jensen, S.M.R.; Porter, B.O.; Antonelli, L.R.; et al. Mycobacterial Antigen Driven Activation of CD14++CD16- Monocytes Is a Predictor of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. PLoS Pathog. 2014, 10, e1004433. [Google Scholar] [CrossRef] [Green Version]
- Pean, P.; Nerrienet, E.; Madec, Y.; Borand, L.; Laureillard, D.; Fernandez, M.; Marcy, O.; Sarin, C.; Phon, K.; Taylor, S.; et al. Natural Killer Cell Degranulation Capacity Predicts Early Onset of the Immune Reconstitution Inflammatory Syndrome (IRIS) in HIV-Infected Patients with Tuberculosis. Blood 2012, 119, 3315–3320. [Google Scholar] [CrossRef]
- Wilkinson, K.A.; Walker, N.F.; Meintjes, G.; Deffur, A.; Nicol, M.P.; Skolimowska, K.H.; Matthews, K.; Tadokera, R.; Seldon, R.; Maartens, G.; et al. Cytotoxic Mediators in Paradoxical HIV-Tuberculosis Immune Reconstitution Inflammatory Syndrome. J. Immunol. 2015, 194, 1748–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, N.F.; Opondo, C.; Meintjes, G.; Jhilmeet, N.; Friedland, J.S.; Elkington, P.T.; Wilkinson, R.J.; Wilkinson, K.A. Invariant Natural Killer T-Cell Dynamics in Human Immunodeficiency Virus-Associated Tuberculosis. Clin. Infect. Dis. 2020, 70, 1865–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, R.P.J.; Meintjes, G.; Wilkinson, K.A.; Graham, C.M.; Marais, S.; Van der Plas, H.; Deffur, A.; Schutz, C.; Bloom, C.; Munagala, I.; et al. HIV-Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome Is Characterized by Toll-like Receptor and Inflammasome Signalling. Nat. Commun. 2015, 6, 8451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.Y.; Yong, Y.K.; Shankar, E.M.; Paukovics, G.; Ellegård, R.; Larsson, M.; Kamarulzaman, A.; French, M.A.; Crowe, S.M. Aberrant Inflammasome Activation Characterizes Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. J. Immunol. 2016, 196, 4052–4063. [Google Scholar] [CrossRef] [Green Version]
- Marais, S.; Lai, R.P.J.; Wilkinson, K.A.; Meintjes, G.; O’Garra, A.; Wilkinson, R.J. Inflammasome Activation Underlying Central Nervous System Deterioration in HIV-Associated Tuberculosis. J. Infect. Dis. 2017, 215, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Herbst, S.; Schaible, U.E.; Schneider, B.E. Interferon Gamma Activated Macrophages Kill Mycobacteria by Nitric Oxide Induced Apoptosis. PLoS ONE 2011, 6, e19105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Chastellier, C. The Many Niches and Strategies Used by Pathogenic Mycobacteria for Survival within Host Macrophages. Immunobiology 2009, 214, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Midha, M.K.; Verma, H.N.; Basu, A.; Rao, K.V.S.; Manivel, V. Characterizing Virulence-Specific Perturbations in the Mitochondrial Function of Macrophages Infected with Mycobacterium Tuberculosis. Sci. Rep. 2013, 3, 1328. [Google Scholar] [CrossRef] [Green Version]
- Marais, S.; Meintjes, G.; Pepper, D.J.; Dodd, L.E.; Schutz, C.; Ismail, Z.; Wilkinson, K.A.; Wilkinson, R.J. Frequency, Severity, and Prediction of Tuberculous Meningitis Immune Reconstitution Inflammatory Syndrome. Clin. Infect. Dis. 2013, 56, 450–460. [Google Scholar] [CrossRef] [Green Version]
- Marais, S.; Wilkinson, K.A.; Lesosky, M.; Coussens, A.K.; Deffur, A.; Pepper, D.J.; Schutz, C.; Ismail, Z.; Meintjes, G.; Wilkinson, R.J. Neutrophil-Associated Central Nervous System Inflammation in Tuberculous Meningitis Immune Reconstitution Inflammatory Syndrome. Clin. Infect. Dis. 2014, 59, 1638–1647. [Google Scholar] [CrossRef] [Green Version]
- Nakiwala, J.K.; Walker, N.F.; Diedrich, C.R.; Worodria, W.; Meintjes, G.; Wilkinson, R.J.; Mayanja-Kizza, H.; Colebunders, R.; Kestens, L.; Wilkinson, K.A.; et al. Neutrophil Activation and Enhanced Release of Granule Products in HIV-TB Immune Reconstitution Inflammatory Syndrome. J. Acquir. Immune. Defic. Syndr. 2018, 77, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, F.; Su, W.; Li, Q.; Li, J.; Ji, J.; Deng, Y.; Zhou, Y.; Wang, X.; Yang, H.; et al. Zinc Finger and Interferon-Stimulated Genes Play a Vital Role in TB-IRIS Following HAART in AIDS. Per. Med. 2018, 15, 251–269. [Google Scholar] [CrossRef] [Green Version]
- Tadokera, R.; Meintjes, G.A.; Wilkinson, K.A.; Skolimowska, K.H.; Walker, N.; Friedland, J.S.; Maartens, G.; Elkington, P.T.G.; Wilkinson, R.J. Matrix Metalloproteinases and Tissue Damage in HIV-Tuberculosis Immune Reconstitution Inflammatory Syndrome. Eur. J. Immunol. 2014, 44, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, E.M.; Vignesh, R.; Velu, V.; Murugavel, K.G.; Sekar, R.; Balakrishnan, P.; Lloyd, C.A.; Saravanan, S.; Solomon, S.; Kumarasamy, N. Does CD4+ CD25+ Foxp3+ Cell (Treg) and IL-10 Profile Determine Susceptibility to Immune Reconstitution Inflammatory Syndrome (IRIS) in HIV Disease? J. Inflamm. 2008, 5, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalan, N.; Andrade, B.B.; Swaminathan, S. Tuberculosis-Immune Reconstitution Inflammatory Syndrome in HIV: From Pathogenesis to Prediction. Expert. Rev. Clin. Immunol. 2014, 10, 631–645. [Google Scholar] [CrossRef]
- Lai, R.P.J.; Meintjes, G.; Wilkinson, R.J. HIV-1 Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. Semin. Immunopathol. 2016, 38, 185–198. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, I.; Peterson, K.; Jeffries, D.; Whittle, H.; de Silva, T.; Rowland-Jones, S.; Jaye, A.; de Jong, B.C. Immune Reconstitution Inflammatory Syndrome and the Influence of T Regulatory Cells: A Cohort Study in The Gambia. PLoS ONE 2012, 7, e39213. [Google Scholar] [CrossRef] [Green Version]
- Martin-Blondel, G.; Bauer, J.; Uro-Coste, E.; Biotti, D.; Averseng-Peaureaux, D.; Fabre, N.; Dumas, H.; Bonneville, F.; Lassmann, H.; Marchou, B.; et al. Therapeutic Use of CCR5 Antagonists Is Supported by Strong Expression of CCR5 on CD8(+) T Cells in Progressive Multifocal Leukoencephalopathy-Associated Immune Reconstitution Inflammatory Syndrome. Acta Neuropathol. 2015, 129, 463–465. [Google Scholar] [CrossRef]
- Silveira-Mattos, P.S.; Narendran, G.; Akrami, K.; Fukutani, K.F.; Anbalagan, S.; Nayak, K.; Subramanyam, S.; Subramani, R.; Vinhaes, C.L.; Souza, D.O.; et al. Differential Expression of CXCR3 and CCR6 on CD4+ T-Lymphocytes with Distinct Memory Phenotypes Characterizes Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. Sci. Rep. 2019, 9, 1502. [Google Scholar] [CrossRef] [Green Version]
- Tibúrcio, R.; Narendran, G.; Barreto-Duarte, B.; Queiroz, A.T.L.; Araújo-Pereira, M.; Anbalagan, S.; Nayak, K.; Ravichandran, N.; Subramani, R.; Antonelli, L.R.V.; et al. Frequency of CXCR3+ CD8+ T-Lymphocyte Subsets in Peripheral Blood Is Associated With the Risk of Paradoxical Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome Development in Advanced HIV Disease. Front. Immunol. 2022, 13, 873985. [Google Scholar] [CrossRef]
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and Mycobacterium Tuberculosis Co-Infection. Nat. Rev. Microbiol. 2018, 16, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.A.; Meintjes, G.; Seldon, R.; Goliath, R.; Wilkinson, R.J. Immunological Characterisation of an Unmasking TB-IRIS Case. S. Afr. Med. J. 2012, 102, 512–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawn, S.D.; Wainwright, H.; Orrell, C. Fatal Unmasking Tuberculosis Immune Reconstitution Disease with Bronchiolitis Obliterans Organizing Pneumonia: The Role of Macrophages. AIDS 2009, 23, 143–145. [Google Scholar] [CrossRef]
- Bell, L.C.K.; Pollara, G.; Pascoe, M.; Tomlinson, G.S.; Lehloenya, R.J.; Roe, J.; Meldau, R.; Miller, R.F.; Ramsay, A.; Chain, B.M.; et al. In Vivo Molecular Dissection of the Effects of HIV-1 in Active Tuberculosis. PLoS Pathog. 2016, 12, e1005469. [Google Scholar] [CrossRef] [Green Version]
- Meintjes, G.; Stek, C.; Blumenthal, L.; Thienemann, F.; Schutz, C.; Buyze, J.; Ravinetto, R.; van Loen, H.; Nair, A.; Jackson, A.; et al. Prednisone for the Prevention of Paradoxical Tuberculosis-Associated IRIS. N. Engl. J. Med. 2018, 379, 1915–1925. [Google Scholar] [CrossRef]
- Drechsler, H.; Ayers, C.; Cutrell, J.; Maalouf, N.; Tebas, P.; Bedimo, R. Current Use of Statins Reduces Risk of HIV Rebound on Suppressive HAART. PLoS ONE 2017, 12, e0172175. [Google Scholar] [CrossRef] [Green Version]
- Eckard, A.R.; McComsey, G.A. The Role of Statins in the Setting of HIV Infection. Curr. HIV/AIDS Rep. 2015, 12, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uthman, O.A.; Okwundu, C.; Gbenga, K.; Volmink, J.; Dowdy, D.; Zumla, A.; Nachega, J.B. Optimal Timing of Antiretroviral Therapy Initiation for HIV-Infected Adults With Newly Diagnosed Pulmonary Tuberculosis: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2015, 163, 32–39. [Google Scholar] [CrossRef]
- Shahani, L.; Hamill, R.J. Therapeutics Targeting Inflammation in the Immune Reconstitution Inflammatory Syndrome. Transl. Res. 2016, 167, 88–103. [Google Scholar] [CrossRef]
- Abay, S.M.; Deribe, K.; Reda, A.A.; Biadgilign, S.; Datiko, D.; Assefa, T.; Todd, M.; Deribew, A. The Effect of Early Initiation of Antiretroviral Therapy in TB/HIV-Coinfected Patients: A Systematic Review and Meta-Analysis. J. Int. Assoc. Provid. AIDS Care 2015, 14, 560–570. [Google Scholar] [CrossRef] [Green Version]
- Meintjes, G.; Wilkinson, R.J.; Morroni, C.; Pepper, D.J.; Rebe, K.; Rangaka, M.X.; Oni, T.; Maartens, G. Randomized Placebo-Controlled Trial of Prednisone for Paradoxical Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. AIDS 2010, 24, 2381–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy. Available online: https://www.who.int/publications-detail-redirect/9789241550062 (accessed on 25 November 2022).
- Durovni, B.; Cavalcante, S. Preventive Therapy for HIV-Associated Tuberculosis. Curr. Opin. HIV AIDS 2018, 13, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Hakim, J.; Musiime, V.; Szubert, A.J.; Mallewa, J.; Siika, A.; Agutu, C.; Walker, S.; Pett, S.L.; Bwakura-Dangarembizi, M.; Lugemwa, A.; et al. Enhanced Prophylaxis plus Antiretroviral Therapy for Advanced HIV Infection in Africa. N. Engl. J. Med. 2017, 377, 233–245. [Google Scholar] [CrossRef]
- Valin, N.; Pacanowski, J.; Denoeud, L.; Lacombe, K.; Lalande, V.; Fonquernie, L.; Girard, P.-M.; Meynard, J.-L. Risk Factors for “unmasking Immune Reconstitution Inflammatory Syndrome” Presentation of Tuberculosis Following Combination Antiretroviral Therapy Initiation in HIV-Infected Patients. AIDS 2010, 24, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, P.C.; Reichman, L.B.; Castro, K.G. Parallels and Mutual Lessons in Tuberculosis and COVID-19 Transmission, Prevention, and Control. Emerg. Infect. Dis. 2021, 27, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Kasaeva, T.; Swaminathan, S. COVID-19’s Devastating Effect on Tuberculosis Care—A Path to Recovery. N. Engl. J. Med. 2022, 386, 1490–1493. [Google Scholar] [CrossRef]
- Song, W.-M.; Zhao, J.-Y.; Zhang, Q.-Y.; Liu, S.-Q.; Zhu, X.-H.; An, Q.-Q.; Xu, T.-T.; Li, S.-J.; Liu, J.-Y.; Tao, N.-N.; et al. COVID-19 and Tuberculosis Coinfection: An Overview of Case Reports/Case Series and Meta-Analysis. Front. Med. (Lausanne) 2021, 8, 657006. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Chi, Y.; Ge, Y.; Wu, B.; Zhang, W.; Wu, T.; Wen, T.; Liu, J.; Guo, X.; Huang, C.; Jiao, Y.; et al. Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. J. Infect. Dis. 2020, 222, 746–754. [Google Scholar] [CrossRef]
- Seddiki, N.; French, M. COVID-19 and HIV-Associated Immune Reconstitution Inflammatory Syndrome: Emergence of Pathogen-Specific Immune Responses Adding Fuel to the Fire. Front. Immunol. 2021, 12, 649567. [Google Scholar] [CrossRef] [PubMed]
- Cancio, M.; Ciccocioppo, R.; Rocco, P.R.M.; Levine, B.L.; Bronte, V.; Bollard, C.M.; Weiss, D.; Boelens, J.J.; Hanley, P.J. Emerging Trends in COVID-19 Treatment: Learning from Inflammatory Conditions Associated with Cellular Therapies. Cytotherapy 2020, 22, 474–481. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with COVID-19 Pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, T.; Tieng, A.; Chilimuri, S.; Franchin, G. A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 8865954. [Google Scholar] [CrossRef]
- Merchant, E.A.; Flint, K.; Barouch, D.H.; Blair, B.M. Co-Infection with Coronavirus Disease 2019, Previously Undiagnosed Human Immunodeficiency Virus, Pneumocystis Jirovecii Pneumonia and Cytomegalovirus Pneumonitis, with Possible Immune Reconstitution Inflammatory Syndrome. IDCases 2021, 24, e01153. [Google Scholar] [CrossRef]
- Mertens, J.; Laghrib, Y.; Kenyon, C. A Case of Steroid-Responsive, COVID-19 Immune Reconstitution Inflammatory Syndrome Following the Use of Granulocyte Colony-Stimulating Factor. Open. Forum. Infect. Dis. 2020, 7, ofaa326. [Google Scholar] [CrossRef]
- Garcia-Carretero, R.; Vazquez-Gomez, O.; Ordoñez-Garcia, M. Delayed Immune Reconstitution Inflammatory Syndrome in an Immunosuppressed Patient With SARS-CoV-2. Cureus 2021, 13, E19481. [Google Scholar] [CrossRef]
- Lalonde, C.S.; Zhuang, T.Z.; Aldredge, A.A.; Adelman, M.W.; AbouYabis, A.N.; McLemore, M.L.; Auld, S.C. Abstract 444: COVID-IRIS: Immune Reconstitution after G-CSF Administration for Neutropenia during Acute COVID-19 Infection. Cancer Res. 2022, 82, 444. [Google Scholar] [CrossRef]

| Author and Year of Publication | Country | Frequency (%) |
|---|---|---|
| Haddow, L.J., 2012 [45] | South Africa | 13.7 |
| Achenbach. C.J., 2012 [46] | USA | 16 |
| Limmahakhun, S., 2012 [47] | Thailand | 6.1 |
| Worodria, W., 2012 [48] | Uganda | 20.9 |
| Ali, K., 2012 [49] | Ethiopia | 22.4 |
| Narendran, G., 2013 [50] | India | 54.2 |
| Tan, H.Y., 2014 [20] | Malaysia | 9.4 |
| Xue, M., 2020 [51] | China | 22.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vignesh, R.; Balakrishnan, P.; Tan, H.Y.; Yong, Y.K.; Velu, V.; Larsson, M.; Shankar, E.M. Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome—An Extempore Game of Misfiring with Defense Arsenals. Pathogens 2023, 12, 210. https://doi.org/10.3390/pathogens12020210
Vignesh R, Balakrishnan P, Tan HY, Yong YK, Velu V, Larsson M, Shankar EM. Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome—An Extempore Game of Misfiring with Defense Arsenals. Pathogens. 2023; 12(2):210. https://doi.org/10.3390/pathogens12020210
Chicago/Turabian StyleVignesh, Ramachandran, Pachamuthu Balakrishnan, Hong Yien Tan, Yean Kong Yong, Vijayakumar Velu, Marie Larsson, and Esaki M. Shankar. 2023. "Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome—An Extempore Game of Misfiring with Defense Arsenals" Pathogens 12, no. 2: 210. https://doi.org/10.3390/pathogens12020210
APA StyleVignesh, R., Balakrishnan, P., Tan, H. Y., Yong, Y. K., Velu, V., Larsson, M., & Shankar, E. M. (2023). Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome—An Extempore Game of Misfiring with Defense Arsenals. Pathogens, 12(2), 210. https://doi.org/10.3390/pathogens12020210








