Borrelia miyamotoi: A Comprehensive Review
Abstract
:1. Introduction
2. Background
3. Disease
4. Diagnosis
4.1. Microscopy
4.2. PCR
4.3. Serodiagnosis
5. Human Cases and Infection Prevalence
6. Ticks
| Country | Tick |
|---|---|
| Austria | I. ricinus [74] |
| Belarus | I. ricinus [75] |
| Belgium | I. ricinus [76,77] |
| Canada | I. scapularis [78] |
| China | I. persulcatus [79], H. longicornis [57], H. concinna [58] |
| Czechia | I. ricinus [80] |
| Denmark | I. ricinus [81] |
| Estonia | I. ricinus, I. persulcatus [82] |
| Finland | I. ricinus [83,84,85] |
| France | I. ricinus [81,86,87] |
| Germany | I. ricinus [76,88] |
| Ireland | I. ricinus [89] |
| Italy | I. ricinus [76] |
| Japan | I. persulcatus [4], I. ovatus [90] |
| Moldova | I. ricinus [91] |
| Mongolia | I. persulcatus [90,92] |
| Netherlands | I. ricinus [76,81,93,94] |
| Norway | I. ricinus [95] |
| Poland | I. ricinus [96,97] |
| Portugal | I. ricinus [98] |
| Russia | I. persulcatus [99] |
| Serbia | I. ricinus [100] |
| Slovakia | I. ricinus [59,101], H. inermis [59] |
| South Korea | I. nipponensis [55] |
| Spain | I. ricinus [102] |
| Sweden | I. ricinus [67,76] |
| Switzerland | I. ricinus [103] |
| Turkey | I. ricinus [104] |
| Ukraine | I. ricinus [105] |
| United Kingdom | I. ricinus [76] |
| United States | I. pacificus [106], I. scapularis [107] |
| Questing Tick Prevalence | B. miyamotoi Prevalence | |
|---|---|---|
| I. scapularis | 28.0% | 1.1% |
| I. pacificus | 14.8% | 0.7% |
| I. ricinus | 52.2% | 1.0% |
| I. persulcatus | 5.0% | 2.8% |
7. Animal Infections
| % Positive | Countries | |
|---|---|---|
| Small Mammals | ||
| Striped field mouse (Apodemus agrarius) | 3.13% (1/32); 13.2% (7/53); 7.0% (11/157) | Austria [120], Croatia [121], Poland [112] |
| Small Japanese field mouse (Apodemus argenteus) | 0.7% (1/137) | Japan [118] |
| Yellow-necked mouse (Apodemus flavicollis) * | 0.7% (1/131); 0.9% (1/102) a & 1.5% (1/67) b; 3.6% (3/84); 2.0% (1/49); 0.4% (1/251) c & 2.2% (1/46) d | Croatia [121], Hungary [111], Poland [112], Romania [122], Slovenia [123] |
| Large Japanese field mouse (Apodemus speciosus) | 2.2% (10/446) | Japan [118] |
| Wood mouse (Apodemus sylvaticus) | 14.3% (3/21) | Netherlands [94] |
| European hedgehog (Erinaceus europaeus) | 5.0% (3/60) | Czechia [124] |
| Common vole (Microtus arvalis) | 12.5% (1/8) | Netherlands [94], Slovakia [59] |
| Meadow vole (Microtus pennsylvanicus) | 0.7% (1/146) | Canada [113] |
| Bank vole (Myodes glareolus) * | 5.5% (4/72); 8.8% (3/34); 3.1% (1/32) | France [86], Netherlands [94], Romania [122], Switzerland [103] |
| Grey red-backed vole (Myodes rufocanus) | 1.0% (2/195) | Japan [118] |
| Jumping mouse (Napaeozapus insignis) | 14.3% (3/21) | Canada [113] |
| Dusky-footed woodrat (Neotoma fuscipes) | 16.7% (1/6) | United States [125] |
| Brush mouse (Peromyscus boylii) | 2.8% (2/71) | United States [125] |
| California mouse (Peromyscus californicus) | 16.7% (4/24) | United States [125] |
| White-footed mouse (Peromyscus leucopus) * | 6.4% (36/556) d & 2.3% (2/86) e; 0.5% (3/625) | United States [69], Canada [126] |
| Deer mouse (Peromyscus maniculatus) | 2.9% (1/34) | Canada [113] |
| Eastern grey squirrel (Sciurus carolinensis) | 25.0% (1/4) | Canada [113] |
| Red squirrel (Sciurus vulgaris) | 13.6% (3/22) | Czechia [124], Hungary [127] |
| Common Shrew (Sorex araneus) | 16.7% (1/6) | Croatia [121] |
| Muller’s giant Sunda rat (Sundamys muelleri) | 33.3% (1/3) | Malaysia [128] |
|
Eastern chipmunk (Tamias striatus) | 15.4% (2/13) | Canada [126] |
| Large mammals | ||
| Père David Deer (Elaphurus davidianus) | 2.3% (1/43) | China [57] |
| Birds | ||
| European greenfinch (Carduelis chloris) | 25% (1/4) | Netherlands [94] |
| Wild turkey (Meleagris gallopavo) | 56.0% (35/60) | United States [109] |
| Great tits (Parus major) | 50% (1/2) | Netherlands [94] |
| Ostrich (Struthio camelus) | 16.7% (1/6) | Czechia [110] |
| Tick | Countries | |
|---|---|---|
| Mid-Sized Mammals | ||
| Beech marten (Martes foina) | I. ricinus | Belgium, Netherlands [129] |
| European pine marten (Martes martes) | I. ricinus | Belgium, Netherlands [129] |
| European polecat (Mustela putorius) | I. ricinus | Belgium, Netherlands [129] |
| Large Mammals | ||
| Cattle (Bos primigenius tarus) | I. ricinus | Germany [130] |
| Dog (Canis lupus familiaris) | I. ovatus, I. hexagonus, I. ricinus, I. persulcatus, Dermacentor reticulatus | Germany [131,132], Japan [133], Latvia [134], Russia [56] |
| Goat (Capra aegagrus hircus) | I. ricinus | Germany [130] |
| Roe deer (Capreolus capreolus) | I. ricinus | Germany [132], Poland [135], Spain [136] |
| Red deer (Cervus elaphus) | I. ricinus | Poland [137] |
| Raccoon dog (Nyctereutes procyonoides) | I. ricinus | Denmark [138] |
| White-tailed deer (Odocoileus virginianus) | I. scapularis | United States [114,115,139] |
| Wild boar (Sus scrofa) | I. ricinus | Poland [136] |
| Birds | ||
| Northern cardinal (Cardinalis cardinalis) | I. dentatus | United States [140] |
| Veery (Catharus fuscescens) | I. scapularis | Canada [126] |
| Hermit Thrush (Catharus guttatus) | I. scapularis, I. dentatus | Canada [126], United States [140] |
| European robin (Erithacus rubecula) | I. ricinus | Netherlands [93], Sweden [67] |
| Song Sparrow (Melospiza melodia) | I. scapularis | Canada [126] |
| Common redstart (Phoenicurus phoeincurus) | I. ricinus | Sweden [67] |
| Common chiffchaff (Phylloscopus collybita) | I. ricinus | Netherlands [93] |
| Eurasian wren (Troglodytes troglodytes) | I. ricinus | Sweden [67] |
| Common blackbird (Turdus merula) | I. ricinus | Moldova [91], Netherlands [93], Poland [135] |
| American robin (Turdus migratorius) | I. dentatus | United States [140] |
| Song thrush (Turdus philomelos) | I. ricinus | Netherlands [93] |
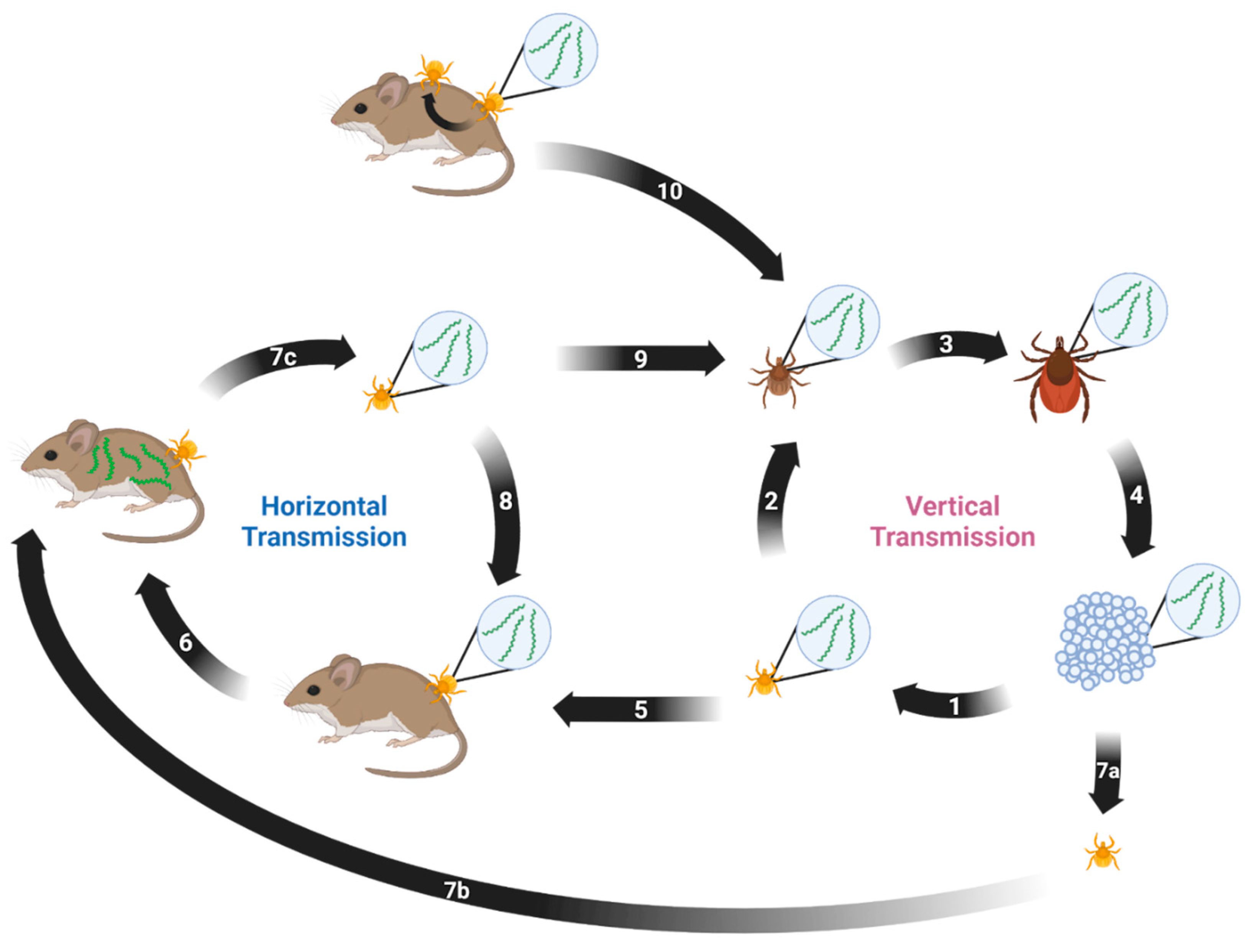
8. Transmission
9. Phylogenetics
10. Laboratory Studies on B. miyamotoi
10.1. Culturing
10.2. Genomics and Pathogenesis
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Charon, N.W.; Goldstein, S.F. Genetics of Motility and Chemotaxis of a Fascinating Group of Bacteria: The Spirochetes. Annu. Rev. Genet. 2002, 36, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme Borreliosis. Nat. Rev. Dis. Prim. 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Hovius, J.W.; Bergström, S. Pathogenesis of Relapsing Fever. Curr. Issues Mol. Biol. 2021, 42, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Takahashi, Y.; Tsuruta, Y.; Matsushita, O.; Ralph, D.; McClelland, M.; Nakao, M. Genetic and Phenotypic Analysis of Borrelia Miyamotoi Sp. Nov., Isolated from the Ixodid Tick Ixodes Persulcatus, the Vector for Lyme Disease in Japan. Int. J. Syst. Bacteriol. 1995, 45, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Bratton, R.L.; Whiteside, J.W.; Hovan, M.J.; Engle, R.L.; Edwards, F.D. Diagnosis and Treatment of Lyme Disease. Mayo Clin. Proc. 2008, 83, 566–571. [Google Scholar] [CrossRef]
- Halperin, J.J. Nervous System Lyme Disease. Curr. Infect. Dis. Rep. 2015, 17, 445. [Google Scholar] [CrossRef]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. Erratum: The Clinical Assessment, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America (Clinical Infectious Diseases (2006) 43, (1089–1134)). Clin. Infect. Dis. 2007, 45, 941. [Google Scholar] [CrossRef]
- Marques, A. Lyme Neuroborreliosis. Contin. (Minneap Minn) 2015, 21, 1729–1744. [Google Scholar] [CrossRef]
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V.V.; Fish, D.; Krause, P.J. Humans Infected with Relapsing Fever Spirochete Borrelia Miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816–1823. [Google Scholar] [CrossRef]
- NIAID Emerging Infectious Diseases/Pathogens. Available online: https://www.niaid.nih.gov/research/emerging-infectious-diseases-pathogens (accessed on 9 January 2023).
- Krause, P.J.; Fish, D.; Narasimhan, S.; Barbour, A.G. Borrelia Miyamotoi Infection in Nature and in Humans. Clin. Microbiol. Infect. 2015, 21, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Delaney, S.L.; Murray, L.A.; Aasen, C.E.; Bennett, C.E.; Brown, E.; Fallon, B.A. Borrelia Miyamotoi Serology in a Clinical Population with Persistent Symptoms and Suspected Tick-Borne Illness. Front. Med. 2020, 7, 567350. [Google Scholar] [CrossRef]
- Molloy, P.J.; Telford, S.R.; Chowdri, H.R.; Lepore, T.J.; Gugliotta, J.L.; Weeks, K.E.; Hewins, M.E.; Goethert, H.K.; Berardi, V.P. Borrelia Miyamotoi Disease in the Northeastern United States a Case Series. Ann. Intern. Med. 2015, 163, 91–98. [Google Scholar] [CrossRef]
- Barbour, A.G. Multiple and Diverse vsp and Vlp Sequences in Borrelia Miyamotoi, a Hard Tick-Borne Zoonotic Pathogen. PLoS ONE 2016, 11, e0146283. [Google Scholar] [CrossRef]
- Wagemakers, A.; Koetsveld, J.; Narasimhan, S.; Wickel, M.; Deponte, K.; Bleijlevens, B.; Jahfari, S.; Sprong, H.; Karan, L.S.; Sarksyan, D.S.; et al. Variable Major Proteins as Targets for Specific Antibodies against Borrelia Miyamotoi. J. Immunol. 2016, 196, 4185–4195. [Google Scholar] [CrossRef]
- Crowder, C.D.; Langeroudi, A.G.; Estabragh, A.S.; Lewis, E.R.G.; Marcsisin, R.A.; Barbour, A.G. Pathogen and Host Response Dynamics in a Mouse Model of Borrelia Hermsii Relapsing Fever. Vet. Sci. 2016, 3, 19. [Google Scholar] [CrossRef]
- Barbour, A.G.; Hayes, S.F. Biology of Borrelia Species. Microbiol. Rev. 1986, 50, 381–400. [Google Scholar] [CrossRef]
- Mason, L.M.K.; Koetsveld, J.; Trentelman, J.J.A.; Kaptein, T.M.; Hoornstra, D.; Wagemakers, A.; Fikrig, M.M.; Ersoz, J.I.; Oei, A.; Geijtenbeek, T.B.H.; et al. Borrelia Miyamotoi Activates Human Dendritic Cells and Elicits T Cell Responses. J. Immunol. 2020, 204, 386–393. [Google Scholar] [CrossRef]
- Lynn, G.E.; Breuner, N.E.; Eisen, L.; Hojgaard, A.; Replogle, A.J.; Eisen, R.J. An Immunocompromised Mouse Model to Infect Ixodes Scapularis Ticks with the Relapsing Fever Spirochete, Borrelia Miyamotoi. Ticks Tick. Borne Dis. 2019, 10, 352–359. [Google Scholar] [CrossRef]
- Gandhi, S.; Narasimhan, S.; Workineh, A.; Mamula, M.; Yoon, J.; Krause, P.J.; Farhadian, S.F. Borrelia Miyamotoi Meningoencephalitis in an Immunocompetent Patient. Open Forum Infect. Dis. 2022, 9, ofac295. [Google Scholar] [CrossRef]
- Hovius, J.W.R.; De Wever, B.; Sohne, M.; Brouwer, M.C.; Coumou, J.; Wagemakers, A.; Oei, A.; Knol, H.; Narasimhan, S.; Hodiamont, C.J.; et al. A Case of Meningoencephalitis by the Relapsing Fever Spirochaete Borrelia Miyamotoi in Europe. Lancet 2013, 382, 658. [Google Scholar] [CrossRef] [Green Version]
- Gugliotta, J.L.; Goethert, H.K.; Berardi, V.P.; Telford, S.R. Meningoencephalitis from Borrelia Miyamotoi in an Immunocompromised Patient. N. Engl. J. Med. 2013, 368, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Boden, K.; Lobenstein, S.; Hermann, B.; Margos, G.; Fingerle, V. Borrelia Miyamotoi-Associated Neuroborreliosis in Immunocompromised Person. Emerg. Infect. Dis. 2016, 22, 1617–1620. [Google Scholar] [CrossRef] [PubMed]
- Henningsson, A.J.; Asgeirsson, H.; Hammas, B.; Karlsson, E.; Parke, Å.; Hoornstra, D.; Wilhelmsson, P.; Hovius, J.W. Two Cases Of Borrelia miyamotoi Meningitis, Sweden, 2018. Emerg. Infect. Dis. 2019, 25, 2017–2020. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, S.S.; Ard, K.L.; Schaefer, P.W.; Branda, J.A. Case 32-2020: A 63-Year-Old Man with Confusion, Fatigue, and Garbled Speech. N. Engl. J. Med. 2020, 383, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Hoornstra, D.; Azagi, T.; van Eck, J.A.; Wagemakers, A.; Koetsveld, J.; Spijker, R.; Platonov, A.E.; Sprong, H.; Hovius, J.W. Prevalence and Clinical Manifestation of Borrelia Miyamotoi in Ixodes Ticks and Humans in the Northern Hemisphere: A Systematic Review and Meta-Analysis. Lancet Microbe 2022, 3, e772–e786. [Google Scholar] [CrossRef] [PubMed]
- Koetsveld, J.; Manger, A.; Hoornstra, D.; Draga, R.O.; Oei, A.; Kolyasnikova, N.M.; Toporkova, M.G.; Sarksyan, D.S.; Wagemakers, A.; Platonov, A.E.; et al. In Vitro Antimicrobial Susceptibility of Clinical Isolates of Borrelia Miyamotoi. Antimicrob. Agents Chemother. 2018, 62, e00419–e00518. [Google Scholar] [CrossRef]
- Rodino, K.G.; Theel, E.S.; Pritt, B.S. Tick-Borne Diseases in the United States. Clin. Chem. 2020, 66, 537–548. [Google Scholar] [CrossRef]
- Telford, S.R.; Goethert, H.K.; Molloy, P.J.; Berardi, V. Blood Smears Have Poor Sensitivity for Confirming Borrelia Miyamotoi Disease. J. Clin. Microbiol. 2019, 57, e01468–e01518. [Google Scholar] [CrossRef]
- Karan, L.; Makenov, M.; Kolyasnikova, N.; Stukolova, O.; Toporkova, M.; Olenkova, O. Dynamics of Spirochetemia and Early PCR Detection of Borrelia Miyamotoi. Emerg. Infect. Dis. 2018, 24, 860–867. [Google Scholar] [CrossRef]
- Replogle, A.J.; Sexton, C.; Young, J.; Kingry, L.C.; Schriefer, M.E.; Dolan, M.; Johnson, T.L.; Connally, N.P.; Padgett, K.A.; Petersen, J.M. Isolation of Borrelia Miyamotoi and Other Borreliae Using a Modified BSK Medium. Sci. Rep. 2021, 11, 1926. [Google Scholar] [CrossRef]
- Sage, K.M.; Johnson, T.L.; Teglas, M.B.; Nieto, N.C.; Schwan, T.G. Ecological Niche Modeling and Distribution of Ornithodoros Hermsi Associated with Tick-Borne Relapsing Fever in Western North America. PLoS Negl. Trop. Dis. 2017, 11, e0006047. [Google Scholar] [CrossRef]
- Xu, G.; Luo, C.-Y.; Ribbe, F.; Pearson, P.; Ledizet, M.; Rich, S.M. Borrelia Miyamotoi in Human-Biting Ticks, United States, 2013–2019. Emerg. Infect. Dis. 2021, 27, 3193–3195. [Google Scholar] [CrossRef]
- Dibernardo, A.; Cote, T.; Ogden, N.H.; Lindsay, L.R. The Prevalence of Borrelia Miyamotoi Infection, and Co-Infections with Other Borrelia Spp. in Ixodes Scapularis Ticks Collected in Canada. Parasites Vectors 2014, 7, 183. [Google Scholar] [CrossRef]
- Dietrich, E.A.; Replogle, A.J.; Sheldon, S.W.; Petersen, J.M. Simultaneous Detection and Differentiation of Clinically Relevant Relapsing Fever Borrelia with Semimultiplex Real-Time PCR. J. Clin. Microbiol. 2021, 59, e0298120. [Google Scholar] [CrossRef]
- Aguero-Rosenfeld, M.E.; Nowakowski, J.; Bittker, S.; Cooper, D.; Nadelman, R.B.; Wormser, G.P. Evolution of the Serologic Response to Borrelia Burgdorferi in Treated Patients with Culture-Confirmed Erythema Migrans. J. Clin. Microbiol. 1996, 34, 1–9. [Google Scholar] [CrossRef]
- Koetsveld, J.; Kolyasnikova, N.M.; Wagemakers, A.; Stukolova, O.A.; Hoornstra, D.; Sarksyan, D.S.; Toporkova, M.G.; Henningsson, A.J.; Hvidsten, D.; Ang, W.; et al. Serodiagnosis of Borrelia Miyamotoi Disease by Measuring Antibodies against GlpQ and Variable Major Proteins. Clin. Microbiol. Infect. 2018, 24, 1338.e1–1338.e7. [Google Scholar] [CrossRef]
- Landry, M.L. Immunoglobulin M for Acute Infection: True or False? Clin. Vaccine Immunol. 2016, 23, 540–545. [Google Scholar] [CrossRef]
- Schriefer, M.E. Lyme Disease Diagnosis: Serology. Clin. Lab. Med. 2015, 35, 797–814. [Google Scholar] [CrossRef]
- Molloy, P.J.; Weeks, K.E.; Todd, B.; Wormser, G.P. Seroreactivity to the C6 Peptide in Borrelia Miyamotoi Infections Occurring in the Northeastern United States. Clin. Infect. Dis. 2018, 66, 1407–1410. [Google Scholar] [CrossRef]
- Reiter, M.; Stelzer, T.; Schötta, A.M.; Markowicz, M.; Leschnik, M.; Harsch, A.; Reiß, E.; Kneusel, R.E.; Stockinger, H.; Stanek, G. Glycerophosphodiester Phosphodiesterase Identified as Non-Reliable Serological Marker for Borrelia Miyamotoi Disease. Microorganisms 2020, 8, 1846. [Google Scholar] [CrossRef]
- Mayer, C.; Kluj, R.M.; Mühleck, M.; Walter, A.; Unsleber, S.; Hottmann, I.; Borisova, M. Bacteria’s Different Ways to Recycle Their Own Cell Wall. Int. J. Med. Microbiol. 2019, 309, 151326. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Narasimhan, S.; Wormser, G.P.; Rollend, L.; Fikrig, E.; Lepore, T.; Barbour, A.; Fish, D. Human Borrelia Miyamotoi Infection in the United States. N. Engl. J. Med. 2013, 368, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Narasimhan, S.; Wormser, G.P.; Barbour, A.G.; Platonov, A.E.; Brancato, J.; Lepore, T.; Dardick, K.; Mamula, M.; Rollend, L.; et al. Borrelia Miyamotoi Sensu Lato Seroreactivity and Seroprevalence in the Northeastern United States. Emerg. Infect. Dis. 2014, 20, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.R.; Hardham, J.M.; Barbour, A.G.; Norris, S.J. Antigenic Variation in Lyme Disease Borreliae by Promiscuous Recombination of VMP-like Sequence Cassettes. Cell 1997, 89, 275–285. [Google Scholar] [CrossRef]
- Liang, F.; Alvarez, A.; Gu, Y.; Nowling, J.; Ramamoorthi, R.; Philipp, M. An Immunodominant Conserved Region within the Variable Domain of VlsE, the Variable Surface Antigen of Borrelia Burgdorferi. J. Immunol. 1999, 163, 5566–5573. [Google Scholar] [CrossRef]
- Harris, E.K.; Brandt, K.S.; Van Gundy, T.J.; Goodrich, I.; Wormser, G.P.; Armstrong, B.A.; Gilmore, R.D. Characterization of a Borrelia Miyamotoi Membrane Antigen (BmaA) for Serodiagnosis of Borrelia Miyamotoi Disease. Ticks Tick Borne Dis. 2020, 11, 101476. [Google Scholar] [CrossRef]
- Tokarz, R.; Tagliafierro, T.; Caciula, A.; Mishra, N.; Thakkar, R.; Chauhan, L.V.; Sameroff, S.; Delaney, S.; Wormser, G.P.; Marques, A.; et al. Identification of Immunoreactive Linear Epitopes of Borrelia Miyamotoi. Ticks Tick Borne Dis. 2020, 11, 101314. [Google Scholar] [CrossRef]
- Koetsveld, J.; Platonov, A.E.; Kuleshov, K.; Wagemakers, A.; Hoornstra, D.; Ang, W.; Szekeres, S.; van Duijvendijk, G.L.A.; Fikrig, E.; Embers, M.E.; et al. Borrelia Miyamotoi Infection Leads to Cross-Reactive Antibodies to the C6 Peptide in Mice and Men. Clin. Microbiol. Infect. 2020, 26, 513.e1–513.e6. [Google Scholar] [CrossRef]
- Scoles, G.A.; Papero, M.; Beati, L.; Fish, D. A Relapsing Fever Group Spirochete Transmitted by Ixodes Scapularis Ticks. Vector Borne Zoonotic Dis. 2001, 1, 21–34. [Google Scholar] [CrossRef]
- Franck, M.; Ghozzi, R.; Pajaud, J.; Lawson-Hogban, N.E.; Mas, M.; Lacout, A.; Perronne, C. Borrelia Miyamotoi: 43 Cases Diagnosed in France by Real-Time PCR in Patients with Persistent Polymorphic Signs and Symptoms. Front. Med. 2020, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Franck, M.; Ghozzi, R.; Pajaud, J.; Lawson-Hogban, N.E.; Mas, M.; Lacout, A.; Perronne, C. Response: Commentary: Borrelia Miyamotoi: 43 Cases Diagnosed in France by Real-Time PCR in Patients with Persistent Polymorphic Signs and Symptoms. Front. Med. 2020, 7, 586694. [Google Scholar] [CrossRef]
- Wagemakers, A.; Sprong, H.; Platonov, A.; Hovius, J.W. Commentary: Borrelia Miyamotoi: 43 Cases Diagnosed in France by Real-Time PCR in Patients with Persistent Polymorphic Signs and Symptoms. Front. Med. 2020, 7, 474. [Google Scholar] [CrossRef]
- Boyer, P.H.; Koetsveld, J.; Zilliox, L.; Sprong, H.; Talagrand-Reboul, É.; Hansmann, Y.; De Martino, S.J.; Boulanger, N.; Hovius, J.W.; Jaulhac, B. Assessment of Borrelia Miyamotoi in Febrile Patients and Ticks in Alsace, an Endemic Area for Lyme Borreliosis in France. Parasites Vectors 2020, 13, 199. [Google Scholar] [CrossRef]
- Kim, C.M.; Seo, J.W.; Kim, D.M.; Yun, N.R.; Park, J.W.; Chung, J.K.; Song, H.J. Detection of Borrelia Miyamotoi in Ixodes Nipponensis in Korea. PLoS ONE 2019, 14, e0220465. [Google Scholar] [CrossRef]
- Livanova, N.N.; Fomenko, N.V.; Akimov, I.A.; Ivanov, M.J.; Tikunova, N.V.; Armstrong, R.; Konyaev, S.V. Dog Survey in Russian Veterinary Hospitals: Tick Identification and Molecular Detection of Tick-Borne Pathogens. Parasites Vectors 2018, 11, 591. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Z.; Kelly, P.; Li, J.; Ren, Y.; Wang, C. Borrelia Miyamotoi Sensu Lato in Pere David Deer and Haemaphysalis Longicornis Ticks. Emerg. Infect. Dis. 2018, 24, 928–931. [Google Scholar] [CrossRef]
- Jiang, B.G.; Jia, N.; Jiang, J.F.; Zheng, Y.C.; Chu, Y.L.; Jiang, R.R.; Wang, Y.W.; Liu, H.B.; Wei, R.; Zhang, W.H.; et al. Borrelia Miyamotoi Infections in Humans and Ticks, Northeastern China. Emerg. Infect. Dis. 2018, 24, 236–241. [Google Scholar] [CrossRef]
- Heglasová, I.; Rudenko, N.; Golovchenko, M.; Zubriková, D.; Miklisová, D.; Stanko, M. Ticks, Fleas and Rodent-Hosts Analyzed for the Presence of Borrelia Miyamotoi in Slovakia: The First Record of Borrelia Miyamotoi in a Haemaphysalis Inermis Tick. Ticks Tick Borne Dis. 2020, 11, 101456. [Google Scholar] [CrossRef]
- Breuner, N.E.; Ford, S.L.; Hojgaard, A.; Osikowicz, L.M.; Parise, C.M.; Rosales Rizzo, M.F.; Bai, Y.; Levin, M.L.; Eisen, R.J.; Eisen, L. Failure of the Asian Longhorned Tick, Haemaphysalis Longicornis, to Serve as an Experimental Vector of the Lyme Disease Spirochete, Borrelia Burgdorferi Sensu Stricto. Ticks Tick Borne Dis. 2020, 11, 101311. [Google Scholar] [CrossRef]
- Rudolf, I.; Hubálek, Z. Effect of the Salivary Gland and Midgut Extracts from Ixodes Ricinus and Dermacentor Reticulatus (Acari: Ixodidae) on the Growth of Borrelia Garinii in Vitro. Folia Parasitol. 2003, 50, 159–160. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Lehane, A.; Maes, S.E.; Graham, C.B.; Jones, E.; Delorey, M.; Eisen, R.J. Prevalence of Single and Coinfections of Human Pathogens in Ixodes Ticks from Five Geographical Regions in the United States, 2013–2019. Ticks Tick Borne Dis. 2021, 12, 101637. [Google Scholar] [CrossRef] [PubMed]
- Lynn, G.E.; Breuner, N.E.; Hojgaard, A.; Oliver, J.; Eisen, L.; Eisen, R.J. A Comparison of Horizontal and Transovarial Transmission Efficiency of Borrelia Miyamotoi by Ixodes Scapularis. Ticks Tick Borne Dis. 2022, 13, 102003. [Google Scholar] [CrossRef]
- Kurokawa, C.; Lynn, G.E.; Pedra, J.H.F.; Pal, U.; Narasimhan, S.; Fikrig, E. Interactions between Borrelia Burgdorferi and Ticks. Nat. Rev. Microbiol. 2020, 18, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, P.; Lindblom, P.; Fryland, L.; Ernerudh, J.; Forsberg, P.; Lindgren, P.E. Prevalence, Diversity, and Load of Borrelia Species in Ticks That Have Fed on Humans in Regions of Sweden and Åland Islands, Finland with Different Lyme Borreliosis Incidences. PLoS ONE 2013, 8, e81433. [Google Scholar] [CrossRef]
- Wilhelmsson, P.; Jaenson, T.G.T.; Olsen, B.; Waldenström, J.; Lindgren, P.E. Migratory Birds as Disseminators of Ticks and the Tick-Borne Pathogens Borrelia Bacteria and Tick-Borne Encephalitis (TBE) Virus: A Seasonal Study at Ottenby Bird Observatory in South-Eastern Sweden. Parasites Vectors 2020, 13, 607. [Google Scholar] [CrossRef]
- Strnad, M.; Hönig, V.; Ružek, D.; Gribhoffer, L.; Rego, R.O.M. Europe-Wide Meta-Analysis of Borrelia Burgdorferi Sensu Lato Prevalence in Questing Ixodes Ricinus Ticks Martin. Appl. Environ. Microbiol. 2017, 83, e00609–e00617. [Google Scholar] [CrossRef]
- Barbour, A.G.; Bunikis, J.; Travinsky, B.; Hoen, A.G.; Diuk-Wasser, M.A.; Fish, D.; Tsao, J.I. Niche Partitioning of Borrelia Burgdorferi and Borrelia Miyamotoi in the Same Tick Vector and Mammalian Reservoir Species. Am. J. Trop. Med. Hyg. 2009, 81, 1120–1131. [Google Scholar] [CrossRef]
- De Silva, A.M.; Fikrig, E. Growth and Migration of Borrelia Burgdorferi in Ixodes Ticks during Blood Feeding. Am. J. Trop. Med. Hyg. 1995, 53, 397–404. [Google Scholar] [CrossRef]
- Schwan, T.G.; Raffel, S.J. Transovarial Transmission of Borrelia Hermsii by Its Tick Vector and Reservoir Host Ornithodoros Hermsi. Microorganisms 2021, 9, 1978. [Google Scholar] [CrossRef]
- Ramamoorthi, N.; Narasimhan, S.; Pal, U.; Bao, F.; Yang, X.F.; Fish, D.; Anguita, J.; Norgard, M.V.; Kantor, F.S.; Anderson, J.F.; et al. The Lyme Disease Agent Exploits a Tick Protein to Infect the Mammalian Host. Nature 2005, 436, 573–577. [Google Scholar] [CrossRef]
- Schwan, T.G.; Raffel, S.J.; Battisti, J.M. Transgenic Functional Complementation with a Transmission-Associated Protein Restores Spirochete Infectivity by Tick Bite. Ticks Tick Borne Dis. 2020, 11, 101377. [Google Scholar] [CrossRef]
- Schötta, A.M.; Stelzer, T.; Stanek, G.; Stockinger, H.; Wijnveld, M. Bacteria and Protozoa with Pathogenic Potential in Ixodes Ricinus Ticks in Viennese Recreational Areas. Wien. Klin. Wochenschr. 2022. [Google Scholar] [CrossRef]
- Kniazeva, V.; Pogotskaya, Y.; Higgs, S.; Krasko, A. The Prevalence of Different Human Pathogenic Microorganisms Transmitted by Ixodes Tick Vectors in Belarus. Vector Borne Zoonotic Dis. 2021, 21, 6–10. [Google Scholar] [CrossRef]
- Garcia-Vozmediano, A.; Tomassone, L.; Fonville, M.; Bertolotti, L.; Heylen, D.; Fabri, N.D.; Medlock, J.M.; Nijhof, A.M.; Hansford, K.M.; Sprong, H.; et al. The Genetic Diversity of Rickettsiella Symbionts in Ixodes Ricinus Throughout Europe. Microb. Ecol. 2022, 84, 613–626. [Google Scholar] [CrossRef]
- Lernout, T.; De Regge, N.; Tersago, K.; Fonville, M.; Suin, V.; Sprong, H. Prevalence of Pathogens in Ticks Collected from Humans through Citizen Science in Belgium. Parasites Vectors 2019, 12, 550. [Google Scholar] [CrossRef]
- Burrows, H.; Talbot, B.; McKay, R.; Slatculescu, A.; Logan, J.; Thickstun, C.; Lindsay, L.R.; Dibernardo, A.; Koffi, J.K.; Ogden, N.H.; et al. A Multi-Year Assessment of Blacklegged Tick (Ixodes Scapularis) Population Establishment and Lyme Disease Risk Areas in Ottawa, Canada, 2017–2019. PLoS ONE 2021, 16, e0246484. [Google Scholar] [CrossRef]
- Gaowa; Wulantuya; Sato, K.; Liu, D.; Cui, Y.; Yin, X.; Zhang, L.; Li, H.; Wang, T.; Liu, R.; et al. Surveillance of Borrelia Miyamotoi-Carrying Ticks and Genomic Analysis of Isolates in Inner Mongolia, China. Parasites Vectors 2021, 14, 368. [Google Scholar] [CrossRef]
- Janeček, J.; Nováková, M.; Oppelt, J.; Pospíšilová, P.; Cunha, A.; Silva, A.C.; Dantong, L.; Šmajs, D. Complete Chromosomal Sequences of Two Borrelia Miyamotoi Samples Obtained from Ixodes Ricinus Eggs in Czechia. Microbiol. Resour. Announc. 2020, 9, 11–13. [Google Scholar] [CrossRef]
- Michelet, L.; Delannoy, S.; Devillers, E.; Umhang, G.; Aspan, A.; Juremalm, M.; Chirico, J.; van der Wal, F.J.; Sprong, H.; Boye Pihl, T.P.; et al. High-Throughput Screening of Tick-Borne Pathogens in Europe. Front. Cell. Infect. Microbiol. 2014, 4, 103. [Google Scholar] [CrossRef]
- Geller, J.; Nazarova, L.; Katargina, O.; Järvekülg, L.; Fomenko, N.; Golovljova, I. Detection and Genetic Characterization of Relapsing Fever Spirochete Borrelia Miyamotoi in Estonian Ticks. PLoS ONE 2012, 7, e51914. [Google Scholar] [CrossRef] [PubMed]
- Sormunen, J.J.; Penttinen, R.; Klemola, T.; Hänninen, J.; Vuorinen, I.; Laaksonen, M.; Sääksjärvi, I.E.; Ruohomäki, K.; Vesterinen, E.J. Tick-Borne Bacterial Pathogens in Southwestern Finland. Parasites Vectors 2016, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Sormunen, J.J.; Andersson, T.; Aspi, J.; Bäck, J.; Cederberg, T.; Haavisto, N.; Halonen, H.; Hänninen, J.; Inkinen, J.; Kulha, N.; et al. Monitoring of Ticks and Tick-Borne Pathogens through a Nationwide Research Station Network in Finland. Ticks Tick Borne Dis. 2020, 11, 101449. [Google Scholar] [CrossRef] [PubMed]
- Zakham, F.; Jääskeläinen, A.J.; Castrén, J.; Sormunen, J.J.; Uusitalo, R.; Smura, T.; Von Troil, G.; Kuivanen, S.; Sironen, T.; Vapalahti, O. Molecular Detection and Phylogenetic Analysis of Borrelia Miyamotoi Strains from Ticks Collected in the Capital Region of Finland. Ticks Tick Borne Dis. 2021, 12, 101608. [Google Scholar] [CrossRef] [PubMed]
- Cosson, J.F.; Michelet, L.; Chotte, J.; Le Naour, E.; Cote, M.; Devillers, E.; Poulle, M.L.; Huet, D.; Galan, M.; Geller, J.; et al. Genetic Characterization of the Human Relapsing Fever Spirochete Borrelia Miyamotoi in Vectors and Animal Reservoirs of Lyme Disease Spirochetes in France. Parasites Vectors 2014, 7, 233. [Google Scholar] [CrossRef]
- Lejal, E.; Marsot, M.; Chalvet-Monfray, K.; Cosson, J.F.; Moutailler, S.; Vayssier-Taussat, M.; Pollet, T. A Three-Years Assessment of Ixodes Ricinus-Borne Pathogens in a French Peri-Urban Forest. Parasites Vectors 2019, 12, 551. [Google Scholar] [CrossRef]
- Rǎileanu, C.; Tauchmann, O.; Vasić, A.; Wöhnke, E.; Silaghi, C. Borrelia Miyamotoi and Borrelia Burgdorferi (Sensu Lato) Identification and Survey of Tick-Borne Encephalitis Virus in Ticks from North-Eastern Germany. Parasites Vectors 2020, 13, 106. [Google Scholar] [CrossRef]
- Lambert, J.S.; Cook, M.J.; Healy, J.E.; Murtagh, R.; Avramovic, G.; Lee, S.H. Metagenomic 16S RRNA Gene Sequencing Survey of Borrelia Species in Irish Samples of Ixodes Ricinus Ticks. PLoS ONE 2019, 14, e0209881. [Google Scholar] [CrossRef]
- Iwabu-Itoh, Y.; Bazartseren, B.; Naranbaatar, O.; Yondonjamts, E.; Furuno, K.; Lee, K.; Sato, K.; Kawabata, H.; Takada, N.; Andoh, M.; et al. Tick Surveillance for Borrelia Miyamotoi and Phylogenetic Analysis of Isolates in Mongolia and Japan. Ticks Tick Borne Dis. 2017, 8, 850–857. [Google Scholar] [CrossRef]
- Morozov, A.; Tischenkov, A.; Silaghi, C.; Proka, A.; Toderas, I.; Movila, A.; Frickmann, H.; Poppert, S. Prevalence of Bacterial and Protozoan Pathogens in Ticks Collected from Birds in the Republic of Moldova. Microorganisms 2022, 10, 1111. [Google Scholar] [CrossRef]
- Lagunova, E.K.; Liapunova, N.A.; Tuul, D.; Otgonsuren, G.; Nomin, D.; Erdenebat, N.; Abmed, D.; Danchinova, G.A.; Sato, K.; Kawabata, H.; et al. Co-Infections with Multiple Pathogens in Natural Populations of Ixodes Persulcatus Ticks in Mongolia. Parasites Vectors 2022, 15, 236. [Google Scholar] [CrossRef] [PubMed]
- Heylen, D.; Fonville, M.; Docters Van Leeuwen, A.; Stroo, A.; Duisterwinkel, M.; Van Wieren, S.; Diuk-Wasser, M.; De Bruin, A.; Sprong, H. Pathogen Communities of Songbird-Derived Ticks in Europe’s Low Countries. Parasites Vectors 2017, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Wagemakers, A.; Jahfari, S.; de Wever, B.; Spanjaard, L.; Starink, M.V.; de Vries, H.J.C.; Sprong, H.; Hovius, J.W. Borrelia Miyamotoi in Vectors and Hosts in The Netherlands. Ticks Tick Borne Dis. 2017, 8, 370–374. [Google Scholar] [CrossRef]
- Kjelland, V.; Rollum, R.; Korslund, L.; Slettan, A.; Tveitnes, D. Borrelia Miyamotoi Is Widespread in Ixodes Ricinus Ticks in Southern Norway. Ticks Tick Borne Dis. 2015, 6, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Kiewra, D.; Stańczak, J.; Richter, M. Ixodes Ricinus Ticks (Acari, Ixodidae) as a Vector of Borrelia Burgdorferi Sensu Lato and Borrelia Miyamotoi in Lower Silesia, Poland—Preliminary Study. Ticks Tick Borne Dis. 2014, 5, 892–897. [Google Scholar] [CrossRef]
- Kubiak, K.; Szymańska, H.; Dmitryjuk, M.; Dzika, E. Abundance of Ixodes Ricinus Ticks (Acari: Ixodidae) and the Diversity of Borrelia Species in Northeastern Poland. Int. J. Environ. Res. Public Health 2022, 19, 7378. [Google Scholar] [CrossRef]
- Nunes, M.; Parreira, R.; Lopes, N.; Maia, C.; Carreira, T.; Sousa, C.; Faria, S.; Campino, L.; Vieira, M.L. Molecular Identification of Borrelia Miyamotoi in Ixodes Ricinus from Portugal. Vector-Borne Zoonotic Dis. 2015, 15, 515–517. [Google Scholar] [CrossRef]
- Pukhovskaya, N.M.; Morozova, O.V.; Vysochina, N.P.; Belozerova, N.B.; Ivanov, L.I. Prevalence of Borrelia Burgdorferi Sensu Lato and Borrelia Miyamotoi in Ixodid Ticks in the Far East of Russia. Int. J. Parasitol. Parasites Wildl. 2019, 8, 192–202. [Google Scholar] [CrossRef]
- Banović, P.; Díaz-Sánchez, A.A.; Galon, C.; Simin, V.; Mijatović, D.; Obregón, D.; Moutailler, S.; Cabezas-Cruz, A. Humans Infested with Ixodes Ricinus Are Exposed to a Diverse Array of Tick-Borne Pathogens in Serbia. Ticks Tick Borne Dis. 2021, 12, 101609. [Google Scholar] [CrossRef]
- Hamšíková, Z.; Coipan, C.; Mahríková, L.; Minichová, L.; Sprong, H.; Kazimírová, M. Borrelia Miyamotoi and Co-Infection with Borrelia Afzelii in Ixodes Ricinus Ticks and Rodents from Slovakia. Microb. Ecol. 2017, 73, 1000–1008. [Google Scholar] [CrossRef]
- Remesar, S.; Díaz, P.; Venzal, J.M.; Prieto, A.; Estrada-Peña, A.; López, C.M.; Panadero, R.; Fernández, G.; Díez-Baños, P.; Morrondo, P. Longitudinal Study of Infection with Borrelia Spp. in Questing Ticks from North-Western Spain. Vector-Borne Zoonotic Dis. 2019, 19, 785–792. [Google Scholar] [CrossRef]
- Hornok, S.; Daccord, J.; Takács, N.; Kontschán, J.; Tuska-Szalay, B.; Sándor, A.D.; Szekeres, S.; Meli, M.L.; Hofmann-Lehmann, R. Investigation on Haplotypes of Ixodid Ticks and Retrospective Finding of Borrelia Miyamotoi in Bank Vole (Myodes Glareolus) in Switzerland. Ticks Tick Borne Dis. 2022, 13, 101865. [Google Scholar] [CrossRef]
- Sakakibara, K.; Sen, E.; Sato, K.; Kawabata, H.; Ohashi, N.; Masuzawa, T. Detection and Characterization of the Emerging Relapsing Fever Pathogen, Borrelia Miyamotoi, from the Ixodes Ricinus Tick in the Rural Trakya (Thrace) Region of Northwestern Turkey. Vector-Borne Zoonotic Dis. 2016, 16, 797–799. [Google Scholar] [CrossRef]
- Rogovskyy, A.; Batool, M.; Gillis, D.C.; Holman, P.J.; Nebogatkin, I.V.; Rogovska, Y.V.; Rogovskyy, M.S. Diversity of Borrelia Spirochetes and Other Zoonotic Agents in Ticks from Kyiv, Ukraine. Ticks Tick Borne Dis. 2018, 9, 404–409. [Google Scholar] [CrossRef]
- Lynn, G.E.; Graham, C.B.; Horiuchi, K.; Eisen, L.; Johnson, T.L.; Lane, R.S.; Eisen, R.J. Prevalence and Geographic Distribution of Borrelia Miyamotoi in Host-Seeking Ixodes Pacificus (Acari: Ixodidae) Nymphs in Mendocino County, California. J. Med. Entomol. 2018, 55, 711–716. [Google Scholar] [CrossRef]
- Han, S.; Hickling, G.J.; Ogden, N.H.; Ginsberg, H.S.; Kobbekaduwa, V.; Rulison, E.L.; Beati, L.; Tsao, J.I. Seasonality of Acarological Risk of Exposure to Borrelia Miyamotoi from Questing Life Stages of Ixodes Scapularis Collected from Wisconsin and Massachusetts, USA. Ticks Tick Borne Dis. 2021, 12, 101556. [Google Scholar] [CrossRef]
- Burri, C.; Schumann, O.; Schumann, C.; Gern, L. Are Apodemus Spp. Mice and Myodes Glareolus Reservoirs for Borrelia Miyamotoi, Candidatus Neoehrlichia Mikurensis, Rickettsia Helvetica, R. Monacensis and Anaplasma Phagocytophilum? Ticks Tick Borne Dis. 2014, 5, 245–251. [Google Scholar] [CrossRef]
- Scott, M.C.; Rosen, M.E.; Hamer, S.A.; Baker, E.; Edwards, H.; Crowder, C.; Tsao, J.I.; Hickling, G.J. High-Prevalence Borrelia Miyamotoi [Scapin Wild Turkeys (Meleagris Gallopavo) in Tennessee. J. Med. Entomol. 2010, 47, 1238–1242. [Google Scholar] [CrossRef]
- Hrnková, J.; Golovchenko, M.; Musa, A.S.; Needham, T.; Italiya, J.; Ceacero, F.; Kotrba, R.; Grubhoffer, L.; Rudenko, N.; Cerný, J. Borrelia Spirochetes in European Exotic Farm Animals. Front. Vet. Sci. 2022, 9, 996015. [Google Scholar] [CrossRef]
- Szekeres, S.; Coipan, E.C.; Rigó, K.; Majoros, G.; Jahfari, S.; Sprong, H.; Földvári, G. Eco-Epidemiology of Borrelia Miyamotoi and Lyme Borreliosis Spirochetes in a Popular Hunting and Recreational Forest Area in Hungary. Parasites Vectors 2015, 8, 309. [Google Scholar] [CrossRef] [Green Version]
- Gryczyńska, A.; Sokół, M.; Gortat, T.; Kowalec, M. Borrelia Miyamotoi Infection in Apodemus Spp. Mice Populating an Urban Habitat (Warsaw, Poland). Int. J. Parasitol. Parasites Wildl. 2021, 14, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Zinck, C.B.; Lloyd, V.K. Borrelia Burgdorferi and Borrelia Miyamotoi in Atlantic Canadian Wildlife. PLoS ONE 2022, 17, e0262229. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hickling, G.J.; Tsao, J.I. High Prevalence of Borrelia Miyamotoi among Adult Blacklegged Ticks from White-Tailed Deer. Emerg. Infect. Dis. 2016, 22, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lubelczyk, C.; Hickling, G.J.; Belperron, A.A.; Bockenstedt, L.K.; Tsao, J.I. Vertical Transmission Rates of Borrelia Miyamotoi in Ixodes Scapularis Collected from White-Tailed Deer. Ticks Tick Borne Dis. 2019, 10, 682–689. [Google Scholar] [CrossRef]
- Kurtenbach, K.; Sewell, H.S.; Ogden, N.H.; Randolph, S.E.; Nuttall, P.A. Serum Complement Sensitivity as a Key Factor in Lyme Disease Ecology. Infect. Immun. 1998, 66, 1248–1251. [Google Scholar] [CrossRef]
- Barthold, S.W.; De Souza, M.S.; Janotka, J.L.; Smith, A.L.; Persing, D.H. Chronic Lyme Borreliosis in the Laboratory Mouse. Am. J. Pathol. 1993, 143, 959–972. [Google Scholar]
- Taylor, K.R.; Takano, A.; Konnai, S.; Shimozuru, M.; Kawabata, H.; Tsubota, T. Borrelia Miyamotoi Infections among Wild Rodents Show Age and Month Independence and Correlation with Ixodes Persulcatus Larval Attachment in Hokkaido, Japan. Vector-Borne Zoonotic Dis. 2013, 13, 92–97. [Google Scholar] [CrossRef]
- Krawczyk, A.I.; Van Duijvendijk, G.L.A.; Swart, A.; Heylen, D.; Jaarsma, R.I.; Jacobs, F.H.H.; Fonville, M.; Sprong, H.; Takken, W. Effect of Rodent Density on Tick and Tick-Borne Pathogen Populations: Consequences for Infectious Disease Risk. Parasites Vectors 2020, 13, 34. [Google Scholar] [CrossRef]
- Jeske, K.; Herzig-Straschil, B.; Răileanu, C.; Kunec, D.; Tauchmann, O.; Emirhar, D.; Schmidt, S.; Trimpert, J.; Silaghi, C.; Heckel, G.; et al. Zoonotic Pathogen Screening of Striped Field Mice (Apodemus Agrarius) from Austria. Transbound. Emerg. Dis. 2022, 69, 886–890. [Google Scholar] [CrossRef]
- Tadin, A.; Tokarz, R.; Markotic, A.; Margaletic, J.; Turk, N.; Habu, J.; Svoboda, P.; Vucelja, M.; Desai, A.; Jain, K.; et al. Molecular Survey of Zoonotic Agents in Rodents and Other Small Mammals in Croatia. Am. J. Trop. Med. Hyg. 2016, 94, 466–473. [Google Scholar] [CrossRef]
- Kalmár, Z.; Sándor, A.D.; Matei, I.A.; Ionicǎ, A.; D’Amico, G.; Gherman, C.M.; Mihalca, A.D. Borrelia Spp. in Small Mammals in Romania. Parasites Vectors 2019, 12, 461. [Google Scholar] [CrossRef]
- Cerar, T.; Korva, M.; Avšič-Županc, T.; Ružić-Sabljić, E. Detection, Identification and Genotyping of Borrellia Spp. In Rodents in Slovenia by PCR and Culture. BMC Vet. Res. 2015, 11, 188. [Google Scholar] [CrossRef]
- Majerová, K.; Hönig, V.; Houda, M.; Papežík, P.; Fonville, M.; Sprong, H.; Rudenko, N.; Golovchenko, M.; Bolfíková, B.Č.; Hulva, P.; et al. Hedgehogs, Squirrels, and Blackbirds as Sentinel Hosts for Active Surveillance of Borrelia Miyamotoi and Borrelia Burgdorferi Complex in Urban and Rural Environments. Microorganisms 2020, 8, 1908. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Nieto, N.C.; Bonilla, D.L.; Yoshimizu, M.H.; Padgett, K.A. Borrelia Miyamotoi Infections in Small Mammals, California, USA. Emerg. Infect. Dis. 2018, 24, 2356–2359. [Google Scholar] [CrossRef]
- Dumas, A.; Bouchard, C.; Dibernardo, A.; Drapeau, P.; Robbin Lindsay, L.; Ogden, N.H.; Leighton, P.A. Transmission Patterns of Tick-Borne Pathogens among Birds and Rodents in a Forested Park in Southeastern Canada. PLoS ONE 2022, 17, e0266527. [Google Scholar] [CrossRef]
- Szekeres, S.; Docters van Leeuwen, A.; Tóth, E.; Majoros, G.; Sprong, H.; Földvári, G. Road-Killed Mammals Provide Insight into Tick-Borne Bacterial Pathogen Communities within Urban Habitats. Transbound. Emerg. Dis. 2019, 66, 277–286. [Google Scholar] [CrossRef]
- Lau, A.C.C.; Qiu, Y.; Moustafa, M.A.M.; Nakao, R.; Shimozuru, M.; Onuma, M.; Mohd-Azlan, J.; Tsubota, T. Detection of Borrelia Burgdorferi Sensu Lato and Relapsing Fever Borrelia in Feeding Ixodes Ticks and Rodents in Sarawak, Malaysia: New Geographical Records of Borrelia Yangtzensis and Borrelia Miyamotoi. Pathogens 2020, 9, 846. [Google Scholar] [CrossRef]
- Hofmeester, T.R.; Krawczyk, A.I.; Van Leeuwen, A.D.; Fonville, M.; Montizaan, M.G.E.; Van Den Berge, K.; Gouwy, J.; Ruyts, S.C.; Verheyen, K.; Sprong, H. Role of Mustelids in the Life-Cycle of Ixodid Ticks and Transmission Cycles of Four Tick-Borne Pathogens. Parasites Vectors 2018, 11, 600. [Google Scholar] [CrossRef]
- Richter, D.; Matuschka, F.R. Elimination of Lyme Disease Spirochetes from Ticks Feeding on Domestic Ruminants. Appl. Environ. Microbiol. 2010, 76, 7650–7652. [Google Scholar] [CrossRef]
- Schreiber, C.; Krücken, J.; Beck, S.; Maaz, D.; Pachnicke, S.; Krieger, K.; Gross, M.; Kohn, B.; Von Samson-Himmelstjerna, G. Pathogens in Ticks Collected from Dogs in Berlin/Brandenburg, Germany. Parasites Vectors 2014, 7, 535. [Google Scholar] [CrossRef]
- Regier, Y.; Komma, K.; Weigel, M.; Kraiczy, P.; Laisi, A.; Pulliainen, A.T.; Hain, T.; Kempf, V.A.J. Combination of Microbiome Analysis and Serodiagnostics to Assess the Risk of Pathogen Transmission by Ticks to Humans and Animals in Central Germany 11 Medical and Health Sciences 1108 Medical Microbiology. Parasites Vectors 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Seto, J.; Tanaka, S.; Kawabata, H.; Ito, Y.; Ikeda, T.; Mizuta, K. Detection of Tick-Borne Pathogens in Ticks from Dogs and Cats in the Yamagata Prefecture of Japan in 2018. Jpn. J. Infect. Dis. 2021, 74, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Namina, A.; Capligina, V.; Seleznova, M.; Krumins, R.; Aleinikova, D.; Kivrane, A.; Akopjana, S.; Lazovska, M.; Berzina, I.; Ranka, R. Tick-Borne Pathogens in Ticks Collected from Dogs, Latvia, 2011–2016. BMC Vet. Res. 2019, 15, 398. [Google Scholar] [CrossRef] [PubMed]
- Wodecka, B.; Rymaszewska, A.; Skotarczak, B. Host and Pathogen DNA Identification in Blood Meals of Nymphal Ixodes Ricinus Ticks from Forest Parks and Rural Forests of Poland. Exp. Appl. Acarol. 2014, 62, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Remesar, S.; Venzal, J.M.; Vázquez-López, M.E.; Fernández, G.; López, C.; Díez-Baños, P.; Morrondo, P.; Panadero, R. Occurrence of Borrelia and Borreliella Species in Ixodes Ricinus Collected from Roe Deer in Northwestern Spain. Med. Vet. Entomol. 2019, 33, 427–430. [Google Scholar] [CrossRef]
- Wodecka, B. Znaczenie Jeleni (Cervus Elaphus) w Ekologii Borrelia Burgdorferi Sensu Lato. Wiadomości Parazytol. 2007, 53, 231–237. [Google Scholar] [PubMed]
- Kjær, L.J.; Jensen, L.M.; Chriél, M.; Bødker, R.; Petersen, H.H. The Raccoon Dog (Nyctereutes Procyonoides) as a Reservoir of Zoonotic Diseases in Denmark. Int. J. Parasitol. Parasites Wildl. 2021, 16, 175–182. [Google Scholar] [CrossRef]
- Rosen, M.E.; Hamer, S.A.; Gerhardt, R.R.; Jones, C.J.; Muller, L.I.; Scott, M.C.; Hickling, G.J. Borrelia Burgdorferi Not Detected in Widespread Ixodes Scapularis (Acari: Ixodidae) Collected from White-Tailed Deer in Tennessee. J. Med. Entomol. 2012, 49, 1473–1480. [Google Scholar] [CrossRef]
- Hamer, S.A.; Hickling, G.J.; Keith, R.; Sidge, J.L.; Walker, E.D.; Tsao, J.I. Associations of Passerine Birds, Rabbits, and Ticks with Borrelia Miyamotoi and Borrelia Andersonii in Michigan, U.S.A. Parasites Vectors 2012, 5, 231. [Google Scholar] [CrossRef]
- Rollend, L.; Fish, D.; Childs, J.E. Transovarial Transmission of Borrelia Spirochetes by Ixodes Scapularis: A Summary of the Literature and Recent Observations. Ticks Tick Borne Dis. 2013, 4, 46–51. [Google Scholar] [CrossRef]
- Breuner, N.E.; Dolan, M.C.; Replogle, A.J.; Sexton, C.; Hojgaard, A.; Boegler, K.A.; Clark, R.J.; Eisen, L. Transmission of Borrelia Miyamotoi Sensu Lato Relapsing Fever Group Spirochetes in Relation to Duration of Attachment by Ixodes Scapularis Nymphs. Ticks Tick Borne Dis. 2017, 8, 677–681. [Google Scholar] [CrossRef]
- Breuner, N.E.; Hojgaard, A.; Replogle, A.J.; Boegler, K.A.; Eisen, L. Transmission of the Relapsing Fever Spirochete, Borrelia Miyamotoi, by Single Transovarially-Infected Larval Ixodes Scapularis Ticks. Ticks Tick Borne Dis. 2018, 9, 1464–1467. [Google Scholar] [CrossRef]
- Hojgaard, A.; Osikowicz, L.M.; Maes, S.; Eisen, L.; Eisen, R.J. Detection of Genetic Variability in Borrelia Miyamotoi (Spirochaetales: Spirochaetaceae) between and within the Eastern and Western United States. J. Med. Entomol. 2021, 58, 2154–2160. [Google Scholar] [CrossRef]
- Nakao, R.; Kasama, K.; Boldbaatar, B.; Ogura, Y.; Kawabata, H.; Toyoda, A.; Hayashi, T.; Takano, A.; Maeda, K. The Evolution of Hard Tick-Borne Relapsing Fever Borreliae Is Correlated with Vector Species Rather than Geographical Distance. BMC Ecol. Evol. 2021, 21, 105. [Google Scholar] [CrossRef]
- Kelly, R. Cultivation of Borrelia Hernsii. Science 1971, 173, 443–444. [Google Scholar] [CrossRef]
- Barbour, A.G. Isolation and Cultivation of Lyme Disease Spirochetes. Yale J. Biol. Med. 1984, 57, 521–525. [Google Scholar]
- Preac-Mursic, V.; Wilske, B.; Schierz, G. European Borrelia Burgdorferi Isolated from Humans and Ticks Culture Conditions and Antibiotic Susceptibility. Zent. Bakteriol. Mikrobiol. Hyg.—Abt. 1 Orig. A 1986, 263, 112–118. [Google Scholar] [CrossRef]
- Wang, G.; Iyer, R.; Bittker, S.; Cooper, D.; Small, J.; Wormser, G.P.; Schwartz, I. Variations in Barbour-Stoenner-Kelly Culture Medium Modulate Infectivity and Pathogenicity of Borrelia Burgdorferi Clinical Isolates. Infect. Immun. 2004, 72, 6702–6706. [Google Scholar] [CrossRef]
- Margos, G.; Stockmeier, S.; Hizo-Teufel, C.; Hepner, S.; Fish, D.; Dautel, H.; Sing, A.; Dzaferovic, E.; Rieger, M.; Jungnick, S.; et al. Long-Term in Vitro Cultivation of Borrelia Miyamotoi. Ticks Tick Borne Dis. 2015, 6, 181–184. [Google Scholar] [CrossRef]
- Wagemakers, A.; Oei, A.; Fikrig, M.M.; Miellet, W.R.; Hovius, J.W. The Relapsing Fever Spirochete Borrelia Miyamotoi Is Cultivable in a Modified Kelly-Pettenkofer Medium, and Is Resistant to Human Complement. Parasites Vectors 2014, 7, 4–9. [Google Scholar] [CrossRef]
- Casjens, S.; Palmer, N.; Van Vugt, R.; Huang, W.M.; Stevenson, B.; Rosa, P.; Lathigra, R.; Sutton, G.; Peterson, J.; Dodson, R.J.; et al. A Bacterial Genome in Flux: The Twelve Linear and Nine Circular Extrachromosomal DNAs in an Infectious Isolate of the Lyme Disease Spirochete Borrelia Burgdorferi. Mol. Microbiol. 2000, 35, 490–516. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, R.D.; Mikula, S.; Harris, E.K.; Van Gundy, T.J.; Goodrich, I.; Brandt, K.S. Borrelia Miyamotoi Strain LB-2001 Retains Plasmids and Infectious Phenotype throughout Continuous Culture Passages as Evaluated by Multiplex PCR. Ticks Tick Borne Dis. 2021, 12, 101587. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, R.D.; Kneubehl, A.R.; Lopez, J.E.; Armstrong, B.A.; Brandt, K.S.; Van Gundy, T.J. Modification of the Multiplex Plasmid PCR Assay for Borrelia Miyamotoi Strain LB-2001 Based on the Complete Genome Sequence Reflecting Genomic Rearrangements Differing from Strain CT13–2396. Ticks Tick Borne Dis. 2022, 13, 101843. [Google Scholar] [CrossRef] [PubMed]
- Röttgerding, F.; Wagemakers, A.; Koetsveld, J.; Fingerle, V.; Kirschfink, M.; Hovius, J.W.; Zipfel, P.F.; Wallich, R.; Kraiczy, P. Immune Evasion of Borrelia Miyamotoi: CbiA, a Novel Outer Surface Protein Exhibiting Complement Binding and Inactivating Properties. Sci. Rep. 2017, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G. Plasmid Analysis of Borrelia Burgdorferi, the Lyme Disease Agent. J. Clin. Microbiol. 1988, 26, 475–478. [Google Scholar] [CrossRef]
- Schwan, T.G.; Burgdorfer, W.; Garon, C.F. Changes in Infectivity and Plasmid Profile of the Lyme Disease Spirochete, Borrelia Burgdorferi, as a Result of in Vitro Cultivation. Infect. Immun. 1988, 56, 1831–1836. [Google Scholar] [CrossRef]
- Grimm, D.; Elias, A.F.; Tilly, K.; Rosa, P.A. Plasmid Stability during in Vitro Propagation of Borrelia Burgdorferi Assessed at a Clonal Level. Infect. Immun. 2003, 71, 3138–3145. [Google Scholar] [CrossRef]
- Sato, K.; Kumagai, Y.; Sekizuka, T.; Kuroda, M.; Hayashi, T.; Takano, A.; Gaowa; Taylor, K.R.; Ohnishi, M.; Kawabata, H. Vitronectin Binding Protein, BOM1093, Confers Serum Resistance on Borrelia Miyamotoi. Sci. Rep. 2021, 11, 5462. [Google Scholar] [CrossRef]
- Schmidt, F.L.; Sürth, V.; Berg, T.K.; Lin, Y.P.; Hovius, J.W.; Kraiczy, P. Interaction between Borrelia Miyamotoi Variable Major Proteins Vlp15/16 and Vlp18 with Plasminogen and Complement. Sci. Rep. 2021, 11, 4964. [Google Scholar] [CrossRef]
- Booth, C.E.; Powell-Pierce, A.D.; Skare, J.T.; Garcia, B.L. Borrelia Miyamotoi FbpA and FbpB Are Immunomodulatory Outer Surface Lipoproteins with Distinct Structures and Functions. Front. Immunol. 2022, 13, 886733. [Google Scholar] [CrossRef]
- Fischer, J.R.; LeBlanc, K.T.; Leong, J.M. Fibronectin Binding Protein BBK32 of the Lyme Disease Spirochete Promotes Bacterial Attachment to Glycosaminoglycans. Infect. Immun. 2006, 74, 435–441. [Google Scholar] [CrossRef]
- Singh, B.; Su, Y.C.; Riesbeck, K. Vitronectin in Bacterial Pathogenesis: A Host Protein Used in Complement Escape and Cellular Invasion. Mol. Microbiol. 2010, 78, 545–560. [Google Scholar] [CrossRef]
| Strain | Origin | Source | Accession |
|---|---|---|---|
| LB-2001 | North America | I. scapularis | CP006647 |
| CT13-2396 | North America | I. scapularis | NZ_CP017126 |
| C14D4 | North America | Human, blood | NZ_CP010308 |
| CA17-2241 | North America | I. pacificus | NZ_CP021872 |
| FR64b | Japan | A. argenteus, blood | NZ_CP004217 |
| HT31 | Japan | I. persulcatus | NZ_AP024371 |
| HT24 | Japan | I. persulcatus | NZ_AP024372 |
| Hk004 | Japan | I. persulcatus | NZ_AP024373 |
| NB103/1 | Japan | A. argenteus, blood | NZ_AP024374 |
| MYK1 | Japan | I. pavlovskyi | NZ_AP024375 |
| MYK2 | Japan | I. persulcatus | NZ_AP024391 |
| MYK3 | Japan | I. persulcatus | NZ_AP024392 |
| MYK4 | Japan | I. persulcatus | NZ_AP024393 |
| MYK5 | Japan | I. persulcatus | NZ_AP024394 |
| Y14T1 | Japan | I. persulcatus | NZ_AP024398 |
| Y15T1 | Japan | I. persulcatus | NZ_AP024399 |
| Y14T18 | Japan | I. ovatus | NZ_AP024400 |
| Yekat-1 | Russia | Human, plasma | NZ_CP024333 |
| Yekat-6 | Russia | Human, plasma | NZ_CP024316 |
| Yekat-17 | Russia | Human, plasma | NZ_CP037215 |
| Yekat-18 | Russia | Human, plasma | CP037471 |
| Yekat-19 | Russia | Human, plasma | NZ_CP037058 |
| Yekat-21 | Russia | Human, plasma | NZ_CP036914 |
| Yekat-31 | Russia | Human, plasma | NZ_CP036726 |
| Yekat-76 | Russia | Human, plasma | NZ_CP036557 |
| Izh-4 | Russia | Human, plasma | NZ_CP024390 |
| Izh-5 | Russia | Human, plasma | NZ_CP024205 |
| Izh-14 | Russia | Human, plasma | CP024371 |
| Izh-16 | Russia | Human, plasma | CP024351 |
| NL-IR-1 | Europe | I. ricinus, eggs | NZ_CP044783 |
| NL-IR-2 | Europe | I. ricinus, eggs | NZ_CP044625 |
| CZ-F1E | Europe | I. ricinus, eggs | NZ_CP046389 |
| CZ-F190E | Europe | I. ricinus, eggs | NZ_CP046388 |
| M12C4 | Mongolia | I. persulcatus | NZ_AP024395 |
| M15A8 | Mongolia | I. persulcatus | NZ_AP024396 |
| M20E6 | Mongolia | I. persulcatus | NZ_AP024397 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cleveland, D.W.; Anderson, C.C.; Brissette, C.A. Borrelia miyamotoi: A Comprehensive Review. Pathogens 2023, 12, 267. https://doi.org/10.3390/pathogens12020267
Cleveland DW, Anderson CC, Brissette CA. Borrelia miyamotoi: A Comprehensive Review. Pathogens. 2023; 12(2):267. https://doi.org/10.3390/pathogens12020267
Chicago/Turabian StyleCleveland, Dawn W., Cassidy C. Anderson, and Catherine A. Brissette. 2023. "Borrelia miyamotoi: A Comprehensive Review" Pathogens 12, no. 2: 267. https://doi.org/10.3390/pathogens12020267






