Immune Responses in Lung Granulomas during Mtb/HIV Co-Infection: Implications for Pathogenesis and Therapy
Abstract
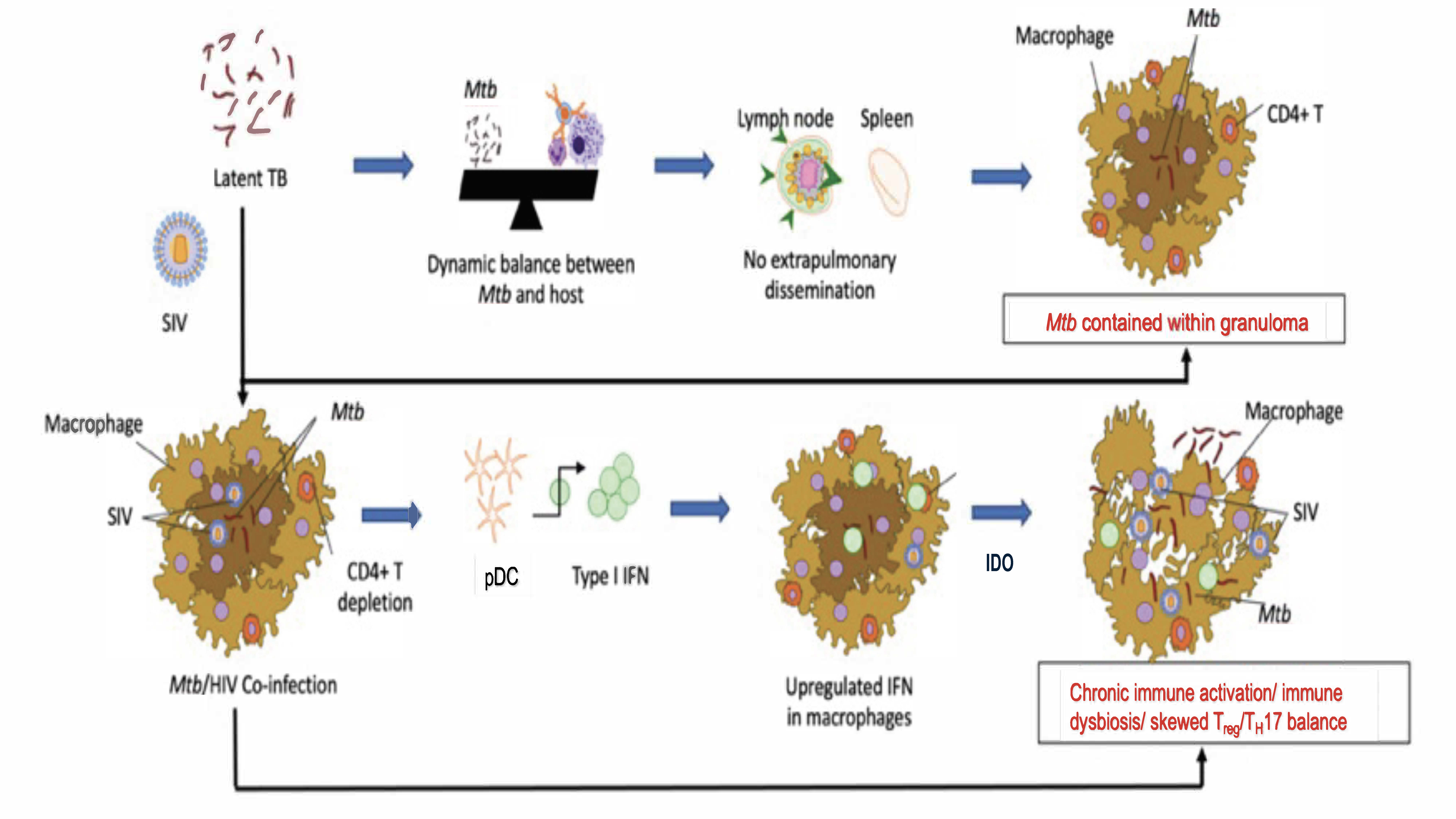
:1. Introduction
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020.
- Wong, N.S.; Leung, C.C.; Chan, K.C.W.; Chan, W.K.; Lin, A.W.C.; Lee, S.S. A longitudinal study on latent TB infection screening and its association with TB incidence in HIV patients. Sci. Rep. 2019, 9, 10093. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.M.; Kammerer, J.S.; Winston, C.A.; Navin, T.R.; Horsburgh, C.R., Jr. Estimated rate of reactivation of latent tuberculosis infection in the United States, overall and by population subgroup. Am. J. Epidemiol. 2014, 179, 216–225. [Google Scholar] [CrossRef]
- Salazar-Gonzalez, J.F.; Salazar, M.G.; Keele, B.F.; Learn, G.H.; Giorgi, E.E.; Li, H.; Decker, J.M.; Wang, S.; Baalwa, J.; Kraus, M.H.; et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 2009, 206, 1273–1289. [Google Scholar] [CrossRef]
- Veazey, R.S.; DeMaria, M.; Chalifoux, L.V.; Shvetz, D.E.; Pauley, D.R.; Knight, H.L.; Rosenzweig, M.; Johnson, R.P.; Desrosiers, R.C.; Lackner, A.A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998, 280, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Adema, G.J.; van Kooyk, Y.; Figdor, C.G. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 2000, 100, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Malaspina, A.; Li, Y.; Chun, T.W.; Lowe, T.; Adelsberger, J.; Baseler, M.; Ehler, L.A.; Liu, S.; Davey, R.T., Jr.; et al. B cells of HIV-1-infected patients bind virions through CD21-complement interactions and transmit infectious virus to activated T cells. J. Exp. Med. 2000, 192, 637–646. [Google Scholar] [CrossRef]
- Gasper-Smith, N.; Crossman, D.M.; Whitesides, J.F.; Mensali, N.; Ottinger, J.S.; Plonk, S.G.; Moody, M.A.; Ferrari, G.; Weinhold, K.J.; Miller, S.E.; et al. Induction of plasma (TRAIL), TNFR-2, Fas ligand, and plasma microparticles after human immunodeficiency virus type 1 (HIV-1) transmission: Implications for HIV-1 vaccine design. J. Virol. 2008, 82, 7700–7710. [Google Scholar] [CrossRef]
- Foreman, T.W.; Mehra, S.; LoBato, D.N.; Malek, A.; Alvarez, X.; Golden, N.A.; Bucsan, A.N.; Didier, P.J.; Doyle-Meyers, L.A.; Russell-Lodrigue, K.E.; et al. CD4+ T-cell-independent mechanisms suppress reactivation of latent tuberculosis in a macaque model of HIV coinfection. Proc. Natl. Acad. Sci. USA 2016, 113, E5636–E5644. [Google Scholar] [CrossRef]
- Bucsan, A.N.; Chatterjee, A.; Singh, D.K.; Foreman, T.W.; Lee, T.H.; Threeton, B.; Kirkpatrick, M.G.; Ahmed, M.; Golden, N.; Alvarez, X.; et al. Mechanisms of reactivation of latent tuberculosis infection due to SIV co-infection. J. Clin. Investig. 2019, 129, 5254–5260. [Google Scholar] [CrossRef]
- McMichael, A.J.; Borrow, P.; Tomaras, G.D.; Goonetilleke, N.; Haynes, B.F. The immune response during acute HIV-1 infection: Clues for vaccine development. Nat. Rev. Immunol. 2010, 10, 11–23. [Google Scholar] [CrossRef]
- Mattapallil, J.J.; Douek, D.C.; Hill, B.; Nishimura, Y.; Martin, M.; Roederer, M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005, 434, 1093–1097. [Google Scholar] [CrossRef]
- Ganatra, S.R.; Bucsan, A.N.; Alvarez, X.; Kumar, S.; Chatterjee, A.; Quezada, M.; Fish, A.I.; Singh, D.K.; Singh, B.; Sharan, R.; et al. Anti-retroviral therapy does not reduce tuberculosis reactivation in a tuberculosis-HIV co-infection model. J. Clin. Investig. 2020, 130, 5171–5179. [Google Scholar] [CrossRef]
- Sharan, R.; Ganatra, S.R.; Bucsan, A.N.; Cole, J.; Singh, D.K.; Alvarez, X.; Gough, M.; Alvarez, C.; Blakley, A.; Ferdin, J.; et al. Antiretroviral therapy timing impacts latent tuberculosis infection reactivation in a tuberculosis/simian immunodeficiency virus coinfection model. J. Clin. Investig. 2021, 132, e153090. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.K.; Lee, E.J.; Manuzak, J.A.; Dillon, S.M.; Beckham, J.D.; McCarter, M.D.; Santiago, M.L.; Wilson, C.C. Microbial exposure alters HIV-1-induced mucosal CD4+ T cell death pathways ex vivo. Retrovirology 2014, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, R.V.; Rodgers, M.A.; Ellis, A.L.; Balgeman, A.J.; Larson, E.C.; Hopkins, F.; Chase, M.R.; Maiello, P.; Fortune, S.M.; Scanga, C.A.; et al. Spontaneous Control of SIV Replication Does Not Prevent T Cell Dysregulation and Bacterial Dissemination in Animals Co-Infected with M. tuberculosis. Microbiol. Spectr. 2022, 10, e0172421. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.C.; Ellis, A.L.; Rodgers, M.A.; Gubernat, A.K.; Gleim, J.L.; Moriarty, R.V.; Balgeman, A.J.; Menezes, Y.K.; Ameel, C.L.; Fillmore, D.J.; et al. Host Immunity to Mycobacterium tuberculosis Infection Is Similar in Simian Immunodeficiency Virus (SIV)-Infected, Antiretroviral Therapy-Treated and SIV-Naive Juvenile Macaques. Infect. Immun. 2023, 91, e0055822. [Google Scholar] [CrossRef]
- Legchenko, A.; Baltassat, J.M.; Bobachev, A.; Martin, C.; Robain, H.; Vouillamoz, J.M. Magnetic resonance sounding applied to aquifer characterization. Ground Water 2004, 42, 363–373. [Google Scholar] [CrossRef]
- Swanson, R.V.; Gupta, A.; Foreman, T.W.; Lu, L.; Choreno-Parra, J.A.; Mbandi, S.K.; Rosa, B.A.; Akter, S.; Das, S.; Ahmed, M.; et al. Antigen-specific B cells direct T follicular-like helper cells into lymphoid follicles to mediate Mycobacterium tuberculosis control. Nat. Immunol. 2023, 24, 855–868. [Google Scholar] [CrossRef]
- Matsumoto, R.; Gray, J.; Rybkina, K.; Oppenheimer, H.; Levy, L.; Friedman, L.M.; Khamaisi, M.; Meng, W.; Rosenfeld, A.M.; Guyer, R.S.; et al. Induction of bronchus-associated lymphoid tissue is an early life adaptation for promoting human B cell immunity. Nat. Immunol. 2023, 24, 1370–1381. [Google Scholar] [CrossRef]
- Diedrich, C.R.; Rutledge, T.; Maiello, P.; Baranowski, T.M.; White, A.G.; Borish, H.J.; Karell, P.; Hopkins, F.; Brown, J.; Fortune, S.M.; et al. SIV and Mycobacterium tuberculosis synergy within the granuloma accelerates the reactivation pattern of latent tuberculosis. PLoS Pathog. 2020, 16, e1008413. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.D.; Harris, L.D.; Klatt, N.R.; Tabb, B.; Pittaluga, S.; Paiardini, M.; Barclay, G.R.; Smedley, J.; Pung, R.; Oliveira, K.M.; et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010, 6, e1001052. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.J.; Sugimoto, C.; Cai, Y.; Merino, K.M.; Mehra, S.; Arainga, M.; Roy, C.J.; Midkiff, C.C.; Alvarez, X.; Didier, E.S.; et al. High Turnover of Tissue Macrophages Contributes to Tuberculosis Reactivation in Simian Immunodeficiency Virus-Infected Rhesus Macaques. J. Infect. Dis. 2018, 217, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Mehra, S.; Alvarez, X.; Didier, P.J.; Doyle, L.A.; Blanchard, J.L.; Lackner, A.A.; Kaushal, D. Granuloma correlates of protection against tuberculosis and mechanisms of immune modulation by Mycobacterium tuberculosis. J. Infect. Dis. 2013, 207, 1115–1127. [Google Scholar] [CrossRef]
- Gautam, U.S.; Foreman, T.W.; Bucsan, A.N.; Veatch, A.V.; Alvarez, X.; Adekambi, T.; Golden, N.A.; Gentry, K.M.; Doyle-Meyers, L.A.; Russell-Lodrigue, K.E.; et al. In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2018, 115, E62–E71. [Google Scholar] [CrossRef]
- McCaffrey, E.F.; Donato, M.; Keren, L.; Chen, Z.; Delmastro, A.; Fitzpatrick, M.B.; Gupta, S.; Greenwald, N.F.; Baranski, A.; Graf, W.; et al. The immunoregulatory landscape of human tuberculosis granulomas. Nat. Immunol. 2022, 23, 814. [Google Scholar] [CrossRef]
- Collins, J.M.; Siddiqa, A.; Jones, D.P.; Liu, K.; Kempker, R.R.; Nizam, A.; Shah, N.S.; Ismail, N.; Ouma, S.G.; Tukvadze, N.; et al. Tryptophan catabolism reflects disease activity in human tuberculosis. JCI Insight 2020, 5, e137131. [Google Scholar] [CrossRef]
- Isa, F.; Collins, S.; Lee, M.H.; Decome, D.; Dorvil, N.; Joseph, P.; Smith, L.; Salerno, S.; Wells, M.T.; Fischer, S.; et al. Mass Spectrometric Identification of Urinary Biomarkers of Pulmonary Tuberculosis. EBioMedicine 2018, 31, 157–165. [Google Scholar] [CrossRef]
- Tornheim, J.A.; Paradkar, M.; Zhao, H.; Kulkarni, V.; Pradhan, N.; Kinikar, A.; Kagal, A.; Gupte, N.; Mave, V.; Gupta, A.; et al. The Kynurenine/Tryptophan Ratio Is a Sensitive Biomarker for the Diagnosis of Pediatric Tuberculosis Among Indian Children. Front. Immunol. 2021, 12, 774043. [Google Scholar] [CrossRef]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef]
- Terness, P.; Bauer, T.M.; Rose, L.; Dufter, C.; Watzlik, A.; Simon, H.; Opelz, G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: Mediation of suppression by tryptophan metabolites. J. Exp. Med. 2002, 196, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Fallarino, F.; Grohmann, U.; Vacca, C.; Orabona, C.; Spreca, A.; Fioretti, M.C.; Puccetti, P. T cell apoptosis by kynurenines. Adv. Exp. Med. Biol. 2003, 527, 183–190. [Google Scholar] [PubMed]
- Puccetti, P. On watching the watchers: IDO and type I/II IFN. Eur. J. Immunol. 2007, 37, 876–879. [Google Scholar] [CrossRef]
- Mehra, S.; Pahar, B.; Dutta, N.K.; Conerly, C.N.; Philippi-Falkenstein, K.; Alvarez, X.; Kaushal, D. Transcriptional reprogramming in nonhuman primate (rhesus macaque) tuberculosis granulomas. PLoS ONE 2010, 5, e12266. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Moodley, C.; Singh, D.K.; Escobedo, R.A.; Sharan, R.; Arora, G.; Ganatra, S.R.; Shivanna, V.; Gonzalez, O.; Hall-Ursone, S.; et al. Inhibition of indoleamine dioxygenase leads to better control of tuberculosis adjunctive to chemotherapy. JCI Insight 2023, 8, e163101. [Google Scholar] [CrossRef]
- Esaulova, E.; Das, S.; Singh, D.K.; Choreno-Parra, J.A.; Swain, A.; Arthur, L.; Rangel-Moreno, J.; Ahmed, M.; Singh, B.; Gupta, A.; et al. The immune landscape in tuberculosis reveals populations linked to disease and latency. Cell Host Microbe 2020, 29, 165–178. [Google Scholar] [CrossRef]
- Akter, S.; Chauhan, K.S.; Dunlap, M.D.; Choreno-Parra, J.A.; Lu, L.; Esaulova, E.; Zuniga, J.; Artyomov, M.N.; Kaushal, D.; Khader, S.A. Mycobacterium tuberculosis infection drives a type I IFN signature in lung lymphocytes. Cell Rep. 2022, 39, 110983. [Google Scholar] [CrossRef]
- Russell, D.G. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 2007, 5, 39–47. [Google Scholar] [CrossRef]
- Khader, S.A.; Rangel-Moreno, J.; Fountain, J.J.; Martino, C.A.; Reiley, W.W.; Pearl, J.E.; Winslow, G.M.; Woodland, D.L.; Randall, T.D.; Cooper, A.M. In a murine tuberculosis model, the absence of homeostatic chemokines delays granuloma formation and protective immunity. J. Immunol. 2009, 183, 8004–8014. [Google Scholar] [CrossRef]
- Slight, S.R.; Khader, S.A. Chemokines shape the immune responses to tuberculosis. Cytokine Growth Factor. Rev. 2013, 24, 105–113. [Google Scholar] [CrossRef]
- Veatch, A.V.; Kaushal, D. Opening Pandora’s Box: Mechanisms of Mycobacterium tuberculosis Resuscitation. Trends Microbiol. 2017, 26, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Sharan, R.; Ganatra, S.R.; Singh, D.K.; Cole, J.; Foreman, T.W.; Thippeshappa, R.; Peloquin, C.A.; Shivanna, V.; Gonzalez, O.; Day, C.L.; et al. Isoniazid and rifapentine treatment effectively reduces persistent M. tuberculosis infection in macaque lungs. J. Clin. Investig. 2022, 132, e161564. [Google Scholar] [CrossRef] [PubMed]
- Hudock, T.A.; Foreman, T.W.; Bandyopadhyay, N.; Gautam, U.S.; Veatch, A.V.; LoBato, D.N.; Gentry, K.M.; Golden, N.A.; Cavigli, A.; Mueller, M.; et al. Hypoxia Sensing and Persistence Genes Are Expressed during the Intragranulomatous Survival of Mycobacterium tuberculosis. Am. J. Respir. Cell Mol. Biol. 2017, 56, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Gideon, H.P.; Hughes, T.K.; Tzouanas, C.N.; Wadsworth, M.H., 2nd; Tu, A.A.; Gierahn, T.M.; Peters, J.M.; Hopkins, F.F.; Wei, J.R.; Kummerlowe, C.; et al. Multimodal profiling of lung granulomas in macaques reveals cellular correlates of tuberculosis control. Immunity 2022, 55, 827–846.e10. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Aladyeva, E.; Das, S.; Singh, B.; Esaulova, E.; Swain, A.; Ahmed, M.; Cole, J.; Moodley, C.; Mehra, S.; et al. Myeloid cell interferon responses correlate with clearance of SARS-CoV-2. Nat. Commun. 2022, 13, 679. [Google Scholar] [CrossRef]
- Gideon, H.P.; Phuah, J.; Myers, A.J.; Bryson, B.D.; Rodgers, M.A.; Coleman, M.T.; Maiello, P.; Rutledge, T.; Marino, S.; Fortune, S.M.; et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog. 2015, 11, e1004603. [Google Scholar] [CrossRef]
- Sharan, R.; Singh, D.K.; Rengarajan, J.; Kaushal, D. Characterizing Early T Cell Responses in Nonhuman Primate Model of Tuberculosis. Front. Immunol. 2021, 12, 706723. [Google Scholar] [CrossRef]
- Lee, Y.J.; Han, S.K.; Park, J.H.; Lee, J.K.; Kim, D.K.; Chung, H.S.; Heo, E.Y. The effect of metformin on culture conversion in tuberculosis patients with diabetes mellitus. Korean J. Intern. Med. 2018, 33, 933–940. [Google Scholar] [CrossRef]
- Rao, M.; Zumla, A.; Maeurer, M. Host-directed therapy: Tuberculosis vaccine development. Lancet Respir. Med. 2015, 3, 172–173. [Google Scholar] [CrossRef]
- Zumla, A.; Rao, M.; Parida, S.K.; Keshavjee, S.; Cassell, G.; Wallis, R.; Axelsson-Robertsson, R.; Doherty, M.; Andersson, J.; Maeurer, M. Inflammation and tuberculosis: Host-directed therapies. J. Intern. Med. 2015, 277, 373–387. [Google Scholar] [CrossRef]
- Wallis, R.S.; Hafner, R. Advancing host-directed therapy for tuberculosis. Nat. Rev. Immunol. 2015, 15, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Hawn, T.R.; Matheson, A.I.; Maley, S.N.; Vandal, O. Host-directed therapeutics for tuberculosis: Can we harness the host? Microbiol. Mol. Biol. Rev. 2013, 77, 608–627. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, R.J. Host-directed therapies against tuberculosis. Lancet Respir. Med. 2014, 2, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.M. Host-Directed Therapies for Tuberculosis. Cold Spring Harb. Perspect. Med. 2015, 5, a021196. [Google Scholar] [CrossRef] [PubMed]
- Maiga, M.; Agarwal, N.; Ammerman, N.C.; Gupta, R.; Guo, H.; Maiga, M.C.; Lun, S.; Bishai, W.R. Successful shortening of tuberculosis treatment using adjuvant host-directed therapy with FDA-approved phosphodiesterase inhibitors in the mouse model. PLoS ONE 2012, 7, e30749. [Google Scholar] [CrossRef]
- Harding, C.V.; Boom, W.H. Regulation of antigen presentation by Mycobacterium tuberculosis: A role for Toll-like receptors. Nat. Rev. Microbiol. 2010, 8, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Maitra, A.; Bates, S.; Shaik, M.; Evangelopoulos, D.; Abubakar, I.; McHugh, T.D.; Lipman, M.; Bhakta, S. Repurposing drugs for treatment of tuberculosis: A role for non-steroidal anti-inflammatory drugs. Br. Med. Bull. 2016, 118, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Vilcheze, C.; Hartman, T.; Weinrick, B.; Jain, P.; Weisbrod, T.R.; Leung, L.W.; Freundlich, J.S.; Jacobs, W.R., Jr. Enhanced respiration prevents drug tolerance and drug resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2017, 114, 4495–4500. [Google Scholar] [CrossRef]
- Napier, R.J.; Rafi, W.; Cheruvu, M.; Powell, K.R.; Zaunbrecher, M.A.; Bornmann, W.; Salgame, P.; Shinnick, T.M.; Kalman, D. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe 2011, 10, 475–485. [Google Scholar] [CrossRef]
- Thomas, S.M.; Garrity, L.F.; Brandt, C.R.; Schobert, C.S.; Feng, G.S.; Taylor, M.W.; Carlin, J.M.; Byrne, G.I. IFN-gamma-mediated antimicrobial response. Indoleamine 2,3-dioxygenase-deficient mutant host cells no longer inhibit intracellular Chlamydia spp. or Toxoplasma growth. J. Immunol. 1993, 150, 5529–5534. [Google Scholar] [CrossRef]
- Makala, L.H.; Baban, B.; Lemos, H.; El-Awady, A.R.; Chandler, P.R.; Hou, D.Y.; Munn, D.H.; Mellor, A.L. Leishmania major attenuates host immunity by stimulating local indoleamine 2,3-dioxygenase expression. J. Infect. Dis. 2011, 203, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Roy, C.R. Host cell depletion of tryptophan by IFNgamma-induced Indoleamine 2,3-dioxygenase 1 (IDO1) inhibits lysosomal replication of Coxiella burnetii. PLoS Pathog. 2019, 15, e1007955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Rubin, E.J. Feast or famine: The host-pathogen battle over amino acids. Cell Microbiol. 2013, 15, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Reddy, M.C.; Ioerger, T.R.; Rothchild, A.C.; Dartois, V.; Schuster, B.M.; Trauner, A.; Wallis, D.; Galaviz, S.; Huttenhower, C.; et al. Tryptophan biosynthesis protects mycobacteria from CD4 T-cell-mediated killing. Cell 2013, 155, 1296–1308. [Google Scholar] [CrossRef]
- Kondrikov, D.; Elmansi, A.; Bragg, R.T.; Mobley, T.; Barrett, T.; Eisa, N.; Kondrikova, G.; Schoeinlein, P.; Aguilar-Perez, A.; Shi, X.M.; et al. Kynurenine inhibits autophagy and promotes senescence in aged bone marrow mesenchymal stem cells through the aryl hydrocarbon receptor pathway. Exp. Gerontol. 2020, 130, 110805. [Google Scholar] [CrossRef]
- Negatu, D.A.; Yamada, Y.; Xi, Y.; Go, M.L.; Zimmerman, M.; Ganapathy, U.; Dartois, V.; Gengenbacher, M.; Dick, T. Gut Microbiota Metabolite Indole Propionic Acid Targets Tryptophan Biosynthesis in Mycobacterium tuberculosis. mBio 2019, 10, e02781-18. [Google Scholar] [CrossRef]
- Negatu, D.A.; Gengenbacher, M.; Dartois, V.; Dick, T. Indole Propionic Acid, an Unusual Antibiotic Produced by the Gut Microbiota, With Anti-inflammatory and Antioxidant Properties. Front. Microbiol. 2020, 11, 575586. [Google Scholar] [CrossRef]
- Singh, B.; Singh, D.K.; Ganatra, S.R.; Escobedo, R.A.; Khader, S.; Schlesinger, L.S.; Kaushal, D.; Mehra, S. Myeloid-Derived Suppressor Cells Mediate T Cell Dysfunction in Nonhuman Primate TB Granulomas. mBio 2021, 12, e03189-21. [Google Scholar] [CrossRef]
- Mehra, S.; Foreman, T.W.; Didier, P.J.; Ahsan, M.H.; Hudock, T.A.; Kissee, R.; Golden, N.A.; Gautam, U.S.; Johnson, A.M.; Alvarez, X.; et al. The DosR Regulon Modulates Adaptive Immunity and is Essential for M. tuberculosis Persistence. Am. J. Respir. Crit. Care Med. 2015, 191, 1185–1196. [Google Scholar] [CrossRef]
- Mehra, S.; Golden, N.A.; Dutta, N.K.; Midkiff, C.C.; Alvarez, X.; Doyle, L.A.; Asher, M.; Russell-Lodrigue, K.; Monjure, C.; Roy, C.J.; et al. Reactivation of latent tuberculosis in rhesus macaques by coinfection with simian immunodeficiency virus. J. Med. Primatol. 2011, 40, 233–243. [Google Scholar] [CrossRef]
- Sonnenberg, P.; Glynn, J.R.; Fielding, K.; Murray, J.; Godfrey-Faussett, P.; Shearer, S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J. Infect. Dis. 2005, 191, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sharan, R.; Bucsan, A.N.; Ganatra, S.; Paiardini, M.; Mohan, M.; Mehra, S.; Khader, S.A.; Kaushal, D. Chronic Immune Activation in TB/HIV Co-infection. Trends Microbiol. 2020, 28, 619–632. [Google Scholar] [CrossRef]
- Paiardini, M.; Muller-Trutwin, M. HIV-associated chronic immune activation. Immunol. Rev. 2013, 254, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Klatt, N.R.; Funderburg, N.T.; Brenchley, J.M. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013, 21, 6–13. [Google Scholar] [CrossRef]
- Jambo, K.C.; Banda, D.H.; Kankwatira, A.M.; Sukumar, N.; Allain, T.J.; Heyderman, R.S.; Russell, D.G.; Mwandumba, H.C. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol. 2014, 7, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.; Castilla, V.; Sanz, J.; Gaspar, G.; Condes, E.; Barros, C.; Cervero, M.; Torres, R.; Guijarro, C. Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J. Acquir. Immune Defic. Syndr. 2009, 50, 148–152. [Google Scholar] [CrossRef]
- Walker, N.F.; Meintjes, G.; Wilkinson, R.J. HIV-1 and the immune response to TB. Future Virol. 2013, 8, 57–80. [Google Scholar] [CrossRef]
- Sutherland, J.S.; Young, J.M.; Peterson, K.L.; Sanneh, B.; Whittle, H.C.; Rowland-Jones, S.L.; Adegbola, R.A.; Jaye, A.; Ota, M.O. Polyfunctional CD4(+) and CD8(+) T cell responses to tuberculosis antigens in HIV-1-infected patients before and after anti-retroviral treatment. J. Immunol. 2010, 184, 6537–6544. [Google Scholar] [CrossRef]
- Gupta, A.; Wood, R.; Kaplan, R.; Bekker, L.G.; Lawn, S.D. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: Comparison with rates in the community. PLoS ONE 2012, 7, e34156. [Google Scholar] [CrossRef]
- Wilkinson, K.A.; Schneider-Luftman, D.; Lai, R.; Barrington, C.; Jhilmeet, N.; Lowe, D.M.; Kelly, G.; Wilkinson, R.J. Antiretroviral Treatment-Induced Decrease in Immune Activation Contributes to Reduced Susceptibility to Tuberculosis in HIV-1/Mtb Co-infected Persons. Front. Immunol. 2021, 12, 645446. [Google Scholar] [CrossRef]
- Sandler, N.G.; Sereti, I. Can early therapy reduce inflammation? Curr. Opin. HIV AIDS 2014, 9, 72–79. [Google Scholar] [CrossRef]
- Abah, I.O.; Ncube, N.B.Q.; Bradley, H.A.; AgbaJi, O.O.; Kanki, P. Antiretroviral Therapy-associated Adverse Drug Reactions and their Effects on Virologic Failure- A Retrospective Cohort Study in Nigeria. Curr. HIV Res. 2018, 16, 436–446. [Google Scholar] [CrossRef]
- Correa-Macedo, W.; Fava, V.M.; Orlova, M.; Cassart, P.; Olivenstein, R.; Sanz, J.; Xu, Y.Z.; Dumaine, A.; Sindeaux, R.H.; Yotova, V.; et al. Alveolar macrophages from persons living with HIV show impaired epigenetic response to Mycobacterium tuberculosis. J. Clin. Investig. 2021, 131, e148013. [Google Scholar] [CrossRef] [PubMed]
- Slight, S.R.; Rangel-Moreno, J.; Gopal, R.; Lin, Y.; Fallert Junecko, B.A.; Mehra, S.; Selman, M.; Becerril-Villanueva, E.; Baquera-Heredia, J.; Pavon, L.; et al. CXCR5(+) T helper cells mediate protective immunity against tuberculosis. J. Clin. Investig. 2013, 123, 712–726. [Google Scholar] [CrossRef]
- Boasso, A.; Vaccari, M.; Fuchs, D.; Hardy, A.W.; Tsai, W.P.; Tryniszewska, E.; Shearer, G.M.; Franchini, G. Combined effect of antiretroviral therapy and blockade of IDO in SIV-infected rhesus macaques. J. Immunol. 2009, 182, 4313–4320. [Google Scholar] [CrossRef]
- Page, E.E.; Greathead, L.; Metcalf, R.; Clark, S.A.; Hart, M.; Fuchs, D.; Pantelidis, P.; Gotch, F.; Pozniak, A.; Nelson, M.; et al. Loss of Th22 cells is associated with increased immune activation and IDO-1 activity in HIV-1 infection. J. Acquir. Immune Defic. Syndr. 2014, 67, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, A.; Garg, S.; Khanna, A.; Narayana, C.; Dwivedi, V.P.; Joshi, N.; e Anam, Z.; Singh, N.; Singhal, J.; Kaushik, S.; et al. Pathogen induced subversion of NAD(+) metabolism mediating host cell death: A target for development of chemotherapeutics. Cell Death Discov. 2021, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Adekambi, T.; Ibegbu, C.C.; Kalokhe, A.S.; Yu, T.; Ray, S.M.; Rengarajan, J. Distinct effector memory CD4+ T cell signatures in latent Mycobacterium tuberculosis infection, BCG vaccination and clinically resolved tuberculosis. PLoS ONE 2012, 7, e36046. [Google Scholar] [CrossRef]
- Adekambi, T.; Ibegbu, C.C.; Cagle, S.; Kalokhe, A.S.; Wang, Y.F.; Hu, Y.; Day, C.L.; Ray, S.M.; Rengarajan, J. Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response. J. Clin. Investig. 2015, 125, 1827–1838. [Google Scholar] [CrossRef]
- Berthelier, V.; Tixier, J.M.; Muller-Steffner, H.; Schuber, F.; Deterre, P. Human CD38 is an authentic NAD(P)+ glycohydrolase. Biochem. J. 1998, 330 Pt 3, 1383–1390. [Google Scholar] [CrossRef]
- Sun, J.; Siroy, A.; Lokareddy, R.K.; Speer, A.; Doornbos, K.S.; Cingolani, G.; Niederweis, M. The tuberculosis necrotizing toxin kills macrophages by hydrolyzing NAD. Nat. Struct. Mol. Biol. 2015, 22, 672–678. [Google Scholar] [CrossRef]
- Blumenthal, A.; Nagalingam, G.; Huch, J.H.; Walker, L.; Guillemin, G.J.; Smythe, G.A.; Ehrt, S.; Britton, W.J.; Saunders, B.M. M. tuberculosis induces potent activation of IDO-1, but this is not essential for the immunological control of infection. PLoS ONE 2012, 7, e37314. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Gutierrez, N.M.; Marzuki, M.B.; Lu, X.; Foreman, T.W.; Paleja, B.; Lee, B.; Balachander, A.; Chen, J.; Tsenova, L.; et al. Host sirtuin 1 regulates mycobacterial immunopathogenesis and represents a therapeutic target against tuberculosis. Sci. Immunol. 2017, 2, eaaj1789. [Google Scholar] [CrossRef]
- Singhal, A.; Jie, L.; Kumar, P.; Hong, G.S.; Leow, M.K.; Paleja, B.; Tsenova, L.; Kurepina, N.; Chen, J.; Zolezzi, F.; et al. Metformin as adjunct antituberculosis therapy. Sci. Transl. Med. 2014, 6, 263ra159. [Google Scholar] [CrossRef] [PubMed]
- Sadria, M.; Layton, A.T. Interactions among mTORC, AMPK and SIRT: A computational model for cell energy balance and metabolism. Cell Commun. Signal 2021, 19, 57. [Google Scholar] [CrossRef]
- Scully, E.P.; Bryson, B.D. Unlocking the complexity of HIV and Mycobacterium tuberculosis coinfection. J. Clin. Investig. 2021, 131, e154407. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaushal, D.; Singh, D.K.; Mehra, S. Immune Responses in Lung Granulomas during Mtb/HIV Co-Infection: Implications for Pathogenesis and Therapy. Pathogens 2023, 12, 1120. https://doi.org/10.3390/pathogens12091120
Kaushal D, Singh DK, Mehra S. Immune Responses in Lung Granulomas during Mtb/HIV Co-Infection: Implications for Pathogenesis and Therapy. Pathogens. 2023; 12(9):1120. https://doi.org/10.3390/pathogens12091120
Chicago/Turabian StyleKaushal, Deepak, Dhiraj K. Singh, and Smriti Mehra. 2023. "Immune Responses in Lung Granulomas during Mtb/HIV Co-Infection: Implications for Pathogenesis and Therapy" Pathogens 12, no. 9: 1120. https://doi.org/10.3390/pathogens12091120





