Unrevealing the Mystery of Latent Leishmaniasis: What Cells Can Host Leishmania?
Abstract
:1. Background
2. Professional Phagocytes as the Leishmania Primary Host Cells
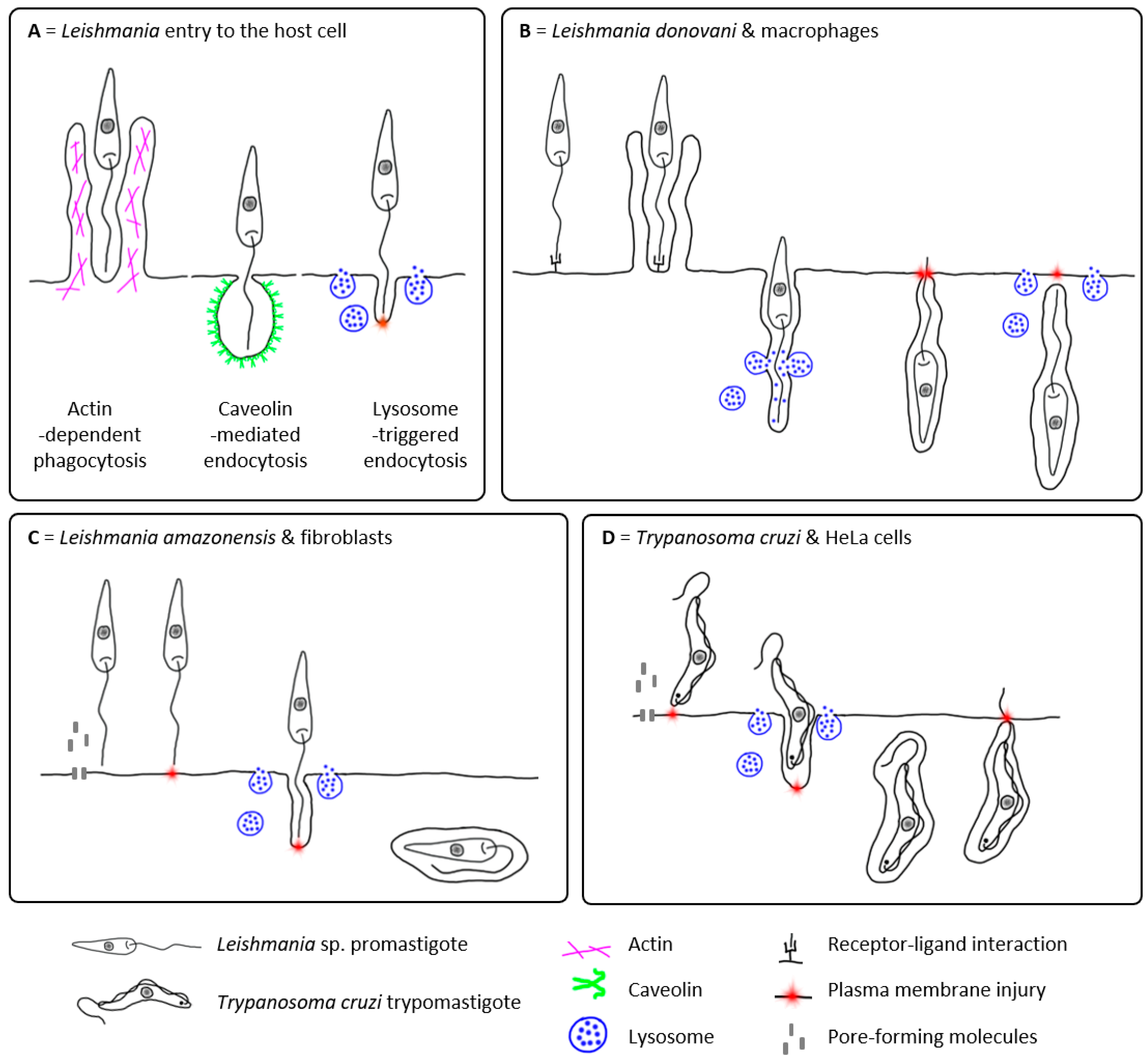
2.1. Passive Entry of Leishmania into the Host Cell
2.2. Active Entry of Leishmania into the Host Cell?
3. Other Potential Host Cells of Leishmania
| Host Cell/Origin | Leishmania Species | Main Outcome | Detection Method | Reference | |
|---|---|---|---|---|---|
| PROFESSIONAL PHAGOCYTES | |||||
| Dendritic cells (DCs) | |||||
| Langerhans cells from mouse skin ❖ | L. major PMs | No or low PMs uptake | LM/Diff-Quik, TEM, FL/AO+EtBr | [53,54,55] | |
| L. major AMs | AMs uptake and internalisation, no or weak multiplication | LM/Diff-Quik, TEM, FL/AO+EtBr, ICC | [54,56,57] | ||
| Mouse lymph node DCs ♦○ | L. major AMs | Presence of AMs | LM/G, IHC | [56,58,59] | |
| Mouse spleen DCs ❖ | L. major PMs/AMs, L. m. mexicana PMs | PMs/AMs uptake | LM/G | [59,60] | |
| Mouse bone marrow DCs ❖ | L. major PMs/AMs, L. mexicana PMs, L. amazonensis AMs/PMs | PMs/AMs uptake in all; multiplication reported only in L. amazonensis | LM/Diff-Quik, ICC, FC | [61,62,63,64] | |
| L. infantum PMs/PMs (CFSE) | PMs uptake, transformation into AMs | LM/G, FC/CFSE-PMs | [65] | ||
| Human immature monocyte-derived DCs❖ | L. amazonensis, L. braziliensis, L. infantum PMs | PMs uptake, internalisation | CLSM/Dapi | [25] | |
| L. donovani PMs | PMs uptake, transformation into AMs | LM/MGG | [66] | ||
| Mast cells (MCs) | |||||
| Mouse peritoneal MCs ❖ | L. tropica, L. donovani PMs (CFSE) | PMs uptake in L. tropica, but not in L. donovani | FC+CLSM/CFSE-PMs | [22,67] | |
| Mouse bone marrow MCs ❖ | L. major, L. infantum PMs | PMs uptake, transformation into AMs, multiplication leading to cell lysis and AMs release | LM/MGG | [21] | |
| Eosinophils | |||||
| Human peripheral eosinophils ❖ | L. donovani PMs | PMs uptake and killing after 2 h p.i. | LM/Diff-Quik | [68] | |
| L. donovani AMs | AMs uptake, not efficient killing | LM, TEM | [69] | ||
| Rat peritoneal eosinophils ❖ | L. major PMs | PMs uptake and killing | LM/MGG, ICC | [70] | |
| Rat peritoneal eosinophils ♦○❖ | L. m. amazonensis PMs/AMs | PMs/AMs uptake and killing | TEM | [71] | |
| Mouse eosinophils in skin lesion ♦○ | L. m. mexicana AMs | AMs uptake, not efficient killing | TEM | [72] | |
| Histiocyte-like cells | [7] | ||||
| Sticker dog sarcoma 503 cells ❖ | L. donovani, L. mexicana, L. m. mexicana, L. braziliensis, L. b. pifanoi, L. t. major PMs/AMs | PMs/AMs uptake, multiplication, continuous passages | LM/G, TEM | [73,74,75,76,77,78] | |
| L. m. mexicana PMs | PMs uptake, transformation into AMs, multiplication after day 3 p.i., transformation into PMs | LM/G, TEM | [43] | ||
| L. adleri, L. hoogstraali, L. agamae PMs | Low PMs uptake, transformation into AMs | LM/G | [43] | ||
| NON-PROFESSIONAL PHAGOCYTES | |||||
| Lymphocytes | |||||
| Human B (Daudi) and T (HUT78) cells ❖ | L. donovani PMs/AMs | PMs/AMs uptake, PMs transformation into AMs, viability up to 2 weeks after infection with AMs | LM/G, TEM | [79] | |
| Fibrocytes | |||||
| Mouse peripheral blood fibrocytes ❖ | L. amazonensis PMs | PMs uptake, transformation into AMs, low multiplication, clearance by 72 h p.i. | LM/G, FL/Dapi, TEM, SEM | [80] | |
| Fibroblasts | |||||
| Canine skin fibroblasts ♦□ | Leishmania sp. | Presence of AMs | TEM, LM/HE, G, PAS | [81] | |
| L. donovani | Presence of AMs | IHC | [82] | ||
| Human skin fibroblasts ♦□ | L. tropica | Presence of AMs | LM/G, TEM | [83,84] | |
| Leishmania sp. (cutaneous) | Presence of AMs | TEM | [85] | ||
| Human skin fibroblasts ❖ | L. amazonensis PMs | PMs uptake, transformation into AMs, multiplication | TEM | [14] | |
| L. m. amazonensis AMs | AMs uptake, multiplication, killing of AMs by day 8 p.i. | LM/G, TEM, ICC | [12] | ||
| Leishmania sp. (mucocutaneous), L. donovani PMs | PMs uptake in Leishmania sp. (not in L. donovani), transformation into AMs, no or low multiplication, decline during a 3-week period p.i. | LM, TEM, SEM | [86] | ||
| Human foreskin fibroblasts ❖ | L. donovani PMs | PMs uptake, transformation into AMs, no multiplication, viability up to day 14 p.i. | LM, TEM, SEM | [87] | |
| L. major PMs (SPIONs) | PMs uptake | LM/SPIONs-PMs+Prussian blue, TEM | [88] | ||
| L. major PMs (AO, Dil) | PMs uptake | FL/AO-PMs, Dil-PMs | [89] | ||
| Mouse skin fibroblasts ♦○ | L. amazonensis PMs | AMs presence | LM/HE, Lennert’s G | [90] | |
| Mouse skin fibroblasts ❖ | L. major PMs/AMs | PMs/AMs uptake | ICC | [9] | |
| L. amazonensis PMs | PMs uptake and killing of PMs after day 3 p.i. | LM/G, TEM, FL/Dapi | [91] | ||
| L. infantum, L. mexicana PMs | PMs uptake, transformation into AMs, multiplication | LM/G, TEM | [10] | ||
| Hamster skin fibroblasts ❖ | L. infantum, L. mexicana PMs | PMs uptake, transformation into AMs, multiplication | LM/G, TEM | [10] | |
| Rat skin fibroblasts ❖ | L. infantum, L. mexicana PMs | PMs uptake, transformation into AMs, no multiplication | LM/G, TEM | [10] | |
| Human fibroblasts in lymph node ♦□ | Leishmania sp. | AMs presence | LM/G | [92] | |
| Mouse fibroblasts in lymph node ❖ | L. major PMs/AMs | PMs/AMs uptake | TEM, ICC | [9] | |
| Draining lymph nodes of healed mice (presumably fibroblasts) ♦○ | L. major PMs | Presence of AMs, parasite survival or limited killing | IHC | [9] | |
| Mouse embryonic fibroblasts ❖ | L. donovani PMs | PMs uptake, transformation into AMs, efficient host defence via IFN-inducible guanylate binding proteins | LM/G, CLSM/Dapi | [93] | |
| L. amazonensis PMs (RFP) | PMs uptake, transformation into AMs, multiplication | LM/HE, TEM, CLSM+FC/RFP-PMs | [8] | ||
| L. major PMs | PMs uptake | CLSM/Dapi | [94] | ||
| Mouse tumour fibroblasts (L cells) ❖ | L. amazonensis, L. major AMs (GFP) | AMs uptake, internalisation (low in L. major), multiplication (not in L. major) | CLSM/GFP-AMs | [15] | |
| Fibroblasts from embryonic chick brain ❖ | L. donovani AMs | AMs uptake, viability up to day 17 p.i., transformation into PMs | LM/G | [11] | |
| Fibroblast-like cells from embryonic chick muscle ❖ | L. donovani/presumably AMs | AMs uptake, no multiplication, degeneration after day 20 p.i. | LM/HE | [95] | |
| Mouse perineurial cells ♦○ | L. amazonensis PMs | Presence of AMs | TEM | [96] | |
| Adipocytes | |||||
| Mouse brown and white adipose tissue ♦○ | L. infantum PMs | PMs uptake, viable AMs present for up to 40 weeks p.i. | IHC, qPCR | [46] | |
| Mouse adipocytes derived from primary pre-adipocytes from subcutaneous white adipose tissue ❖ | L. infantum PMs (GFP) | PMs uptake (further progress not reported) | TEM, qPCR, CLSM/GFP-AMs | [46] | |
| Human adipocytes derived from adipose tissue primary progenitor cells ❖ | L. infantum PMs (GFP) | PMs uptake (further progress not reported) | qPCR, CLSM/GFP-AMs | [46] | |
| Mouse adipocytes differentiated in vitro from 3T3-L1 fibroblasts ❖ | L. amazonensis, L. braziliensis PMs/AMs | PMs/AMs uptake, PMs transformation into AMs, viability up to 144 h p.i. and ability to transform into PMs | LM/G, FL/Dapi, TEM | [45] | |
| L. amazonensis AMs (GFP) | AMs uptake, viability up to 144 h p.i. | FL/GFP-AMs | [45] | ||
| Epithelial cells | |||||
| Human epithelial cells of eccrine sweat gland (HIV patient) ♦□ | Leishmania sp., L. infantum | AMs presence | LM/HE | [97,98] | |
| Human retinal pigmented epithelial cells (ARPE-19) ❖ | L. amazonensis PMs | PMs uptake, internalisation | LM/G, IHC, TEM | [99] | |
| Human amnion epithelium ❖ | L. donovani, L. b. pifanoi PMs | PMs uptake, transformation into AMs, clearance by day 29–32 p.i. | LM/G | [100] | |
| L. donovan PMs | PMs uptake, transformation into AMs, multiplication (not clear whether PMS or AMs) | LM/G | [101] | ||
| A549 (human adenocarcinomic alveolar basal epithelium) cells ❖ | L. donovani PMs | PMs uptake, transformation into AMs, efficient defence via IFN-inducible guanylate binding proteins | LM/G, CLSM/Dapi | [93] | |
| HeLa (human cervix carcinoma) cells ❖ | L. t. major PMs | PMs uptake, transformation into AMs, multiplication, destruction of host cells after day 3 | LM | [102] | |
| L. donovani PMs | PMs uptake, transformation into AMs, decline after 5 h p.i. | LM/G | [103] | ||
| LLC-MK2 (rhesus monkey kidney epithelium) cells ❖ | L. donovani AMs | AMs uptake, multiplication | LM/G | [104] | |
| Vero (monkey kidney) cells ❖ | L. chagasi, L. braziliensis PMs | PMs uptake, transformation into AMs, multiplication | LM/G, TEM | [105,106] | |
| Chinese hamster ovary cells ❖ | L. amazonensis AMs | AMs uptake, multiplication | IHC, TEM | [42] | |
| C. burnetii-infected Vero cells ❖ | L. amazonensis AMs | AMs uptake, multiplication | LM, TEM | [107] | |
| C. burnetii-infected Chinese hamster ovary cells ❖ | L. amazonensis AMs | AMs uptake, multiplication | LM, TEM, CLSM/PI | [107,108] | |
| Mesenchymal stem cells (MSCs) | |||||
| Mouse bone marrow MSCs ♦○❖ | L. infantum PMs | PMs uptake, transformation into AMs | LM/G, ICC, FC | [109] | |
| Human adipose tissue MSCs ❖ | L. donovani, L. infantum, L. major, L. tropica PMs | PMs uptake, transformation into AMs but AMs present only at day 1 p.i.; at day 7, 14, 21, and 28 only PMs detected | LM/G, microcapillary culture method, PCR | [16] | |
| Myocytes | |||||
| Canine skeletal/smooth muscles ♦□ | L. infantum, Leishmania sp. | Presence of AMs within myofibres | LM/HE, IHC | [110,111] | |
| Mouse skeletal muscles ♦○ | L. amazonensis AMs | Presence of AMs within myofibres | LM/HE | [112] | |
| Turtle heart cells ❖ | L. m. mexicana, L. adleri, L. hoogstraali PMs | PMs uptake (lower in L. adleri and L. hoogstraali), transformation into AMs (further progress not reported) | TEM (L. mexicana only), LM/G | [43] | |
| Endothelial cells | |||||
| Human endothelial cells of blood vessels ♦□ | L. donovani, Leishmania sp. | Presence of AMs | LM | [81,113,114] | |
| Human endothelial cells of capillaries ♦○ | L. tropica PMs | Presence of AMs | LM | [115] | |
| Human microvascular endothelial (HMEC-1) cell line ❖ | L. infantum PMs | No uptake of PMs | LM/G | [116] | |
| Keratinocytes | |||||
| Human keratinocytes (HIV patient) ♦□ | L. infantum | AMs presence | LM/HE | [98] | |
| Human keratinocytes ❖ | L. infantum, L. major PMs | PMs uptake, transformation into AMs at low levels, no multiplication | LM/G, CLSM/Dapi | [117] | |
| Unidentified cells in primary cultures | |||||
| Hamster kidney cells ❖ | L. braziliensis, L. donovani PMs | PMs uptake, transformation into AMs, multiplication (not in L. donovani) | LM/G | [118,119], as cited in [7,49] | |
| Chicken embryo muscles ♦○ | L. t. major PMs | PMs uptake, transformation into AMs, multiplication, destruction of host cells after day 3 | LM | [102] | |
3.1. Fibroblasts
3.2. Adipocytes
3.3. Mesenchymal Stems Cells
3.4. Myocytes
3.5. Endothelial Cells
3.6. Keratinocytes
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMs | Amastigotes |
| AO | Acridine orange |
| ASM | Acid sphingomyelinase |
| CFSE | Carboxyfluorescein N-succinimidyl ester |
| CLSM | Confocal laser scanning microscopy |
| DCs | Dendritic cells |
| EtBr | Ethidium bromide |
| FC | Flow cytometry |
| FL | Fluorescence microscopy |
| G | Giemsa |
| GFP | Green fluorescent protein |
| HE | Haematoxylin-eosin |
| HMEC-1 | Human microvascular endothelial cell line |
| ICC | Immunocytochemistry |
| IHC | Immunohistochemistry |
| LAMP | Lysosomal membrane-associated protein |
| LM | Light microscopy |
| L-SIGN | Liver/lymph node-specific ICAM-3 grabbing nonintegrin |
| MCs | Mast cells |
| MGG | May–Grünwald–Giemsa |
| MSCs | Mesenchymal stems cells |
| NO | Nitric oxide |
| PAS | Periodic acid-Schiff |
| PCR | Polymerase chain reaction |
| PI | Propidium iodide |
| PMs | Promastigotes |
| p.i. | Post inoculation |
| qPCR | Quantitative polymerase chain reaction |
| RFP | Red fluorescent protein |
| SEM | Scanning electron microscopy |
| SPIONs | Superparamagnetic iron oxide nanoparticles |
| TEM | Transmission electron microscopy |
References
- Kolářová, I.; Valigurová, A. Hide-and-seek: A game played between parasitic protists and their hosts. Microorganisms 2021, 9, 2434. [Google Scholar] [CrossRef]
- Conceição-Silva, F.; Morgado, F.N. Leishmania spp-host interaction: There is always an onset, but is there an end? Front. Cell. Infect. Microbiol. 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Morgado, F.N.; Schubach, A.; Vasconcellos, E.; Azeredo-Coutinho, R.B.; Valete-Rosalino, C.M.; Quintella, L.P.; Santos, G.; Salgueiro, M.; Palmeiro, M.R.; Conceição-Silva, F. Signs of an in situ inflammatory reaction in scars of human American tegumentary leishmaniasis. Parasite Immunol. 2010, 32, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valencia, A.J.; Daza-Rivera, C.F.; Rosales-Chilama, M.; Cossio, A.; Casadiego Rincón, E.J.; Desai, M.M.; Saravia, N.G.; Gómez, M.A. Clinical and parasitological factors in parasite persistence after treatment and clinical cure of cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2017, 11, e0005713. [Google Scholar] [CrossRef]
- Walker, D.M.; Oghumu, S.; Gupta, G.; McGwire, B.S.; Drew, M.E.; Satoskar, A.R. Mechanisms of cellular invasion by intracellular parasites. Cell. Mol. Life Sci. 2014, 71, 1245–1263. [Google Scholar] [CrossRef]
- Chaves, M.M.; Lee, S.H.; Kamenyeva, O.; Ghosh, K.; Peters, N.C.; Sacks, D. The role of dermis resident macrophages and their interaction with neutrophils in the early establishment of Leishmania major infection transmitted by sand fly bite. PLoS Pathog. 2020, 16, e1008674. [Google Scholar] [CrossRef]
- Rittig, M.G.; Bogdan, C. Leishmania-host-cell interaction: Complexities and alternative views. Parasitol. Today 2000, 16, 292–297. [Google Scholar] [CrossRef]
- Cavalcante-Costa, V.S.; Costa-Reginaldo, M.; Queiroz-Oliveira, T.; Oliveira, A.C.S.; Couto, N.F.; Dos Anjos, D.O.; Lima-Santos, J.; Andrade, L.O.; Horta, M.F.; Castro-Gomes, T. Leishmania amazonensis hijacks host cell lysosomes involved in plasma membrane repair to induce invasion in fibroblasts. J. Cell. Sci. 2019, 132, jcs226183. [Google Scholar] [CrossRef]
- Bogdan, C.; Donhauser, N.; Döring, R.; Röllinghoff, M.; Diefenbach, A.; Rittig, M.G. Fibroblasts as host cells in latent leishmaniosis. J. Exp. Med. 2000, 191, 2121–2130. [Google Scholar] [CrossRef]
- Minero, M.A.; Chinchilla, M.; Guerrero, O.M.; Castro, A. Infection of skin fibroblasts in animals with different levels of sensitivity to Leishmania infantum and Leishmania mexicana (Kinetoplastida: Trypanosomatidae). Rev. Biol. Trop. 2004, 52, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, T.W.; Palczuk, N.C. Leishmania in the chick embryo. IV. Effects of embryo age and hatching, and behavior of L. donovani in cultures of chick fibroblasts. Exp. Parasitol. 1975, 37, 398–404. [Google Scholar] [CrossRef]
- Dedet, J.P.; Ryter, A.; Vogt, E.; Hosli, P.; Da Silva, L.P. Uptake and killing of Leishmania mexicana amazonensis amastigotes by human skin fibroblasts. Ann. Trop. Med. Parasitol. 1983, 77, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host-pathogen interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Corte-Real, S.; Santos, C.B.; Meirelles, M.N. Differential expression of the plasma membrane Mg2+ ATPase and Ca2+ ATPase activity during adhesion and interiorization of Leishmania amazonensis in fibroblasts in vitro. J. Submicrosc. Cytol. Pathol. 1995, 27, 359–366. [Google Scholar] [PubMed]
- Orikaza, C.M.; Pessoa, C.C.; Paladino, F.V.; Florentino, P.T.V.; Barbiéri, C.L.; Goto, H.; Ramos-Sanchez, E.M.; Silveira, J.F.D.; Rabinovitch, M.; Mortara, R.A.; et al. Dual host-intracellular parasite transcriptome of enucleated cells hosting Leishmania amazonensis: Control of half-life of host cell transcripts by the parasite. Infect. Immun. 2020, 88, e00261-20. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Bagirova, M.; Elcicek, S.; Koc, R.C.; Baydar, S.Y.; Findikli, N.; Oztel, O.N. Adipose tissue-derived mesenchymal stem cells as a new host cell in latent leishmaniasis. Am. J. Trop. Med. Hyg. 2011, 85, 535–539. [Google Scholar] [CrossRef]
- Carneiro, M.B.; Peters, N.C. The paradox of a phagosomal lifestyle: How innate host cell-Leishmania amazonensis interactions lead to a progressive chronic disease. Front. Immunol. 2021, 12, 728848. [Google Scholar] [CrossRef]
- Rabinovitch, M. Professional and non-professional phagocytes: An introduction. Trends Cell Biol. 1995, 5, 85–87. [Google Scholar] [CrossRef]
- Laskay, T.; van Zandbergen, G.; Solbach, W. Neutrophil granulocytes as host cells and transport vehicles for intracellular pathogens: Apoptosis as infection-promoting factor. Immunobiology 2008, 213, 183–191. [Google Scholar] [CrossRef]
- van Zandbergen, G.; Klinger, M.; Mueller, A.; Dannenberg, S.; Gebert, A.; Solbach, W.; Laskay, T. Cutting edge: Neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J. Immunol. 2004, 173, 6521–6525. [Google Scholar] [CrossRef] [Green Version]
- Bidri, M.; Vouldoukis, I.; Mossalayi, M.D.; Debré, P.; Guillosson, J.J.; Mazier, D.; Arock, M. Evidence for direct interaction between mast cells and Leishmania parasites. Parasite Immunol. 1997, 19, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, N.; Srivastava, R.; Selvapandiyan, A.; Puri, N. Host mast cells in leishmaniasis: Friend or foe? Trends Parasitol. 2020, 36, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, N.E.; Wilson, M.E. Eosinophils and mast cells in leishmaniasis. Immunol. Res. 2014, 59, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, M.; Soto, M.; Iborra, S.; Sancho, D. Leishmania hijacks myeloid cells for immune escape. Front. Microbiol. 2018, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Rebouças, A.; Silva, T.S.; Medina, L.S.; Paredes, B.D.; Aragão, L.S.; Souza, B.S.F.; Borges, V.M.; Schriefer, A.; Veras, P.S.T.; Brodskyn, C.I.; et al. Leishmania-induced dendritic cell migration and its potential contribution to parasite dissemination. Microorganisms 2021, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, B.P.; Beaumann, M.; Heyde, S.; Regli, I.B.; Müller, A.J.; Tacchini-Cottier, F. Frontline Science: Leishmania mexicana amastigotes can replicate within neutrophils. J. Leukoc. Biol. 2017, 102, 1187–1198. [Google Scholar] [CrossRef]
- Passelli, K.; Billion, O.; Tacchini-Cottier, F. The impact of neutrophil recruitment to the skin on the pathology induced by Leishmania infection. Front. Immunol. 2021, 12, 649348. [Google Scholar] [CrossRef]
- Andrade, L.O. Chapter Nine—Plasma membrane repair involvement in parasitic and other pathogen infections. In Current Topics in Membranes; Andrade, L.O., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 84, pp. 217–238. [Google Scholar] [CrossRef]
- Forestier, C.-L.; Machu, C.; Loussert, C.; Pescher, P.; Späth, G.F. Imaging host cell-Leishmania interaction dynamics implicates parasite motility, lysosome recruitment, and host cell wounding in the infection process. Cell Host Microbe 2011, 9, 319–330. [Google Scholar] [CrossRef]
- Kima, P.E. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int. J. Parasitol. 2007, 37, 1087–1096. [Google Scholar] [CrossRef] [Green Version]
- Ueno, N.; Wilson, M.E. Receptor-mediated phagocytosis of Leishmania: Implications for intracellular survival. Trends Parasitol. 2012, 28, 335–344. [Google Scholar] [CrossRef]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.S.; Rodriguez, N.E.; Adesse, D.; Garzoni, L.R.; Esper, L.; Lisanti, M.P.; Burk, R.D.; Albanese, C.; Van Doorslaer, K.; Weiss, L.M.; et al. Recent developments in the interactions between caveolin and pathogens. In Caveolins and Caveolae. Advances in Experimental Medicine and Biology; Jasmin, J.F., Frank, P.G., Lisanti, M.P., Eds.; Springer: New York, NY, USA, 2012; Volume 729, pp. 65–82. [Google Scholar] [CrossRef]
- Kumar, G.A.; Karmakar, J.; Mandal, C.; Chattopadhyay, A. Leishmania donovani internalizes into host cells via caveolin-mediated endocytosis. Sci. Rep. 2019, 9, 12636. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, N.E.; Gaur Dixit, U.; Allen, L.-A.H.; Wilson, M.E. Stage-specific pathways of Leishmania infantum chagasi entry and phagosome maturation in macrophages. PLoS ONE 2011, 6, e19000. [Google Scholar] [CrossRef] [PubMed]
- Ueno, N.; Bratt, C.L.; Rodriguez, N.E.; Wilson, M.E. Differences in human macrophage receptor usage, lysosomal fusion kinetics and survival between logarithmic and metacyclic Leishmania infantum chagasi promastigotes. Cell. Microbiol. 2009, 11, 1827–1841. [Google Scholar] [CrossRef]
- Rodríguez, N.E.; Gaur, U.; Wilson, M.E. Role of caveolae in Leishmania chagasi phagocytosis and intracellular survival in macrophages. Cell. Microbiol. 2006, 8, 1106–1120. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Cortez, M.; Flannery, A.R.; Tam, C.; Mortara, R.A.; Andrews, N.W. Trypanosoma cruzi subverts the sphingomyelinase-mediated plasma membrane repair pathway for cell invasion. J. Exp. Med. 2011, 208, 909–921. [Google Scholar] [CrossRef]
- Andrews, N.W. Lysosomes and the plasma membrane: Trypanosomes reveal a secret relationship. J. Cell Biol. 2002, 158, 389–394. [Google Scholar] [CrossRef]
- Seeberg, J.C.; Loibl, M.; Moser, F.; Schwegler, M.; Büttner-Herold, M.; Daniel, C.; Engel, F.B.; Hartmann, A.; Schlötzer-Schrehardt, U.; Goppelt-Struebe, M.; et al. Non-professional phagocytosis: A general feature of normal tissue cells. Sci. Rep. 2019, 9, 11875. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.M.; Lisanti, M.P. The caveolin proteins. Genome Biol. 2004, 5, 214. [Google Scholar] [CrossRef]
- Morehead, J.; Coppens, I.; Andrews, N.W. Opsonization modulates Rac-1 activation during cell entry by Leishmania amazonensis. Infect. Immun. 2002, 70, 4571–4580. [Google Scholar] [CrossRef]
- Lewis, D.H. Infection of tissue culture cells of low phagocytic ability by Leishmania mexicana mexicana. Ann. Trop. Med. Parasitol. 1974, 68, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R.; Strangways-Dixon, J. Leishmania mexicana: The epidemiology of dermal leishmaniasis in British Honduras. Trans. R. Soc. Trop. Med. Hyg. 1963, 57, 242–265. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.; Minori, K.; Consonni, S.R.; Andrews, N.W.; Miguel, D.C. Causative agents of American tegumentary leishmaniasis are able to infect 3T3-L1 adipocytes in vitro. Front. Cell. Infect. Microbiol. 2022, 12, 824494. [Google Scholar] [CrossRef] [PubMed]
- Schwing, A.; Pisani, D.F.; Pomares, C.; Majoor, A.; Lacas-Gervais, S.; Jager, J.; Lemichez, E.; Marty, P.; Boyer, L.; Michel, G. Identification of adipocytes as target cells for Leishmania infantum parasites. Sci. Rep. 2021, 11, 21275. [Google Scholar] [CrossRef]
- Noronha, F.S.M.; Cruz, J.S.; Beirão, P.S.L.; Horta, M.F. Macrophage damage by Leishmania amazonensis cytolysin: Evidence of pore formation on cell membrane. Infect. Immun. 2000, 68, 4578–4584. [Google Scholar] [CrossRef]
- Castro-Gomes, T.; Almeida-Campos, F.R.; Calzavara-Silva, C.E.; da Silva, R.A.; Frézard, F.; Horta, M.F. Membrane binding requirements for the cytolytic activity of Leishmania amazonensis leishporin. FEBS Lett. 2009, 583, 3209–3214. [Google Scholar] [CrossRef]
- Chang, K.P.; Fish, W.R. Leishmania. In In Vitro Cultivation of Protozoan Parasites; Jenson, P., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 11–153. [Google Scholar] [CrossRef]
- Miranda, A.A.; Sarmiento, L.; Caldas, M.L.; Zapata, C.; Bello, F.J. Morphology and cytochemistry of Aedes aegypti’s cell cultures (Diptera: Culicidae) and susceptibility to Leishmania panamensis (Kinetoplastida: Trypanosomatidae). Rev. Biol. Trop. 2008, 56, 447–458. [Google Scholar]
- Zapata Lesmes, A.C.; Cárdenas Castro, E.; Bello, F. Characterization of cell cultures derived from Lutzomyia spinicrassa (Diptera: Psychodidae) and their susceptibility to infection with Leishmania (Viannia) braziliensis. Med. Sci. Monit. 2005, 11, BR457–BR464. [Google Scholar]
- Dedet, J.P.; Gaudin, O.G. Leishmania donovani multiplication in a cell line of Aedes albopictus. Trans. R. Soc. Trop. Med. Hyg. 1976, 70, 535–536. [Google Scholar] [CrossRef]
- Locksley, R.M.; Heinzel, F.P.; Fankhauser, J.E.; Nelson, C.S.; Sadick, M.D. Cutaneous host defense in leishmaniasis: Interaction of isolated dermal macrophages and epidermal Langerhans cells with the insect-stage promastigote. Infect. Immun. 1988, 56, 336–342. [Google Scholar] [CrossRef]
- von Stebut, E.; Belkaid, Y.; Jakob, T.; Sacks, D.L.; Udey, M.C. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin–derived dendritic cells: Implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 1998, 188, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Mbow, M.L.; DeKrey, G.K.; Titus, R.G. Leishmania major induces differential expression of costimulatory molecules on mouse epidermal cells. Eur. J. Immunol. 2001, 31, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Moll, H.; Flohé, S.; Röllinghoff, M. Dendritic cells in Leishmania major-immune mice harbor persistent parasites and mediate an antigen-specific T cell immune response. Eur. J. Immunol. 1995, 25, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.; Fuchs, H.; Rappersberger, K.; Röllinghoff, M.; Moll, H. Parasitism of epidermal Langerhans cells in experimental cutaneous leishmaniasis with Leishmania major. J. Infect. Dis. 1993, 167, 418–425. [Google Scholar] [CrossRef]
- Stenger, S.; Donhauser, N.; Thüring, H.; Röllinghoff, M.; Bogdan, C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J. Exp. Med. 1996, 183, 1501–1514. [Google Scholar] [CrossRef]
- Williams, R.O. Invasion of murine dendritic cells by Leishmania major and L. mexicana mexicana. J. Parasitol. 1988, 74, 186–187. [Google Scholar] [CrossRef]
- Henri, S.; Curtis, J.; Hochrein, H.; Vremec, D.; Shortman, K.; Handman, E. Hierarchy of susceptibility of dendritic cell subsets to infection by Leishmania major: Inverse relationship to interleukin-12 production. Infect. Immun. 2002, 70, 3874–3880. [Google Scholar] [CrossRef]
- Contreras, I.; Estrada, J.A.; Guak, H.; Martel, C.; Borjian, A.; Ralph, B.; Shio, M.T.; Fournier, S.; Krawczyk, C.M.; Olivier, M. Impact of Leishmania mexicana infection on dendritic cell signaling and functions. PLoS Negl. Trop. Dis. 2014, 8, e3202. [Google Scholar] [CrossRef]
- Boggiatto, P.M.; Jie, F.; Ghosh, M.; Gibson-Corley, K.N.; Ramer-Tait, A.E.; Jones, D.E.; Petersen, C.A. Altered dendritic cell phenotype in response to Leishmania amazonensis amastigote infection is mediated by MAP kinase, ERK. Am. J. Pathol. 2009, 174, 1818–1826. [Google Scholar] [CrossRef] [Green Version]
- Prina, E.; Abdi, S.Z.; Lebastard, M.; Perret, E.; Winter, N.; Antoine, J.-C. Dendritic cells as host cells for the promastigote and amastigote stages of Leishmania amazonensis: The role of opsonins in parasite uptake and dendritic cell maturation. J. Cell Sci. 2004, 117, 315–325. [Google Scholar] [CrossRef]
- Xin, L.; Li, K.; Soong, L. Down-regulation of dendritic cell signaling pathways by Leishmania amazonensis amastigotes. Mol. Immunol. 2008, 45, 3371–3382. [Google Scholar] [CrossRef]
- Margaroni, M.; Agallou, M.; Vasilakaki, A.; Karagkouni, D.; Skoufos, G.; Hatzigeorgiou, A.G.; Karagouni, E. Transcriptional profiling of Leishmania infantum infected dendritic cells: Insights into the role of immunometabolism in host-parasite interaction. Microorganisms 2022, 10, 1271. [Google Scholar] [CrossRef] [PubMed]
- Donaghy, L.; Cabillic, F.; Corlu, A.; Rostan, O.; Toutirais, O.; Guguen-Guillouzo, C.; Guiguen, C.; Gangneux, J.P. Immunostimulatory properties of dendritic cells after Leishmania donovani infection using an in vitro model of liver microenvironment. PLoS Negl. Trop. Dis. 2010, 4, e703. [Google Scholar] [CrossRef]
- Naqvi, N.; Ahuja, K.; Selvapandiyan, A.; Dey, R.; Nakhasi, H.; Puri, N. Role of mast cells in clearance of Leishmania through extracellular trap formation. Sci. Rep. 2017, 7, 13240. [Google Scholar] [CrossRef]
- Pearson, R.D.; Uydess, I.L.; Chapman, S.W.; Steigbigel, R.T. Interaction of human eosinophils with Leishmania donovani. Ann. Trop. Med. Parasitol. 1987, 81, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-P. Leishmanicidal mechanisms of human polymorphonuclear phagocytes. Am. J. Trop. Med. Hyg. 1981, 30, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.H.; Fonseca, S.G.; Romão, P.R.; Figueiredo, F.; Ferreira, S.H.; Cunha, F.Q. Microbicidal activity of eosinophils is associated with activation of the arginine-NO pathway. Parasite Immunol. 1998, 20, 405–412. [Google Scholar] [CrossRef]
- Pimenta, P.F.; Dos Santos, M.A.; De Souza, W. Fine structure and cytochemistry of the interaction between Leishmania mexicana amazonensis and rat neutrophils and eosinophils. J. Submicrosc. Cytol. 1987, 19, 387–395. [Google Scholar]
- Grimaldi, G.J.; Soares, M.J.; Moriearty, P.L. Tissue eosinophilia and Leishmania mexicana mexicana eosinophil interactions in murine cutaneous leishmaniasis. Parasite Immunol. 1984, 6, 397–408. [Google Scholar] [CrossRef]
- Lamy, L.; Samso, A.; Lamy, H. Installation, multiplication et entretien d’une souche de Leishmania donovani en culture cellulaire. Bull. Soc. Path. Exot. 1964, 57, 16–21. [Google Scholar]
- Frothingham, T.E.; Lehtimaki, E. Prolonged growth of Leishmania species in cell culture. J. Parasitol. 1969, 55, 196–199. [Google Scholar] [CrossRef]
- Akiyama, H.J.; McQuillen, N.K. Interaction and transformation of Leishmania donovani within in vitro cultured cells: An electron microscopical study. Am. J. Trop. Med. Hyg. 1972, 21, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Lamy, L.H. La transformation réciproque des formes mastigotes et amastigotes de Leishmania et son déterminisme en présence de cellules vivantes in vitro. Ann. Inst. Pasteur. 1969, 117, 545–555. [Google Scholar]
- Lamy, L.H.; Fromentin, H.; Lamy, H. Comparison, perte et récupération du pouvoir infectieux par des Leishmania en l’absence et en présence de cellules vivantes. Protistologica 1971, 7, 435–437. [Google Scholar]
- Mattock, N.M.; Peters, W. The experimental chemotherapy of leishmaniasis. I: Techniques for the study of drug action in tissue culture. Ann. Trop. Med. Parasitol. 1975, 69, 349–357. [Google Scholar] [CrossRef]
- Manna, P.P.; Basu, A.; Saha, A.; Hassan, M.Q.; Mukherjee, S.; Majumdar, S.; Adhya, S.; Bandyopadhyay, S. Leishmania donovani infects lymphocyte cell lines in vitro. Curr. Sci. 1997, 73, 610–614. [Google Scholar]
- Macedo-Silva, R.M.; dos Santos, C.D.P.; Diniz, V.A.; de Carvalho, J.J.; Guerra, C.; Corte-Real, S. Peripheral blood fibrocytes: New information to explain the dynamics of Leishmania infection. Mem. Inst. Oswaldo Cruz 2014, 109, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.H.; Mozos, E.; Méndez, A.; Pérez, J.; Gómez-Villamandos, J.C. Leishmania infection of canine skin fibroblasts in vivo. Vet. Pathol. 1996, 33, 469–473. [Google Scholar] [CrossRef]
- Ferrer, L.; Rabanal, R.M.; Domingo, M.; Ramos, J.A.; Fondevila, D. Identification of Leishmania donovani amastigotes in canine tissues by immunoperoxidase staining. Res. Vet. Sci. 1988, 44, 194–196. [Google Scholar] [CrossRef]
- Dabiri, S.; Hayes, M.M.M.; Meymandi, S.S.; Basiri, M.; Soleimani, F.; Mousavi, M.R.A. Cytologic features of “dry-type” cutaneous leishmaniasis. Diagn. Cytopathol. 1998, 19, 182–185. [Google Scholar] [CrossRef]
- el-Shoura, S.M.; Tallab, T.M.; Bahamdan, K.A. Human cutaneous leishmaniasis: Ultrastructural interactions between the inflammatory cells and Leishman bodies in the skin lesions. Parasite 1996, 3, 229–236. [Google Scholar] [CrossRef]
- el-Shoura, S.M.; Sheikha, A.K.; Bahamdan, K.A.; Tallab, T.M.; Hassounah, O.A. Visceral and cutaneous leishmaniasis comparative ultrastructure of host-parasite interactions. J. Egypt. Soc. Parasitol. 1995, 25, 861–876. [Google Scholar] [PubMed]
- Chang, K.P. Leishmania infection of human skin fibroblasts in vitro: Absence of phagolysosomal fusion after induced phagocytosis of promastigotes, and their intracellular transformation. Am. J. Trop. Med. Hyg. 1978, 27, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, J.D.; Pearson, R.D. The interaction of Leishmania donovani promastigotes and human fibroblasts in vitro. Am. J. Trop. Med. Hyg. 1985, 34, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Yektaeian, N.; Zare, S.; Radfar, A.H.; Hatam, G. Superparamagnetic iron oxide-labeled Leishmania major can be traced in fibroblasts. J. Parasitol. Res. 2023, 2023, 7628912. [Google Scholar] [CrossRef]
- Yektaeian, N.; Mehrabani, D.; Sepaskhah, M.; Zare, S.; Jamhiri, I.; Hatam, G. Lipophilic tracer Dil and fluorescence labeling of acridine orange used for Leishmania major tracing in the fibroblast cells. Heliyon 2019, 5, e03073. [Google Scholar] [CrossRef]
- de Oliveira Cardoso, F.; da Silva Freitas de Souza, C.; Gonçalves Mendes, V.; Abreu-Silva, A.L.; da Costa, S.C.G.; da Silva Calabrese, K. Immunopathological studies of Leishmania amazonensis infection in resistant and in susceptible mice. J. Infect. Dis. 2010, 201, 1933–1940. [Google Scholar] [CrossRef]
- Hespanhol, R.C.; Soeiro, M.d.N.C.; Meuser, M.B.; Meirelles, M.d.N.S.L.; Côrte-Real, S. The expression of mannose receptors in skin fibroblast and their involvement in Leishmania (L.) amazonensis invasion. J. Histochem. Cytochem. 2005, 53, 35–44. [Google Scholar] [CrossRef]
- Daneshbod, Y.; Daneshbod, K.; Khademi, B.; Negahban, S.; Bedayat, G.R. New cytologic clues in localized Leishmania lymphadenitis. Acta Cytol. 2007, 51, 699–710. [Google Scholar] [CrossRef]
- Haldar, A.K.; Nigam, U.; Yamamoto, M.; Coers, J.; Goyal, N. Guanylate binding proteins restrict Leishmania donovani growth in nonphagocytic cells independent of parasitophorous vacuolar targeting. MBio 2020, 11, e01464-20. [Google Scholar] [CrossRef]
- Hallé, M.; Gomez, M.A.; Stuible, M.; Shimizu, H.; McMaster, W.R.; Olivier, M.; Tremblay, M.L. The Leishmania surface protease GP63 cleaves multiple intracellular proteins and actively participates in p38 mitogen-activated protein kinase inactivation. J. Biol. Chem. 2009, 284, 6893–6908. [Google Scholar] [CrossRef] [PubMed]
- Pai, H.C.; Hu, C.H. Attempts to grow Leishmania donovani in tissue cultures. Proc. Soc. Exp. Biol. Med. 1941, 46, 606–608. [Google Scholar] [CrossRef]
- Vasconcellos, C.; Sotto, M.N. Experimental cutaneous leishmaniasis: Transmission electron microscopy of the inoculation site. Int. J. Exp. Pathol. 1997, 78, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ara, M.; Maillo, C.; Peon, G.; Clavel, A.; Cuesta, J.; Grasa, M.P.; Carapeto, F.J. Visceral leishmaniasis with cutaneous lesions in a patient infected with human immunodeficiency virus. Br. J. Dermatol. 1998, 139, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Perrin, C.; Taillan, B.; Hofman, P.; Mondain, V.; Lefichoux, Y.; Michiels, J.F. Atypical cutaneous histological features of visceral leishmaniasis in acquired immunodeficiency syndrome. Am. J. Dermatopathol. 1995, 17, 145–150. [Google Scholar] [CrossRef]
- Calabrese, K.D.; Silva, L.D.; Carvalho, L.O.P.; Hardoim, D.D.; da Silva-Almeida, M.; Mortara, R.A.; de Souza, C.D.F. Infection of retinal epithelial cells with L. amazonensis impacts in extracellular matrix proteins. Parasitol. Res. 2011, 109, 727–736. [Google Scholar] [CrossRef]
- Frothingham, T.E.; Lehtimaki, E. Leishmania in primary cultures of human amniotic cells. Am. J. Trop. Med. Hyg. 1967, 16, 658–664. [Google Scholar] [CrossRef]
- Belle, E.A. Cultivation of Leishmania donovani in human amnion epithelial cell tissue cultures: A preliminary report. Can. Med. Assoc. J. 1958, 79, 726–728. [Google Scholar]
- Degtiareva, S.M.; Zasukhin, D.N. Cultivation of the causative agent of cutaneous leishmaniasis of the desert type in tissue culture. Med. Parazitol(Mosk). 1959, 28, 706–710. [Google Scholar]
- Miller, H.C. Invasion of Cultured Cells by Leptomonads of Leishmania donovani. Master’s Thesis, Michigan State University of Agriculture and Applied Science, East Lansing, MI, USA, 1966. [Google Scholar]
- Herman, R. Acriflavin-induced dyskinetoplastic Leishmania donovani grown in monkey kidney cell culture. J. Protozool. 1968, 15, 35–44. [Google Scholar] [CrossRef]
- Pessotti, J.H.; Zaverucha Do Valle, T.; Corte-Real, S.; Gonçalves Da Costa, S.C. Interaction of Leishmania (L.) chagasi with the Vero cell line. Parasite 2004, 11, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Walton, B.C.; Brooks, W.H.; Arjona, I. Serodiagnosis of American leishmaniasis by indirect fluorescent antibody test. Am. J. Trop. Med. Hyg. 1972, 21, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Veras, P.S.; Moulia, C.; Dauguet, C.; Tunis, C.T.; Thibon, M.; Rabinovitch, M. Entry and survival of Leishmania amazonensis amastigotes within phagolysosome-like vacuoles that shelter Coxiella burnetii in Chinese hamster ovary cells. Infect. Immun. 1995, 63, 3502–3506. [Google Scholar] [CrossRef] [PubMed]
- Veras, P.S.; de Chastellier, C.; Moreau, M.F.; Villiers, V.; Thibon, M.; Mattei, D.; Rabinovitch, M. Fusion between large phagocytic vesicles: Targeting of yeast and other particulates to phagolysosomes that shelter the bacterium Coxiella burnetii or the protozoan Leishmania amazonensis in Chinese hamster ovary cells. J. Cell Sci. 1994, 107, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.S.; Daifalla, N.; Das, B.; Dias da Silva, V.; Campos-Neto, A. CD271+ mesenchymal stem cells as a possible infectious niche for Leishmania infantum. PLoS ONE 2016, 11, e0162927. [Google Scholar] [CrossRef]
- Vamvakidis, C.D.; Koutinas, A.E.; Saridomichelakis, M.; Kanakoudis, G.; Georgiadis, G. Masticatory and skeletal muscle myositis in canine leishmaniasis (Leishmania infantum). Vet. Rec. 2000, 146, 698–703. [Google Scholar] [CrossRef]
- Naranjo, C.; Fondevila, D.; Leiva, M.; Roura, X.; Peña, T. Detection of Leishmania spp. and associated inflammation in ocular-associated smooth and striated muscles in dogs with patent leishmaniosis. Vet. Ophthalmol. 2010, 13, 139–143. [Google Scholar] [CrossRef]
- Silva-Almeida, M.; Carvalho, L.O.P.; Abreu-Silva, A.L.; d’Escoffier, L.N.; Calabrese, K.S. Leishmania (Leishmania) amazonensis infection: Muscular involvement in BALB/c and C3H.HeN mice. Exp. Parasitol. 2010, 124, 315–318. [Google Scholar] [CrossRef]
- Jarallah, H.M. Pathological effects of Leishmania donovani promastigotes on liver and spleen of experimentally infected BALB/c mice. Med. J. Baby. 2016, 13, 134–140. [Google Scholar]
- Piekarski, G. Protozoen. In Medizinische Parasitologie in Tafeln; Springer: Berlin, Heidelberg, 1987; pp. 5–115. [Google Scholar]
- Adler, S. A Note on the histopathology of a case of experimental cutaneous leishmaniasis. Ann. Trop. Med. Parasitol. 1926, 20, 407–410. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Parapini, S.; Corbett, Y.; Frigerio, R.; Delbue, S.; Modenese, A.; Gramiccia, M.; Ferrante, P.; Taramelli, D.; Basilico, N. Leishmania promastigotes enhance neutrophil recruitment through the production of CXCL8 by endothelial cells. Pathogens 2021, 10, 1380. [Google Scholar] [CrossRef] [PubMed]
- Scorza, B.M.; Wacker, M.A.; Messingham, K.; Kim, P.; Klingelhutz, A.; Fairley, J.; Wilson, M.E. Differential activation of human keratinocytes by Leishmania species causing localized or disseminated disease. J. Investig. Dermatol. 2017, 137, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Lelijveld, J.; Atanasiu, P. Multiplication de Leishmania brasiliensis sur culture cellulaire de rein de hamster. Ann. Inst. Pasteur (Paris) 1966, 110, 788–791. [Google Scholar] [PubMed]
- Lupaşco, G.; Bossie, A.; Dincoulesco, M.; Epurean, E.; Profeta, A. Cultivation and cytopathogenic activity of L. donovani in tissue cultures. Arch. Roum. Pathol. Exp. Microbiol. 1968, 27, 641–650. [Google Scholar] [PubMed]
- Trindade, S.; Rijo-Ferreira, F.; Carvalho, T.; Pinto-Neves, D.; Guegan, F.; Aresta-Branco, F.; Bento, F.; Young, S.A.; Pinto, A.; Van Den Abbeele, J.; et al. Trypanosoma brucei parasites occupy and functionally adapt to the adipose tissue in mice. Cell Host Microbe 2016, 19, 837–848. [Google Scholar] [CrossRef]
- Ferreira, A.V.; Segatto, M.; Menezes, Z.; Macedo, A.M.; Gelape, C.; de Oliveira Andrade, L.; Nagajyothi, F.; Scherer, P.E.; Teixeira, M.M.; Tanowitz, H.B. Evidence for Trypanosoma cruzi in adipose tissue in human chronic Chagas disease. Microbes Infect. 2011, 13, 1002–1005. [Google Scholar] [CrossRef]
- Costales, J.A.; Daily, J.P.; Burleigh, B.A. Cytokine-dependent and-independent gene expression changes and cell cycle block revealed in Trypanosoma cruzi-infected host cells by comparative mRNA profiling. BMC Genom. 2009, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Perry, H.M. Some Observations on the occurrence of Leishmania in the intestinal tissues in Indian kala-azar; on the pathological changes occasioned by their presence, and on their possible significance in this situation. Proc. R. Soc. Med. 1923, 16, 1–8. [Google Scholar] [CrossRef]
- Lugo-Yarbuh, A.; Valera, M.; Alarcón, M.; Moreno, E.; Premoli-Percoco, G.; Colasante, C. Detection of Leishmania (Viannia) braziliensis in vascular endothelium lesions of patients with localized cutaneous leishmaniasis. Investig. Clin. 2003, 44, 61–76. [Google Scholar]
- dos Santos, I.B.; Tortelly, R.; Quintella, L.P.; de Fátima Madeira, M.; Monteiro de Miranda, L.H.; Borges Figueiredo, F.; Carvalhaes de Oliveira Rde, V.; Pacheco Schubach, T.M. Higher sensitivity of immunohistochemistry for bona fide diagnosis of dog Leishmania (Viannia) braziliensis-driven American tegumentary leishmaniasis: Description of an optimized immunohistochemistry method. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 469–476. [Google Scholar] [CrossRef]
- Caparrós, E.; Serrano, D.; Puig-Kröger, A.; Riol, L.; Lasala, F.; Martinez, I.; Vidal-Vanaclocha, F.; Delgado, R.; Rodríguez-Fernández, J.L.; Rivas, L.; et al. Role of the C-type lectins DC-SIGN and L-SIGN in Leishmania interaction with host phagocytes. Immunobiology 2005, 210, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, A.M.; Gaafar, A.; Theander, T.G. Antigen-presenting cells in human cutaneous leishmaniasis due to Leishmania major. Clin. Exp. Immunol. 2008, 99, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, G.; Bird, J.T.; Washam, C.L.; Roys, H.; Bowlin, A.; Byrum, S.D.; Weinkopff, T. In vivo transcriptional analysis of mice infected with Leishmania major unveils cellular heterogeneity and altered transcriptomic profiling at single-cell resolution. PLoS Negl. Trop. Dis. 2022, 16, e0010518. [Google Scholar] [CrossRef]
- Mandell, M.A.; Beverley, S.M. Continual renewal and replication of persistent Leishmania major parasites in concomitantly immune hosts. Proc. Natl. Acad. Sci. USA 2017, 114, E801–E810. [Google Scholar] [CrossRef] [PubMed]
- Dostálová, A.; Volf, P. Leishmania development in sand flies: Parasite-vector interactions overview. Parasit. Vectors 2012, 5, 276. [Google Scholar] [CrossRef]
- Cecílio, P.; Cordeiro-da-Silva, A.; Oliveira, F. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 2022, 5, 305. [Google Scholar] [CrossRef] [PubMed]
- Robles-Loaiza, A.A.; Pinos-Tamayo, E.A.; Mendes, B.; Teixeira, C.; Alves, C.; Gomes, P.; Almeida, J.R. Peptides to tackle leishmaniasis: Current status and future directions. Int. J. Mol. Sci. 2021, 22, 4400. [Google Scholar] [CrossRef]
- Arumugam, S.; Scorza, B.M.; Petersen, C. Visceral leishmaniasis and the skin: Dermal parasite transmission to sand flies. Pathogens 2022, 11, 610. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valigurová, A.; Kolářová, I. Unrevealing the Mystery of Latent Leishmaniasis: What Cells Can Host Leishmania? Pathogens 2023, 12, 246. https://doi.org/10.3390/pathogens12020246
Valigurová A, Kolářová I. Unrevealing the Mystery of Latent Leishmaniasis: What Cells Can Host Leishmania? Pathogens. 2023; 12(2):246. https://doi.org/10.3390/pathogens12020246
Chicago/Turabian StyleValigurová, Andrea, and Iva Kolářová. 2023. "Unrevealing the Mystery of Latent Leishmaniasis: What Cells Can Host Leishmania?" Pathogens 12, no. 2: 246. https://doi.org/10.3390/pathogens12020246
APA StyleValigurová, A., & Kolářová, I. (2023). Unrevealing the Mystery of Latent Leishmaniasis: What Cells Can Host Leishmania? Pathogens, 12(2), 246. https://doi.org/10.3390/pathogens12020246






