Relationship between Biofilm Production and High Somatic Cell Count in Streptococcus agalactiae Isolated from Milk of Cows with Subclinical Mastitis
Abstract
:1. Introduction
2. Material and Methods
2.1. Bacterial Isolates and Molecular Typing
2.2. Biofilm Production
2.3. Adhesion and Invasion Assays for BMEC Cells
3. Results
3.1. Genetic Profile
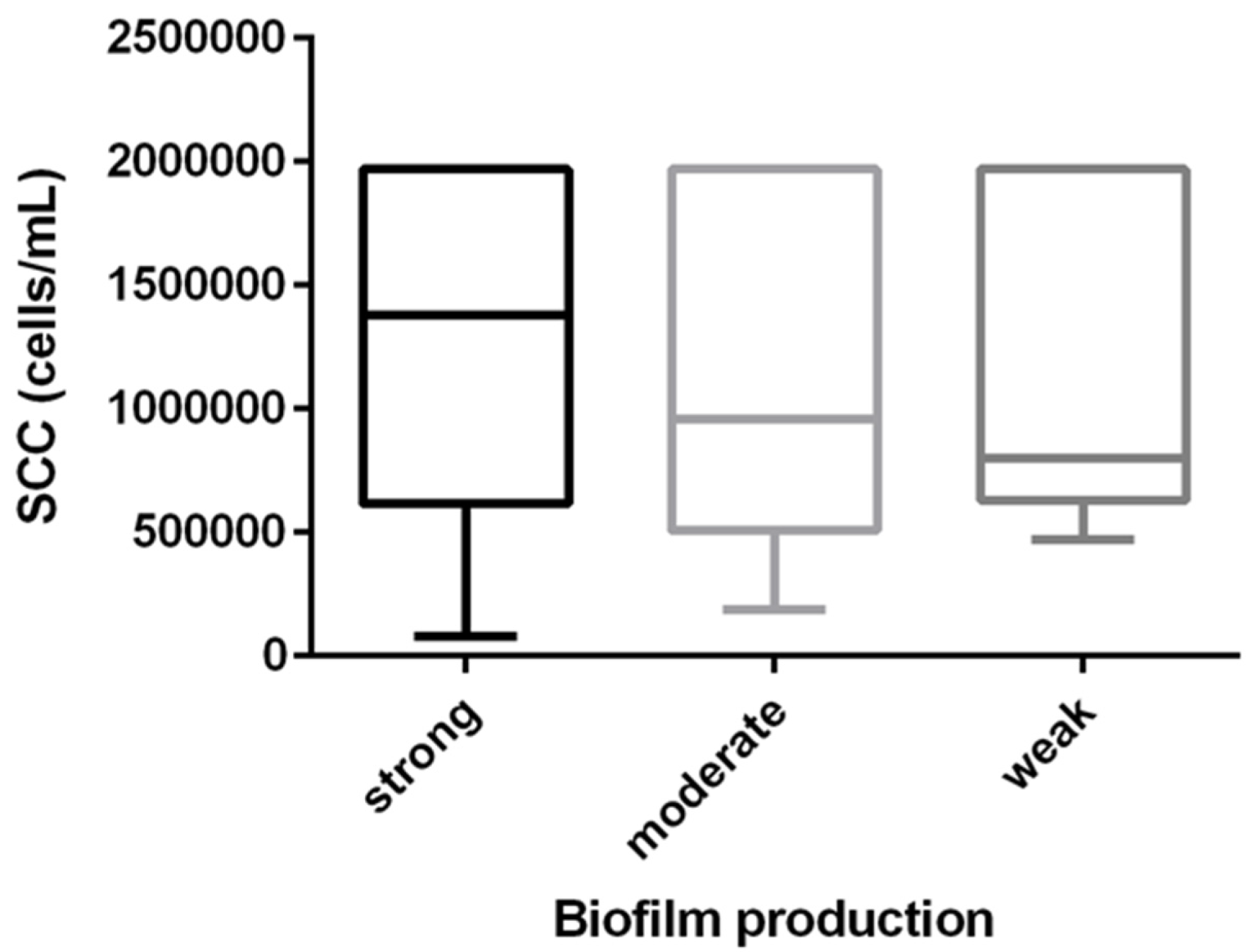
3.2. Biofilm Production and SCC the Relationship
3.3. Cell Adhesion and Invasion
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruegg, P.L. 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 12, 10381–10397. [Google Scholar] [CrossRef]
- Carra, E.; Russo, S.; Micheli, A.; Garbarino, C.; Ricchi, M.; Bergamini, F.; Bassi, P.; Prosperi, A.; Piva, S.; Cricca, M.; et al. Evidence of Common Isolates of Streptococcus agalactiae in Bovines and Humans in Emilia Romagna Region (Northern Italy). Front. Microbiol. 2021, 12, 673126. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Ángel, C.; Jaramillo-Jaramillo, A.S.; Lasso-Rojas, L.M.; Aguilar-Marin, S.B.; Sanchez, J.; Rodriguez-Lecompte, J.C.; Ceballos-Márquez, A.; Zadoks, R.N. Streptococcus agalactiae is not always an obligate intramammary pathogen: Molecular epidemiology of GBS from milk, feces and environment in Colombian dairy herds. PLoS ONE 2018, 13, e0208990. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zhang, B.; Luo, Z.; Lu, B.; Luo, Z.; Zhang, J.; Wang, Y.; Luo, Y.; Yang, Z.; Shen, L.; et al. Molecular typing and prevalence of antibiotic resistance and virulence genes in Streptococcus agalactiae isolated from Chinese dairy cows with clinical mastitis. PLoS ONE 2022, 17, e0268262. [Google Scholar] [CrossRef]
- Kaczorek, E.; Małaczewska, J.; Wójcik, R.; Rękawek, W.; Siwicki, A. Phenotypic and genotypic antimicrobial susceptibility pattern of Streptococcus spp. isolated from cases of clinical mastitis in dairy cattle in Poland. J. Dairy Sci. 2017, 100, 6442–6453. [Google Scholar] [CrossRef] [PubMed]
- Mahmmod, Y.; Klaas, I.; Katholm, J.; Lutton, M.; Zadoks, R. Molecular epidemiology and strain-specific characteristics of Streptococcus agalactiae at the herd and cow level. J. Dairy Sci. 2015, 98, 6913–6924. [Google Scholar] [CrossRef]
- Carvalho-Castro, G.A.; Silva, J.R.; Paiva, L.V.; Custódio, D.A.; Moreira, R.O.; Mian, G.F.; Prado, I.A.; Chalfun-Junior, A.; Costa, G.M. Molecular epidemiology of Streptococcus agalactiae isolated from mastitis in Brazilian dairy herds. Braz. J. Microbiol. 2017, 48, 551–559. [Google Scholar] [CrossRef]
- Keefe, G.P. Streptococcus agalactiae mastitis: A review. Am. Jew. Hist. 1997, 38, 429–437. [Google Scholar]
- Jørgensen, H.; Nordstoga, A.; Sviland, S.; Zadoks, R.; Sølverød, L.; Kvitle, B.; Mørk, T. Streptococcus agalactiae in the environment of bovine dairy herds—Rewriting the textbooks? Vet. Microbiol. 2016, 184, 64–72. [Google Scholar] [CrossRef]
- Amosun, E.A.; Ajuwape, A.T.P.; Adetosoye, A.I. Bovine streptococcal mastitis in Southwest and Northern states of Nigeria. Afr. J Biomed. Res. 2010, 13, 33–37. [Google Scholar]
- Becker, H. Streptococcus agalactiae (group B streptocci). In The Significance of Pathogenic Microorganisms in Raw Milk; International Dairy Federation: Brussels, Belgium, 1994; pp. 43–54. [Google Scholar]
- Bisharat, N.; Crook, D.W.; Leigh, J.; Harding, R.M.; Ward, P.N.; Coffey, T.; Maiden, M.C.; Peto, T.; Jones, N. Hyperinvasive Neonatal Group B Streptococcus Has Arisen from a Bovine Ancestor. J. Clin. Microbiol. 2004, 42, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.C.N.; Ferreira, A.F.M.; Fracalanzza, S.E.L.; Teixeira, L.M.; Pinto, T.C.A. A Perspective on the Potential Zoonotic Role of Streptococcus agalactiae: Searching for a Missing Link in Alternative Transmission Routes. Front. Microbiol. 2018, 9, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopal, L. Understanding the regulation of Group B Streptoccocal virulence factors. Future Microbiol. 2009, 4, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Poyart, C.; Tazi, A.; Réglier-Poupet, H.; Billoet, A.; Tavares, N.; Raymond, J.; Trieu-Cuot, P. Multiplex PCR Assay for Rapid and Accurate Capsular Typing of Group B Streptococci. J. Clin. Microbiol. 2007, 45, 1985–1988. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, C.; do Carmo, R.M. Translocal Ruralism: Mobility and Connectivity in European Rural Spaces; Springer: London, UK; New York, NY, USA, 2012. [Google Scholar]
- Di Xia, F.; Mallet, A.; Caliot, E.; Gao, C.; Trieu-Cuot, P.; Dramsi, S. Capsular polysaccharide of Group B Streptococcus mediates biofilm formation in the presence of human plasma. Microbes Infect. 2015, 17, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Liedtke, J.; Plattes, S.; Lipski, A. Bacterial community composition of biofilms in milking machines of two dairy farms assessed by a combination of culture-dependent and –independent methods. PLoS ONE 2019, 14, e0222238. [Google Scholar] [CrossRef]
- Gogoi-Tiwari, J.; Williams, V.; Waryah, C.B.; Costantino, P.; Al-Salami, H.; Mathavan, S.; Wells, K.; Tiwari, H.K.; Hegde, N.; Isloor, S.; et al. Mammary Gland Pathology Subsequent to Acute Infection with Strong versus Weak Biofilm Forming Staphylococcus aureus Bovine Mastitis Isolates: A Pilot Study Using Non-Invasive Mouse Mastitis Model. PLoS ONE 2017, 12, e0170668. [Google Scholar] [CrossRef]
- Gogoi-Tiwari, J.; Dorji, D.; Tiwari, H.K.; Shirolkar, G.; Aleri, J.W.; Mukkur, T. Phenotypic PIA-Dependent Biofilm Production by Clinical Non-Typeable Staphylococcus aureus Is Not Associated with the Intensity of Inflammation in Mammary Gland: A Pilot Study Using Mouse Mastitis Model. Animals 2021, 11, 3047. [Google Scholar] [CrossRef]
- Watters, C.; Fleming, D.; Bishop, D.; Rumbaugh, K. Host Responses to Biofilm. Prog. Mol. Biol. Transl. Sci. 2016, 142, 193–239. [Google Scholar] [CrossRef] [PubMed]
- Erosini, R.; Margarit, I. Biofilm formation by Streptococcus agalactiae: Influence of environmental conditions and implicated virulence factors. Front. Cell. Infect. Microbiol. 2015, 5, 6. [Google Scholar] [CrossRef]
- Moschioni, M.; Pansegrau, W.; Barocchi, M.A. Adhesion determinants of the Streptococcus species. Microb. Biotechnol. 2009, 3, 370–388. [Google Scholar] [CrossRef] [PubMed]
- Lazzarin, M.; Mu, R.; Fabbrini, M.; Ghezzo, C.; Rinaudo, C.D.; Doran, K.S.; Margarit, I. Contribution of pilus type 2b to invasive disease caused by a Streptococcus agalactiae ST-17 strain. BMC Microbiol. 2017, 17, 148. [Google Scholar] [CrossRef] [Green Version]
- Lauer, P.; Rinaudo, C.D.; Soriani, M.; Margarit, I.; Maione, D.; Rosini, R.; Taddei, A.R.; Mora, M.; Rappuoli, R.; Grandi, G.; et al. Genome Analysis Reveals Pili in Group B Streptococcus. Science 2005, 309, 105. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.E.; Laut, C.; Gaddy, J.A.; Zadoks, R.N.; Dele Davies, H.; Manning, S.D. Association between genotypic diversity and biofilm production in group B Streptococcus. BMC Microbiol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Pang, M.; Sun, L.; He, T.; Bao, H.; Zhang, L.; Zhou, Y.; Zhang, H.; Wei, R.; Liu, Y.; Wang, R. Molecular and virulence characterization of highly prevalent Streptococcus agalactiae circulated in bovine dairy herds. Vet. Res. 2017, 1, 65. [Google Scholar] [CrossRef]
- Nagao, P.E. Streptococcus agalactiae (Group B Streptococci). In Molecular Medical Microbiology; Academic Press: Cambridge, MA, USA, 2015; pp. 1751–1767. [Google Scholar] [CrossRef]
- Rossi, R.; Amarante, A.; Correia, L.; Guerra, S.; Nobrega, D.; Latosinski, G.; Rossi, B.; Rall, V.; Pantoja, J. Diagnostic accuracy of Somaticell, California Mastitis Test, and microbiological examination of composite milk to detect Streptococcus agalactiae intramammary infections. J. Dairy Sci. 2018, 101, 10220–10229. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Albuquerque, P.; Araujo, R.; Ribeiro, J.N.; Tavares, F. Detection and discrimination of common bovine mastitis-causing streptococci. Vet. Microbiol. 2013, 164, 370–377. [Google Scholar] [CrossRef]
- Arpini, C.M.; Cardoso, P.G. Virulence Genes of the Streptococcus agalactiae Associated with Bovine Mastitis in Minas Gerais Livestock Herds, Brazil. Appl. Microbiol. open Access 2016, 2, 1000119. [Google Scholar] [CrossRef]
- Godoy, D.T.; Carvalho-Castro, G.A.; Leal, C.A.; Pereira, U.P.; Leite, R.C.; Figueiredo, H.C. Genetic diversity and new genotyping scheme for fish pathogenic Streptococcus agalactiae. Lett. Appl. Microbiol. 2013, 57, 476–483. [Google Scholar] [CrossRef]
- Bonsaglia, E.C.R.; Latosinski, G.S.; Rossi, R.S.; Rossi, B.F.; Possebon, F.S.; Pantoja, J.C.F.; Júnior, A.F.; Rall, V.L.M. Biofilm production under different atmospheres and growth media by Streptococcus agalactiae isolated from milk of cows with subclinical mastitis. Arch. Microbiol. 2019, 202, 209–212. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Sharma, P.; Lata, H.; Arya, D.K.; Kashyap, A.K.; Kumar, H.; Ali, M.D.A.; Johri, A.K. Role of pilus proteins in adherence and invasion of Streptococcus agalactiae to the lung and cervical epithelial cells. J. Biol. Chem. 2013, 6, 4023–4034. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, U.B.S.; Klaas, I.C.; Boes, J.; Farre, M. The distribution of clones of Streptococcus agalactiae (group B streptococci) among herdspersons and dairy cows demonstrates lack of host specificity for some lineages. Vet. Microbiol. 2019, 235, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Carlin, A.F.; Lewis, A.L.; Varki, A.; Nizet, V. Group B Streptococcal Capsular Sialic Acids Interact with Siglecs (Immunoglobulin-Like Lectins) on Human Leukocytes. J. Bacteriol. 2007, 189, 1231–1237. [Google Scholar] [CrossRef]
- Paveenkittiporn, W.; Ungcharoen, R.; Kerdsin, A. Streptococcus agalactiae infections and clinical relevance in adults, Thailand. Diagn. Microbiol. Infect. Dis. 2020, 97, 115005. [Google Scholar] [CrossRef]
- Alvim, D.C.S.S.; Ferreira, A.F.M.; Leal, M.A.; Oliveira, L.M.A.; Botelho, A.M.N.; Figueiredo, A.M.S.; Fracalanzza, S.E.L.; Teixeira, L.M.; Pinto, T.C.A. Biofilm production and distribution of pilus variants among Streptococcus agalactiae isolated from human and animal sources. Biofouling 2019, 35, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Hänsch, G.M. Host Defence against Bacterial Biofilms: “Mission Impossible”? ISRN Immunol. 2012, 2012, 853123. [Google Scholar] [CrossRef]
- González, J.F.; Hahn, M.M.; Gunn, J.S. Chronic biofilm-based infections: Skewing of the immune response. Pathog. Dis. 2018, 76, fty023. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.-J.; Kang, B.-H.; Choi, B.-K.; Park, I.-S.; Min, B.-H. Clusterin induces the secretion of TNF-α and the chemotactic migration of macrophages. Biochem. Biophys. Res. Commun. 2012, 422, 200–205. [Google Scholar] [CrossRef]
- Gibson, R.L.; Lee, M.K.; Soderland, C.; Chi, E.Y.; E Rubens, C. Group B streptococci invade endothelial cells: Type III capsular polysaccharide attenuates invasion. Infect. Immun. 1993, 61, 478–485. [Google Scholar] [CrossRef]
- Shabayek, S.; Spellerberg, B. Group B Streptococcal colonization, molecular characteristics, and epidemiology. Front. Microbiol. 2018, 9, 437. [Google Scholar] [CrossRef] [PubMed]
| Profile | Isolates (n = 145) | serotype | fbsA | fbsB | pI1 | pI2a | pI2b | hylb | Adhesion BMEC * |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | Ia | + | - | - | - | + | + | 1.5 |
| 2 | 11 | III | + | - | - | - | + | + | 0.3 |
| 3 | 8 | Ia | - | + | - | + | - | + | 0.4 |
| 4 | 20 | III | - | + | - | + | - | + | 1.2 |
| 5 | 3 | III | - | + | - | - | + | - | 1.6 |
| 6 | 1 | III | - | + | - | - | - | + | 0.3 |
| 7 | 27 | III | - | + | - | - | + | + | 0.5 |
| 8 | 1 | Ia | + | - | - | + | - | - | 1.3 |
| 9 | 5 | Ia | + | - | - | - | - | + | 0.6 |
| SCC * (log10cells/mL) | Biofilm (log10 optical density) | ||||||
|---|---|---|---|---|---|---|---|
| gene | Positive | Negative | p-value | Positive | Negative | p-value ** | |
| capIa | 5.98 ± 0.04 | 5.98 ± 0.05 | 0.92 | −0.39 ± 0.03 | −0.38 ± 0.03 | 0.77 | |
| capIII | 5.98 ± 0.05 | 5.98 ± 0.04 | 0.92 | −0.38 ± 0.03 | −0.39 ± 0.03 | 0.77 | |
| fbsA | 5.98 ± 0.04 | 5.98 ± 0.05 | 0.89 | −0.38 ± 0.03 | −0.38 ± 0.03 | 0.99 | |
| fbsB | 5.98 ± 0.05 | 5.98 ± 0.04 | 0.89 | −0.38 ± 0.03 | −0.38 ± 0.03 | 0.99 | |
| pI2a | 5.97 ± 0.07 | 5.98 ± 0.03 | 0.91 | −0.39 ± 0.05 | −0.38 ± 0.03 | 0.84 | |
| pI2b | 5.98 ± 0.05 | 5.98 ± 0.04 | 0.89 | −0.38 ± 0.02 | −0.51 ± 0.10 | 0.19 | |
| hlyB | 5.97 ± 0.03 | 6.30 ± 0.18 | 0.10 | −0.39 ± 0.02 | −0.32 ± 0.13 | 0.63 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonsaglia, E.C.R.; Rossi, R.S.; Latosinski, G.; Rossi, B.F.; Campos, F.C.; Junior, A.F.; Pantoja, J.C.F.; Rall, V.L.M. Relationship between Biofilm Production and High Somatic Cell Count in Streptococcus agalactiae Isolated from Milk of Cows with Subclinical Mastitis. Pathogens 2023, 12, 311. https://doi.org/10.3390/pathogens12020311
Bonsaglia ECR, Rossi RS, Latosinski G, Rossi BF, Campos FC, Junior AF, Pantoja JCF, Rall VLM. Relationship between Biofilm Production and High Somatic Cell Count in Streptococcus agalactiae Isolated from Milk of Cows with Subclinical Mastitis. Pathogens. 2023; 12(2):311. https://doi.org/10.3390/pathogens12020311
Chicago/Turabian StyleBonsaglia, Erika Carolina Romão, Rodolfo S. Rossi, Giulia Latosinski, Bruna Fernanda Rossi, Fernanda Cristina Campos, Ary Fernandes Junior, José Carlos F. Pantoja, and Vera Lucia Mores Rall. 2023. "Relationship between Biofilm Production and High Somatic Cell Count in Streptococcus agalactiae Isolated from Milk of Cows with Subclinical Mastitis" Pathogens 12, no. 2: 311. https://doi.org/10.3390/pathogens12020311







