Recombinant Escherichia coli BL21 with LngA Variants from ETEC E9034A Promotes Adherence to HT-29 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Sequencing of the lngA Gene from Different Clinical ETEC Strains
2.3. Cloning of the lngA Variants into the pJET1.2 Vector
2.4. Transformation of ECBL with the lng Operon and lngA Variants
2.5. Site-Directed Mutagenesis of the lngA Gene
2.6. Genome Sequencing
2.7. Reverse Transcription Polymerase Chain Reaction (RT–PCR)
2.8. SDS–PAGE and Western Blot Analyses
2.9. Assay of Adherence to HT-29 Cells
2.10. Statistical Analysis
3. Results
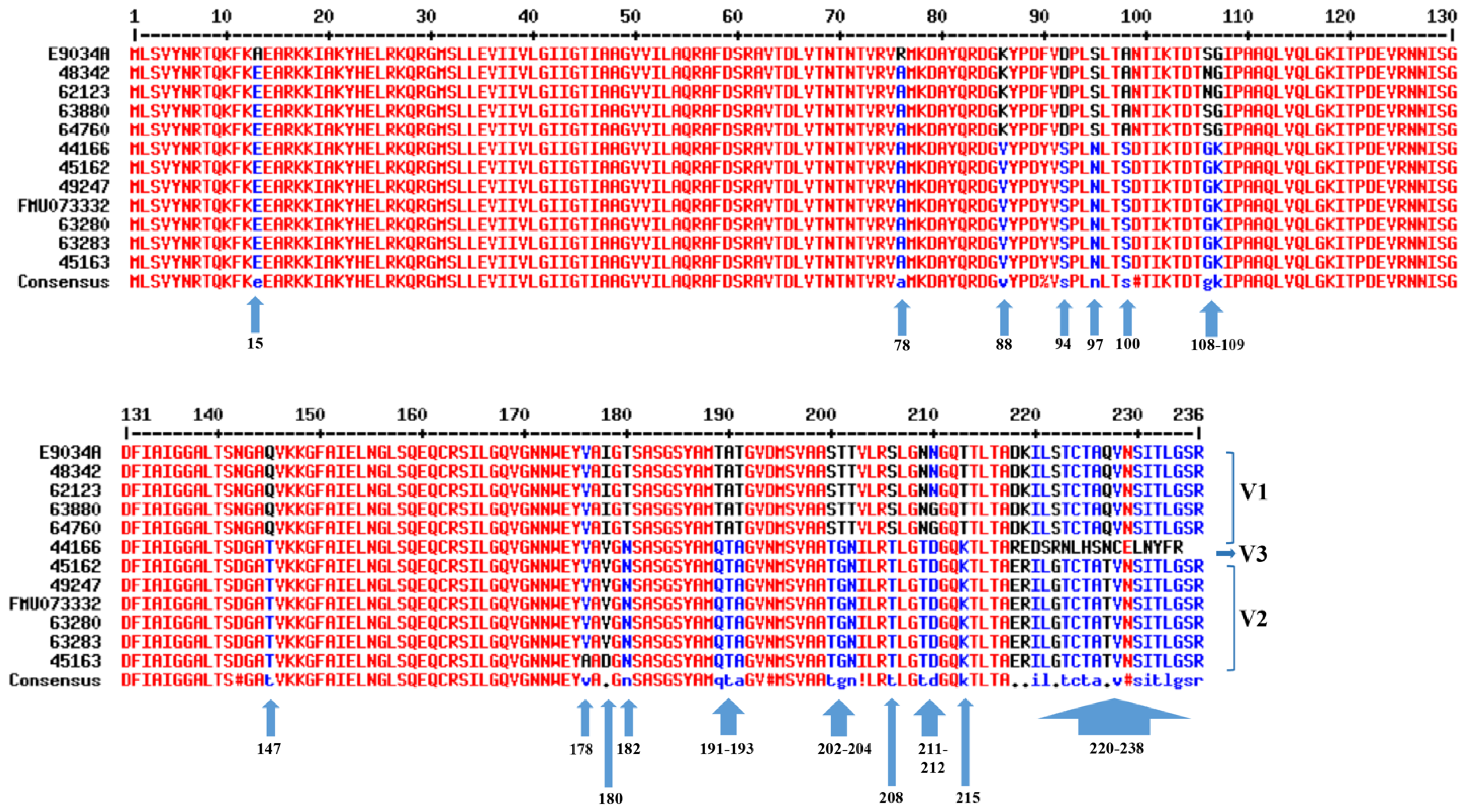
3.1. Sequencing of the lngA Gene from Different Clinical ETEC Strains
3.2. Identification of Variable Regions in the LngA Sequences from Clinical ETEC Strains
3.3. Transcription of Recombinant ECBL Strains with lngA Variants, Site-Specific lngA Mutations and Expression of the lngA Gene
3.4. Adherence to HT-29 Intestinal Cells Was Restored in Recombinant ECBL Strains with lngA Variants
3.5. Recombinant ECBL Strains with Site-Specific Mutations Showed Modified Adherence to HT-29 Intestinal Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Preferred Product Characteristics for Vaccines against Enterotoxigenic Escherichia coli. 2021. Available online: https://www.who.int/publications-detail-redirect/who-preferred-product-characteristics-for-vaccines-against-enterotoxigenic-escherichia-coli (accessed on 19 January 2023).
- Prudden, H.J.; Hasso-Agopsowicz, M.; Black, R.E.; Troeger, C.; Reiner, R.C.; Breiman, R.F.; Jit, M.; Kang, G.; Lamberti, L.; Lanata, C.F.; et al. Meeting Report: WHO Workshop on modelling global mortality and an etiology estimates of enteric pathogens in children under five. Cape Town, 28–29th November 2018. Vaccine 2020, 38, 4792–4800. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Giron, J.A.; Helander, A.; Begum, Y.A.; Asaduzzaman, M.; Xicohtencatl-Cortes, J.; Negrete, E.; Albert, M.J. Human antibody response to longus type IV pilus and study of its prevalence among enterotoxigenic Escherichia coli in Bangladesh by using monoclonal antibodies. J. Infect. Dis. 2000, 181, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Pichel, M.G.; Binsztein, N.; Qadri, F.; Giron, J.A. Type IV longus pilus of enterotoxigenic Escherichia coli: Occurrence and association with toxin types and colonization factors among strains isolated in Argentina. J. Clin. Microbiol. 2002, 40, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Sears, C.L.; Kaper, J.B. Enteric bacterial toxins: Mechanisms of action and linkage to intestinal secretion. Microbiol. Rev. 1996, 60, 167–215. [Google Scholar] [CrossRef]
- Gaastra, W.; Svennerholm, A.M. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 1996, 4, 444–452. [Google Scholar] [CrossRef]
- Isidean, S.D.; Riddle, M.S.; Savarino, S.J.; Porter, C.K. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 2011, 29, 6167–6178. [Google Scholar] [CrossRef]
- Rodea, G.E.; Montiel-Infante, F.X.; Cruz-Córdova, A.; Saldaña-Ahuactzi, Z.; Ochoa, S.A.; Espinosa-Mazariego, K.; Hernández-Castro, R.; Xicohtencatl-Cortes, J. Tracking bioluminescent ETEC during in vivo BALB/c mouse colonization. Front. Cell. Infect. Microbiol. 2017, 7, 187. [Google Scholar] [CrossRef]
- Mazariego-Espinosa, K.; Cruz, A.; Ledesma, M.A.; Ochoa, S.A.; Xicohtencatl-Cortes, J. Longus, a type IV pilus of enterotoxigenic Escherichia coli, is involved in adherence to intestinal epithelial cells. J. Bacteriol. 2010, 192, 2791–2800. [Google Scholar] [CrossRef]
- Cruz-Cordova, A.; Espinosa-Mazariego, K.; Ochoa, S.A.; Saldana, Z.; Rodea, G.E.; Cazares-Dominguez, V.; Rodriguez-Ramirez, V.; Eslava-Campos, C.A.; Navarro-Ocana, A.; Arrellano-Galindo, J.; et al. CS21 positive multidrug-resistant ETEC clinical isolates from children with diarrhea are associated with self-aggregation, and adherence. Front. Microbiol. 2014, 5, 709. [Google Scholar] [CrossRef] [PubMed]
- Saldana-Ahuactzi, Z.; Cruz-Cordova, A.; Rodea, G.E.; Porta, H.; Navarro-Ocana, A.; Eslava-Campos, C.; Cevallos, M.A.; Xicohtencatl-Cortes, J. Genome sequence of enterotoxigenic Escherichia coli strain FMU073332. Genome Announc. 2016, 5, e01600-16. [Google Scholar] [CrossRef] [PubMed]
- Guevara, C.P.; Luiz, W.B.; Sierra, A.; Cruz, C.; Qadri, F.; Kaushik, R.S.; Ferreira, L.C.; Gomez-Duarte, O.G. Enterotoxigenic Escherichia coli CS21 pilus contributes to adhesion to intestinal cells and to pathogenesis under in vivo conditions. Microbiology 2013, 159, 1725–1735. [Google Scholar] [CrossRef]
- Giron, J.A.; Levine, M.M.; Kaper, J.B. Longus: A long pilus ultrastructure produced by human enterotoxigenic Escherichia coli. Mol. Microbiol. 1994, 12, 71–82. [Google Scholar] [CrossRef]
- De la Cruz, M.A.; Ruiz-Tagle, A.; Ares, M.A.; Pacheco, S.; Yáñez, J.A.; Cedillo, L.; Torres, J.; Girón, J.A. The expression of Longus type 4 pilus of enterotoxigenic Escherichia coli is regulated by LngR and LngS and by H-NS, CpxR and CRP global regulators. Environ. Microbiol. 2017, 19, 1761–1775. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Duarte, O.G.; Chattopadhyay, S.; Weissman, S.J.; Giron, J.A.; Kaper, J.B.; Sokurenko, E.V. Genetic diversity of the gene cluster encoding longus, a type IV pilus of enterotoxigenic Escherichia coli. J. Bacteriol. 2007, 189, 9145–9149. [Google Scholar] [CrossRef] [PubMed]
- Kolappan, S.; Roos, J.; Yuen, A.S.; Pierce, O.M.; Craig, L. Structural characterization of CFA/III and Longus type IVb pili from enterotoxigenic Escherichia coli. J. Bacteriol. 2012, 194, 2725–2735. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Cazarez, Z.; Qadri, F.; Albert, M.J.; Giron, J.A. Identification of enterotoxigenic Escherichia coli harboring longus type IV pilus gene by DNA amplification. J. Clin. Microbiol. 2000, 38, 1767–1771. [Google Scholar] [CrossRef]
- Saldaña-Ahuactzi, Z.; Rodea, G.E.; Cruz-Córdova, A.; Rodríguez-Ramírez, V.; Espinosa-Mazariego, K.; González-Montalvo, M.A.; Ochoa, S.A.; González-Pedrajo, B.; Eslava-Campos, C.A.; López-Villegas, E.O.; et al. Effects of lng mutations on LngA expression, processing, and CS21 assembly in enterotoxigenic Escherichia coli E9034A. Front. Microbiol. 2017, 7, 1–19. [Google Scholar] [CrossRef]
- Levine, M.M.; Ristaino, P.; Marley, G.; Smyth, C.; Knutton, S.; Boedeker, E.; Black, R.; Young, C.; Clements, M.L.; Cheney, C.; et al. Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: Morphology, purification, and immune responses in humans. Infect. Immun. 1984, 44, 409–420. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, K.; Parkhill, J.; Crook, J.; Horsnell, T.; Rice, P.; Rajandream, M.-A.; Barrell, B. Artemis: Sequence visualization and annotation. Bioinformatics 2000, 16, 944–945. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, A.P.; Bai, J.; Gomez-Duarte, O.G. The Longus type IV pilus of enterotoxigenic Escherichia coli (ETEC) mediates bacterial self-aggregation and protection from antimicrobial agents. Microb. Pathog. 2010, 48, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 2002, 56, 289–314. [Google Scholar] [CrossRef] [PubMed]
- Foley, J. Mini-review: Strategies for variation and evolution of bacterial antigens. Comput. Struct. Biotechnol. J. 2015, 13, 407–416. [Google Scholar] [CrossRef]
- Seifert, H.S.; Therefore, M. Genetic mechanisms of bacterial antigenic variation. Microbiol. Rev. 1988, 52, 327–336. [Google Scholar] [CrossRef]
- Zhang, C.; Iqbal, J.; Gómez-Duarte, O.G. Murine immunization with CS21 pili or LngA major subunit of enterotoxigenic Escherichia coli (ETEC) elicits systemic and mucosal immune responses and inhibits ETEC gut colonization. Vet. Microbiol. 2017, 202, 90–100. [Google Scholar] [CrossRef]
- McNamara, B.P.; Donnenberg, M.S. Evidence for specificity in type 4 pilus biogenesis by enteropathogenic Escherichia coli. Microbiology (Reading) 2000, 146, 719–729. [Google Scholar] [CrossRef]
- Nassif, X.; Lowy, J.; Stenberg, P.; O’Gaora, P.; Ganji, A.; Therefore, M. Antigenic variation of pilin regulates adhesion of Neisseria meningitidis to human epithelial cells. Mol. Microbiol. 1993, 8, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Nell, S.; Kennemann, L.; Schwarz, S.; Josenhans, C.; Suerbaum, S. Dynamics of Lewis b binding and sequence variation of the babA adhesin gene during chronic Helicobacter pylori infection in humans. MBio 2014, 5, e02281-14. [Google Scholar] [CrossRef] [PubMed]
- Lannergård, J.; Gustafsson, M.C.U.; Waldemarsson, J.; Norrby-Teglund, A.; Stålhammar-Carlemalm, M.; Lindahl, G. The Hypervariable Region of Streptococcus pyogenes M protein escapes antibody attack by antigenic variation and weak immunogenicity. Cell Host Microbe 2011, 10, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Lannergård, J.; Kristensen, B.M.; Gustafsson, M.C.U.; Persson, J.J.; Norrby-Teglund, A.; Stålhammar-Carlemalm, M.; Lindahl, G. Sequence variability is correlated with weak immunogenicity in Streptococcus pyogenes M protein. MicrobiologyOpen 2015, 4, 774–789. [Google Scholar] [CrossRef]
- Zhang, J.R.; Hardham, J.M.; Barbour, A.G.; Norris, S.J. Antigenic variation in lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 1997, 89, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Boddicker, J.D.; Ledeboer, N.A.; Jagnow, J.; Jones, B.D.; Clegg, S. Differential binding to and biofilm formation on, HEp-2 cells by Salmonella enterica Serovar typhimurium is dependent upon allelic variation in the fimH gene of the fim gene cluster. Mol. Microbiol. 2002, 45, 1255–1265. [Google Scholar] [CrossRef]





| ETEC Isolate | Origin | Serotypes | CS21 | LT | ST | CS1 | CS3 | CFA/I | CS8 | Adherence to HT-29 Cells (CFUs/mL) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E9034A | Caribbean | O8:H9 | + | + | + | - | + | + | + | 4–8 * × 106 | Strong |
| 44166 | Mexico | O78:H12 | + | - | + | - | - | + | - | 3 × 106 | Moderate |
| 45162 | Mexico | O78:H12 | + | + | + | - | - | - | - | 2 × 106 | Weak |
| 49247 | Mexico | O6:H16 | + | + | + | - | + | - | - | 42 × 106 | Strong |
| 62123 | Mexico | O6:H16 | + | + | - | - | - | - | - | 40 × 106 | Strong |
| 63280 | Mexico | O78:H12 | + | - | + | - | - | + | - | 5.6 × 106 | Moderate |
| 63880 | Mexico | O6:H16 | + | + | - | - | - | - | - | 3.5 × 106 | Moderate |
| 64760 | Mexico | O8:H− | + | - | + | - | - | - | - | 4.6 × 106 | Moderate |
| FMU073332 | Mexico | O6:H16 | + | + | + | - | + | - | - | 8.5 × 106 | Strong |
| 45163 | Mexico | O78:H12 | + | - | - | - | - | - | - | 9.5 × 106 | Strong |
| 48342 | Mexico | O6:H16 | + | + | + | + | + | - | - | 0.95 × 106 | Weak |
| 63283 | Mexico | O78:H12 | + | - | - | - | - | - | - | 32 × 106 | Strong |
| Strain | Operon lng | Gen lngA | Kanamycin Resistance | Description | Reference |
|---|---|---|---|---|---|
| EBCL | - | - | - | E. coli BL21, suitable for transformation | Lab collection |
| EBCL/pJET 1.2 | - | - | - | E. coli BL21 with pJET 1.2 | This study |
| E9034A | + | + | - | Wild-type ETEC (O8:H9, CS21+, CS3+, STp+ and LT+) | Levine et al. [21] |
| FMU73332 | + | + | - | Wild-type ETEC (O6:H16, CS21+, CS3+, STp+ and LT+) | Cruz-Cordova et al. [12] |
| E9034AΔlngA::km | + | - | + | E9034A with a nonpolar insertional mutation in lngA | Cruz-Cordova et al. [12] |
| Primer | Sequence (5′→3′) | Use | Reference |
|---|---|---|---|
| LngA-F | TGCGGATCCGTGATCTGAAGAAAAATAA | Cloning of lngA in pJET1.2/blunt | Saldana-Ahuactzi et al. [13] |
| LngA-R | TGTGAGAAGGTACTAGCCTATCATATT | ||
| LngAsec-F | CAGATTGGTTGAATCAGTTGTCA | lngA amplification and sequencing | This study |
| LngAsec-R | TGTGAGAAGGTACTAGCCTATCATATT | ||
| LngH-F | AGAGAATTCCCGGGAAAGTACAGGCTG | Amplification of the lngH gene | Saldana-Ahuactzi et al. [13] |
| LngH-R | GAGTCATAGATCGGTAATCCTGAAAGCTTCAT | ||
| lngD-F | GTCCCATGGGGATCCGTTTTCTTCAGAACAATAT | Amplification of the lngD gene | Saldana-Ahuactzi et al. [13] |
| lngD-R | CCATAAGAGCTCCAGCGCAATTTTTTCATC | ||
| 357 | CTCCTACGGGAGGCAGCAG | 16S amplification | Lane [22] |
| 519 | GWATTACCGCGGCKGCTG | 16S amplification |
| Recombinant Strain | Variant of lngA in ETEC Strains | Recombinant and Variant | Denomination | Description | Reference |
|---|---|---|---|---|---|
| ECBLΔlngA E. coli BL21 carrying the plasmid with the lng operon of the CS21 pilus without the lngA gene (ECBLpE9034AΔlngA) | E9034A | ECBLpE9034AΔlngA/pJETE9034A | ECBLΔlngApE9034A | ECBL complemented with the lngA gene variant from the E9034A strain. | This study |
| 48342 | ECBLpE9034AΔlngA/pJET48342 | ECBLΔlngAp48342 | ECBL complemented with the lngA gene variant from the 48342 strain. | This study | |
| 44166 | ECBLpE9034AΔlngA/pJET44166 | ECBLΔlngAp44166 | ECBL complemented with the lngA gene variant from the 44166 strain. | This study | |
| 45162 | ECBLpE9034AΔlngA/pJET45162 | ECBLΔlngAp45162 | ECBL complemented with the lngA gene variant from the 45162 strain. | This study | |
| FMU73332 | ECBLpE9034AΔlngA/pJETFMU073332 | ECBLΔlngApFMU073332 | ECBL complemented with the lngA gene variant from the FMU73332 strain. | This study | |
| 63280 | ECBLpE9034AΔlngA/pJET63280 | ECBLΔlngAp63280 | ECBL complemented with the lngA gene variant from the 63280 strain. | This study |
| Recombinant Strain | Complementation Vector | Specific Mutation | Strains with Site-Specific Mutation | ||
|---|---|---|---|---|---|
| ECBLΔlngA | pJET2.0 lngAFMU073332 | Strain FMU73332 | Amino Acid Substitution in ETEC E9034A | Localization | |
| ECBLΔlngA E. coli BL21 carrying the plasmid with the lng operon of CS21 without the lngA gene (ECBLpE9034AΔlngA) | Plasmid carrying the lngA gene of the FMU073332 strain | QTA | TAT | 191–193 | ECBLΔlngApQTA-TAT |
| GN | TT | 204, 205 | ECBLΔlngApGN-TT | ||
| T | S | 208 | ECBLΔlngApT-S | ||
| G | S | 224 | ECBLΔlngApG-S | ||
| TD | NN | 211, 212 | ECBLΔlngApTD-NN | ||
| K | T | 215 | ECBLΔlngApK-T | ||
| ER | DK | 220, 221 | ECBLΔlngApER-DK | ||
| T | Q | 229 | ECBLΔlngApT-Q | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Mazariego, K.; Saldaña-Ahuactzi, Z.; Ochoa, S.A.; González-Pedrajo, B.; Cevallos, M.A.; Rodríguez-Martínez, R.; Romo-Castillo, M.; Hernández-Castro, R.; Cruz-Córdova, A.; Xicohtencatl-Cortes, J. Recombinant Escherichia coli BL21 with LngA Variants from ETEC E9034A Promotes Adherence to HT-29 Cells. Pathogens 2023, 12, 337. https://doi.org/10.3390/pathogens12020337
Espinosa-Mazariego K, Saldaña-Ahuactzi Z, Ochoa SA, González-Pedrajo B, Cevallos MA, Rodríguez-Martínez R, Romo-Castillo M, Hernández-Castro R, Cruz-Córdova A, Xicohtencatl-Cortes J. Recombinant Escherichia coli BL21 with LngA Variants from ETEC E9034A Promotes Adherence to HT-29 Cells. Pathogens. 2023; 12(2):337. https://doi.org/10.3390/pathogens12020337
Chicago/Turabian StyleEspinosa-Mazariego, Karina, Zeus Saldaña-Ahuactzi, Sara A. Ochoa, Bertha González-Pedrajo, Miguel A. Cevallos, Ricardo Rodríguez-Martínez, Mariana Romo-Castillo, Rigoberto Hernández-Castro, Ariadnna Cruz-Córdova, and Juan Xicohtencatl-Cortes. 2023. "Recombinant Escherichia coli BL21 with LngA Variants from ETEC E9034A Promotes Adherence to HT-29 Cells" Pathogens 12, no. 2: 337. https://doi.org/10.3390/pathogens12020337
APA StyleEspinosa-Mazariego, K., Saldaña-Ahuactzi, Z., Ochoa, S. A., González-Pedrajo, B., Cevallos, M. A., Rodríguez-Martínez, R., Romo-Castillo, M., Hernández-Castro, R., Cruz-Córdova, A., & Xicohtencatl-Cortes, J. (2023). Recombinant Escherichia coli BL21 with LngA Variants from ETEC E9034A Promotes Adherence to HT-29 Cells. Pathogens, 12(2), 337. https://doi.org/10.3390/pathogens12020337









