Orthopoxvirus Zoonoses—Do We Still Remember and Are Ready to Fight?
Abstract
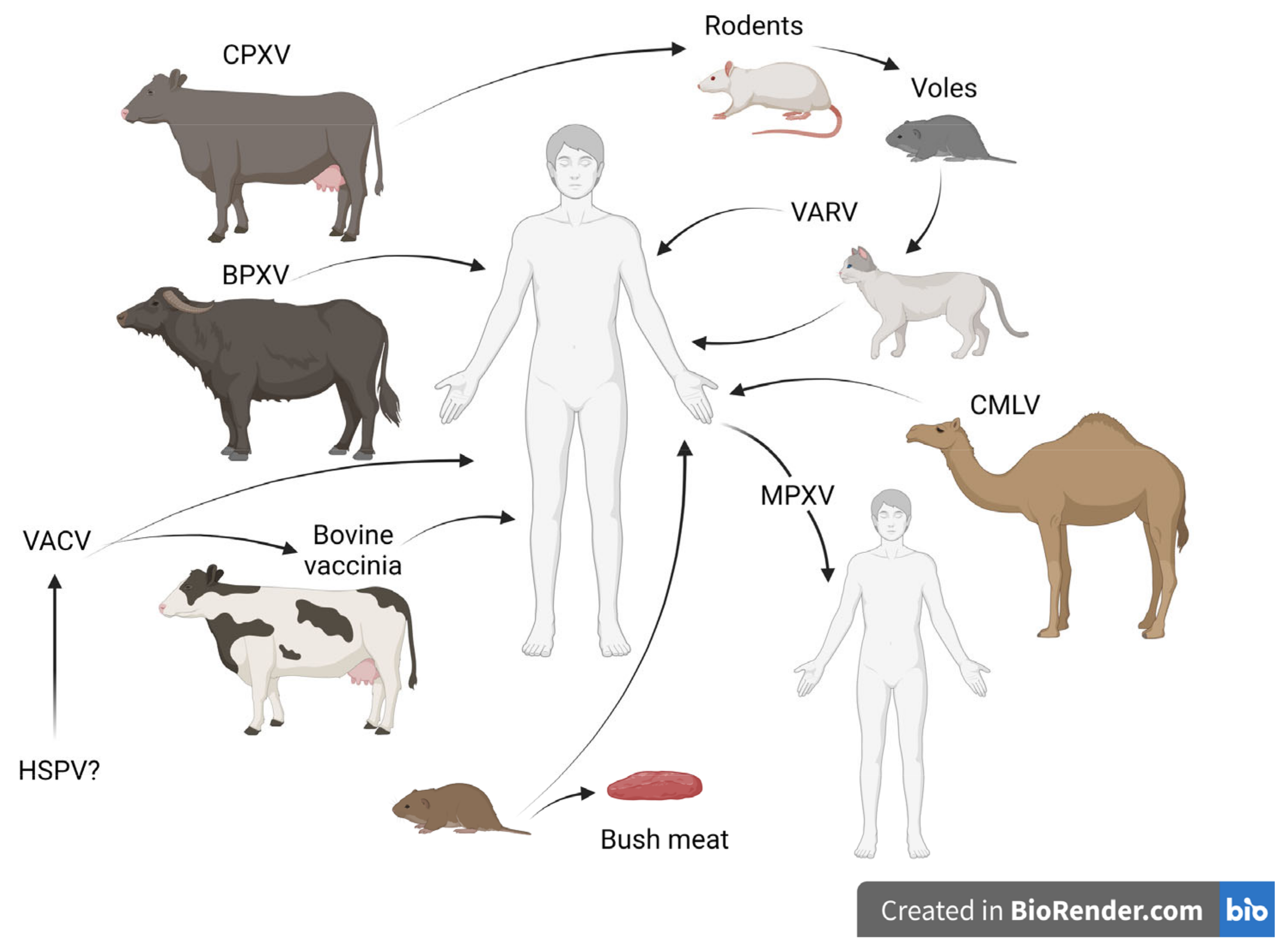
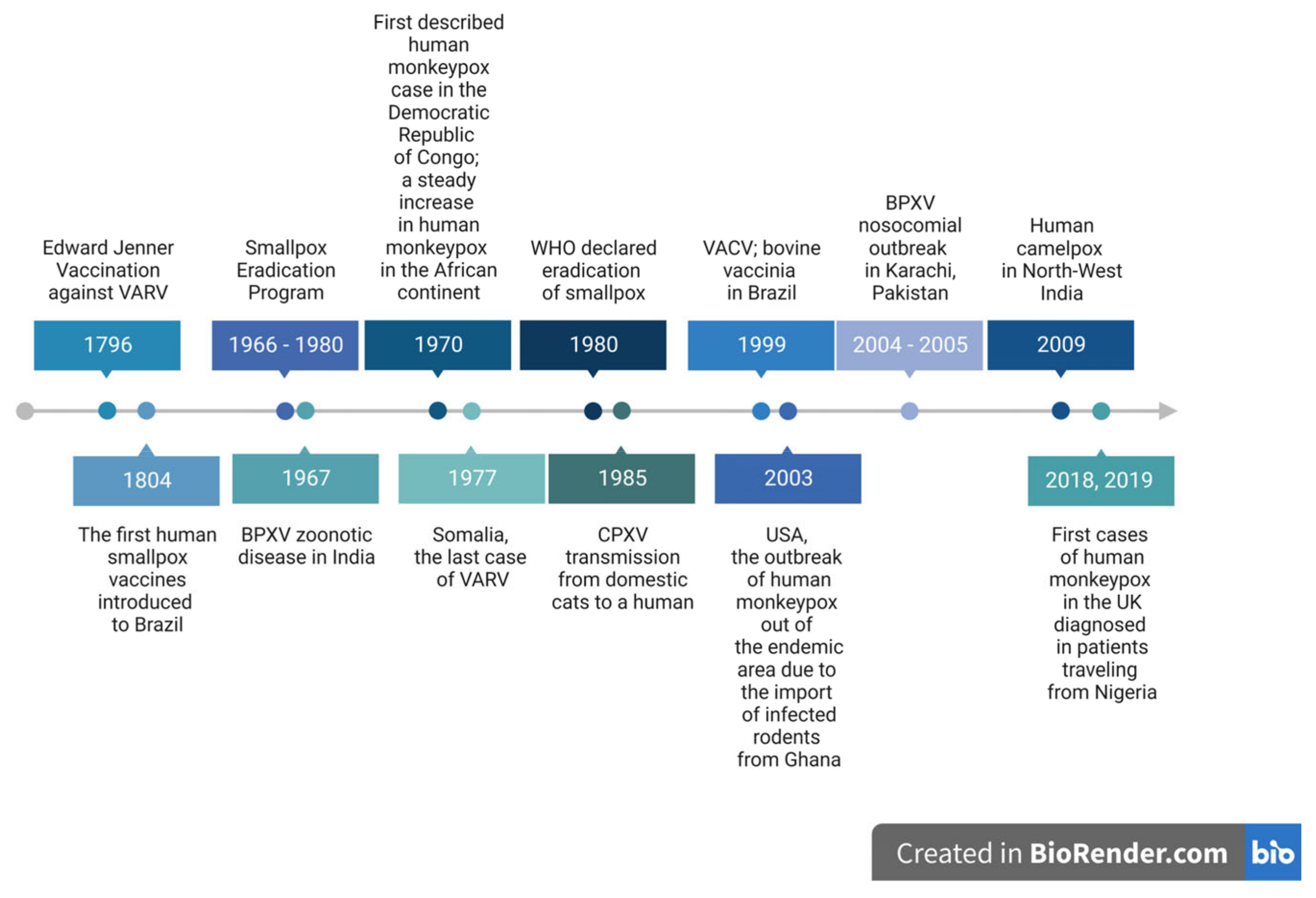
1. Introduction
2. Orthopoxvirus Zoonotic Infections
2.1. Cowpox
2.2. Vaccinia Virus
2.3. Zoonotic Outbreaks Caused by CMLV and BPXV
2.4. Monkeypox
3. Monkeypox Prophylaxis
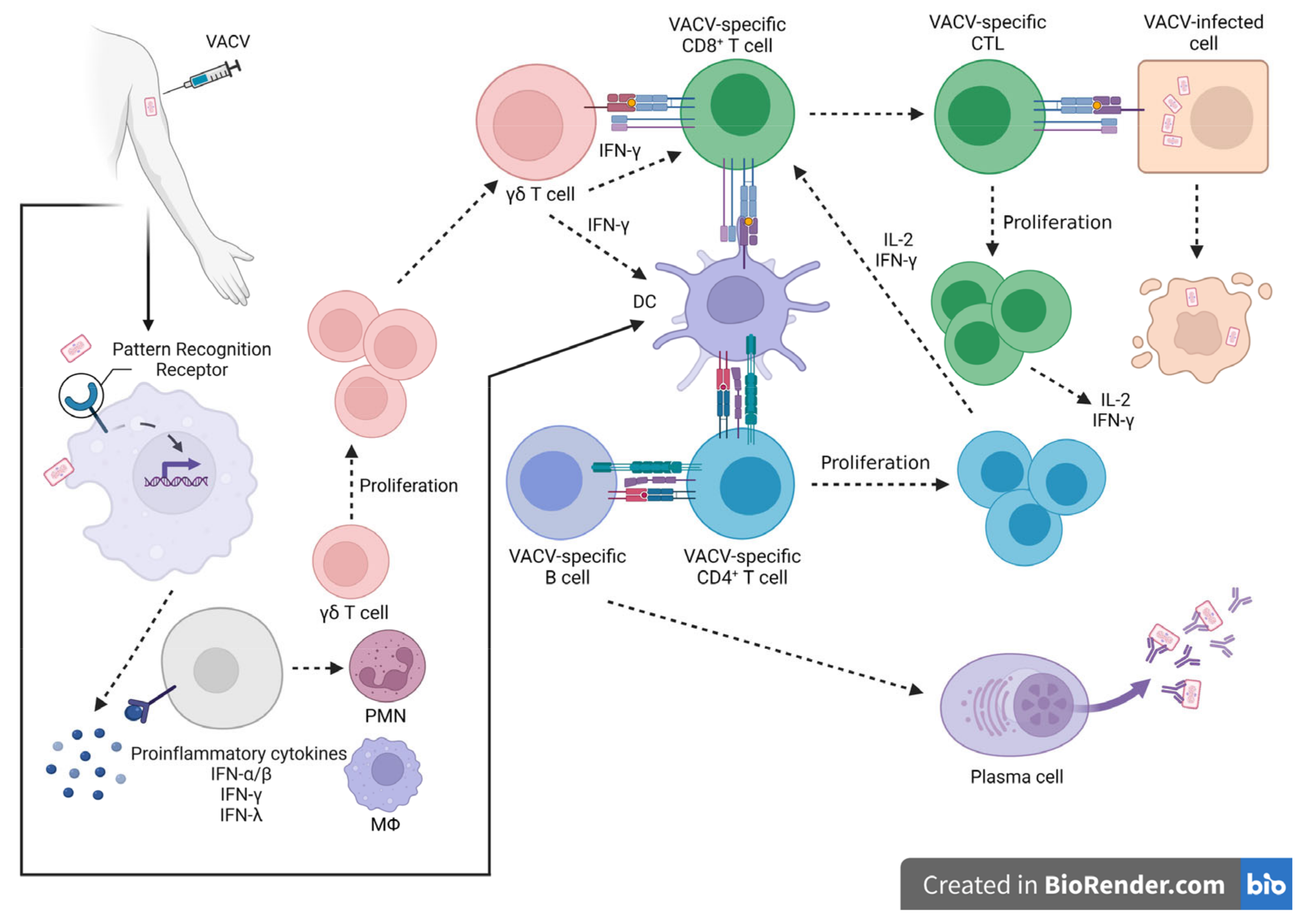
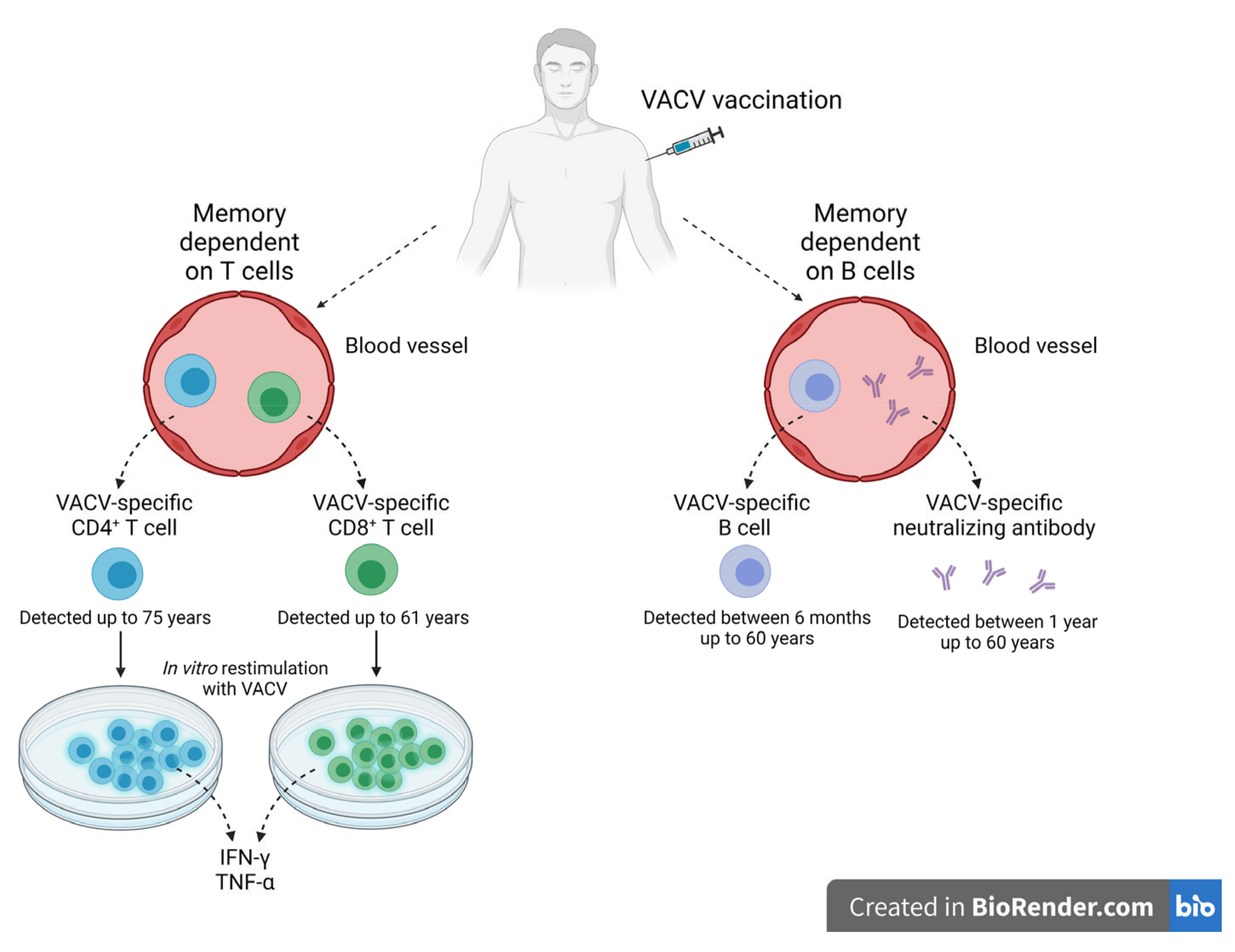
4. Stimulation of the Protective Immune Response—Long Memory against OPXV Infections
Immunological Memory against OPXV Infections
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIM-2 | absent in melanoma |
| AK2015-poxvirus | Alaskapox virus |
| BPXV | Buffalopox virus |
| BV | Bovine vaccinia |
| cDC | conventional dendritic cell |
| cGas | cyclic GMP-AMP synthase |
| CMLV | Camelpox virus |
| CPXV | cowpox virus |
| DAI | DNA-dependent activator of IFN-regulatory factors |
| DNA-PK | DNA-dependent protein kinase |
| DRC | Democratic Republic of Congo |
| ECTV | Ectromelia virus |
| EV | extracellular enveloped virion |
| GBLV | Taterapox virus |
| HSPV | Horsepox virus |
| IFI16 | IFN-γ inducible protein 16, stimulator |
| MHC | major histocompatibility complex |
| MPXV | Monkeypox virus |
| MV | mature virion |
| OPXV | orthopoxvirus |
| PHEIC | public health emergency of international concern |
| PKR | Protein kinase R |
| RPV | Rabbitpox virus |
| SEP | smallpox eradication program |
| STAT | signal transducer and activator of transcription |
| STING | stimulator of interferon genes |
| TLR | toll-like receptor |
| VACV | Vaccinia virus |
| VARV | Variola virus |
| VIG | vaccinia immune globulin |
| VPXV | Volepox virus |
| WHO | World Health Organization |
References
- National Library of Medicine. An Inquiry into the Causes and Effects of the Variolae Vaccinae: A Disease Discovered in Some of the Western Counties of England, Particularly Gloucestershire, and Known by the Name of the Cow Pox—Digital Collections. Available online: https://collections.nlm.nih.gov/catalog/nlm:nlmuid-2559001R-bk (accessed on 8 August 2022).
- Diaz, J.H. The Disease Ecology, Epidemiology, Clinical Manifestations, Management, Prevention, and Control of Increasing Human Infections with Animal Orthopoxviruses. Wilderness Environ. Med. 2021, 32, 528–536. [Google Scholar] [CrossRef] [PubMed]
- ICTV. Poxviridae. Available online: https://ictv.global/report_9th/dsDNA/poxviridae (accessed on 8 August 2022).
- Moss, B. Poxvirus cell entry: How many proteins does it take? Viruses 2012, 4, 688–707. [Google Scholar] [CrossRef]
- Duncan, S.A.; Smith, G.L. Identification and characterization of an extracellular envelope glycoprotein affecting vaccinia virus egress. J. Virol. 1992, 66, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Roper, R.L.; Payne, L.G.; Moss, B. Extracellular vaccinia virus envelope glycoprotein encoded by the A33R gene. J. Virol. 1996, 70, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Van Eijl, H.; Hollinshead, M.; Rodger, G.; Zhang, W.H.; Smith, G.L. The vaccinia virus F12L protein is associated with intracellular enveloped virus particles and is required for their egress to the cell surface. J. Gen. Virol. 2002, 83, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Van Eijl, H.; Hollinshead, M.; Smith, G.L. The vaccinia virus A36R protein is a type Ib membrane protein present on intracellular but not extracellular enveloped virus particles. Virology 2000, 271, 26–36. [Google Scholar] [CrossRef]
- Domi, A.; Weisberg, A.S.; Moss, B. Vaccinia Virus E2L Null Mutants Exhibit a Major Reduction in Extracellular Virion Formation and Virus Spread. J. Virol. 2008, 82, 4215–4226. [Google Scholar] [CrossRef] [PubMed]
- Moor, K.; Diard, M.; Sellin, M.E.; Felmy, B.; Wotzka, S.Y.; Toska, A.; Bakkeren, E.; Arnoldini, M.; Bansept, F.; Co, A.D.; et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature 2017, 544, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Blasco, R.; Moss, B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J. Virol. 1991, 65, 5910–5920. [Google Scholar] [CrossRef] [PubMed]
- Engelstad, M.; Howard, S.T.; Smith, G.L. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology 1992, 188, 801–810. [Google Scholar] [CrossRef]
- Moss, B. Smallpox vaccines: Targets of protective immunity. Immunol. Rev. 2011, 239, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.P.; Rodrigues, R.A.L.; Lima, M.T.; Drumond, B.P.; Abrahão, J.S. Poxvirus host range genes and virus–host spectrum: A critical review. Viruses 2017, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N. An Increasing Danger of Zoonotic Orthopoxvirus Infections. PLoS Pathog. 2013, 9, e1003756. [Google Scholar] [CrossRef]
- Meyer, H.; Ehmann, R.; Smith, G.L. Smallpox in the post-eradication Era. Viruses 2020, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, P.F.; Posch, J.; Richter, K.H.; Henderson, D.A. An airborne outbreak of smallpox in a German hospital and its significance with respect to other recent outbreaks in Europe. Bull. World Health Organ. 1970, 43, 669–679. [Google Scholar]
- WHO. WHA33.4—Global Smallpox Eradication. Available online: https://www.who.int/publications/i/item/WHA33-4 (accessed on 8 August 2022).
- Smith, K.A. Smallpox: Can we still learn from the journey to eradication? Indian J. Med. Res. 2013, 137, 895. [Google Scholar]
- Tack, D.M.; Reynolds, M.G. Zoonotic poxviruses associated with companion animals. Animals 2011, 1, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.I.O.; de Oliveira, J.S.; Kroon, E.G.; de Souza Trindade, G.; Drumond, B.P. Here, there, and everywhere: The wide host range and geographic distribution of zoonotic orthopoxviruses. Viruses 2021, 13, 43. [Google Scholar] [CrossRef]
- Parrino, J.; Graham, B.S. Smallpox vaccines: Past, present, and future. J. Allergy Clin. Immunol. 2006, 118, 1320–1326. [Google Scholar] [CrossRef]
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I.D.; WHO (Eds.) Smallpox and Its Eradication; WHO: Geneva, Switzerland, 1988; pp. 1371–1409. [Google Scholar]
- Henderson, D.A. The eradication of smallpox—An overview of the past, present, and future. Vaccine 2011, 29, D7. [Google Scholar] [CrossRef]
- CDC. Smallpox. Research. Available online: https://www.cdc.gov/smallpox/research/index.html (accessed on 8 August 2022).
- Noyce, R.S.; Lederman, S.; Evans, D.H. Construction of an infectious horsepox virus vaccine from chemically synthesized DNA fragments. PLoS ONE 2018, 13, e0188453. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Ramsay, A.J.; Christensen, C.D.; Beaton, S.; Hall, D.F.; Ramshaw, I.A. Expression of Mouse Interleukin-4 by a Recombinant Ectromelia Virus Suppresses Cytolytic Lymphocyte Responses and Overcomes Genetic Resistance to Mousepox. J. Virol. 2001, 75, 1205–1210. [Google Scholar] [CrossRef]
- Gubser, C.; Smith, G.L. The sequence of camelpox virus shows it is most closely related to variola virus, the cause of smallpox. J. Gen. Virol. 2002, 83, 855–872. [Google Scholar] [CrossRef] [PubMed]
- McFadden, G. Poxvirus tropism. Nat. Rev. Microbiol. 2005, 3, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.L.; Peng, C.; McFadden, G.; Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 2014, 21, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Essbauer, S.; Pfeffer, M.; Meyer, H. Zoonotic poxviruses. Vet. Microbiol. 2010, 140, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Chantrey, J.; Meyer, H.; Baxby, D.; Begon, M.; Bown, K.J.; Hazel, S.M.; Jones, T.; Montgomery, W.I.; Bennett, M. Cowpox: Reservoir hosts and geographic range. Epidemiol. Infect. 1999, 122, 455–460. [Google Scholar] [CrossRef]
- CDC. African Rodent Importation Ban; Monkeypox_ Poxvirus; CDC: Atlanta, GA, USA, 2022. [Google Scholar]
- Enserink, M.U.S. Monkeypox Outbreak Traced to Wisconsin Pet Dealer. Science 2003, 300, 1639. [Google Scholar] [CrossRef]
- Baxby, D.; Bennett, M. Cowpox: A re-evaluation of the risks of human cowpox based on new epidemiological information. In Viral Zoonoses and Food of Animal Origin; Archives of Virology. Supplementa; Springer: Vienna, Austria, 1997; pp. 1–12. [Google Scholar] [CrossRef]
- Becker, C.; Kurth, A.; Hessler, F.; Kramp, H.; Gokel, M.; Hoffmann, R.; Kuczka, A.; Nitsche, A. Kuhpocken bei haltern von farbratten—Ein nicht immer sofort erkanntes krankheitsbild. Dtsch. Arztebl. 2009, 106, 329–334. [Google Scholar] [CrossRef]
- CDC. Poxvirus. Mpox. Mpox in Animals. Available online: https://www.cdc.gov/poxvirus/monkeypox/veterinarian/monkeypox-in-animals.html (accessed on 28 December 2022).
- Crouch, A.C.; Baxby, D.; Mccracken, C.M.; Gaskell, R.M.; Bennett, M. Serological evidence for the reservoir hosts of cowpox virus in British wildlife. Epidemiol. Infect. 1995, 115, 185–191. [Google Scholar] [CrossRef]
- Bennett, M.; Gaskell, R.M.; Gaskell, C.J.; Baxby, D.; Kelly, D.F. Studies on poxvirus infection in cats. Arch. Virol. 1989, 104, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Willemse, A.; Egberink, H.F. Transmission of cowpox virus infection from domestic cat to man. Lancet 1985, 325, 1515. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Gaskell, C.J.; Gaskell, R.M.; Baxby, D.; Gruffydd-Jones, T.J. Poxvirus infection in the domestic cat: Some clinical and epidemiological observations. Vet. Rec. 1986, 118, 387–390. [Google Scholar] [CrossRef]
- Pfeffer, M.; Pfleghaar, S.; von Bomhard, D.; Kaaden, O.R.; Meyer, H. Retrospective investigation of feline cowpox in Germany. Vet. Rec. 2002, 150, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Campe, H.; Zimmermann, P.; Glos, K.; Bayer, M.; Bergemann, H.; Dreweck, C.; Graf, P.; Weber, B.K.; Meyer, H.; Büttner, M.; et al. Cowpox virus transmission from pet rats to humans, Germany. Emerg. Infect. Dis. 2009, 15, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Baxby, D.; Bennett, M.; Getty, B. Human cowpox 1969–93: A review based on 54 cases. Br. J. Dermatol. 1994, 131, 598–607. [Google Scholar] [CrossRef]
- Ninove, L.; Domart, Y.; Vervel, C.; Voinot, C.; Salez, N.; Raoult, D.; Meyer, H.; Capek, I.; Zandotti, C.; Charrel, R.N. Cowpox virus transmission from pet rats to humans, France. Emerg. Infect. Dis. 2009, 15, 781–784. [Google Scholar] [CrossRef]
- Kretzschmar, M.; Wallinga, J.; Teunis, P.; Xing, S.; Mikolajczyk, R. Frequency of adverse events after vaccination with different vaccinia strains. PLoS Med. 2006, 3, 1341–1351. [Google Scholar] [CrossRef]
- Trindade, G.S.; Emerson, G.L.; Carroll, D.S.; Kroon, E.G.; Damon, I.K. Brazilian vaccinia viruses and their origins. Emerg. Infect. Dis. 2007, 13, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.B.; Borges, I.A.; Campos, S.P.S.; Vieira, F.N.; De Ázara, T.M.F.; Marques, F.A.; Costa, G.B.; Luis, A.P.M.F.; De Oliveira, J.S.; Ferreira, P.C.P.; et al. Serologic and molecular evidence of vaccinia virus circulation among small mammals from different biomes, Brazil. Emerg. Infect. Dis. 2017, 23, 931–938. [Google Scholar] [CrossRef]
- Medaglia, M.L.G.; Moussatché, N.; Nitsche, A.; Dabrowski, P.W.; Li, Y.; Damon, I.K.; Lucas, C.G.O.; Arruda, L.B.; Damaso, C.R. Genomic Analysis, Phenotype, and Virulence of the Historical Brazilian Smallpox Vaccine Strain IOC: Implications for the Origins and Evolutionary Relationships of Vaccinia Virus. J. Virol. 2015, 89, 11909–11925. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.S.; Figueiredo, P.d.O.; Costa, G.B.; De Assis, F.L.; Drumond, B.P.; Da Fonseca, F.G.; Nogueira, M.L.; Kroon, E.G.; de Souza Trindade, G. Vaccinia virus natural infections in Brazil: The good, the bad, and the ugly. Viruses 2017, 9, 340. [Google Scholar] [CrossRef]
- Abrahão, J.S.; Campos, R.K.; De Souza Trindade, G.; Da Fonseca, F.G.; Ferreira, P.C.P.; Kroon, E.G. Outbreak of Severe Zoonotic Vaccinia Virus Infection, Southeastern Brazil. Emerg. Infect. Dis. 2015, 21, 695. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.M.A.S.; Maranhão, A.G.K.; Teixeira, A.M.; Fantinato, F.F.S.; Domingues, R.A.S. The Brazilian National Immunization Program: 46 years of achievements and challenges. Cad. Saude Publica 2020, 36, e00222919. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Domingos, I.J.; de Oliveira, J.S.; Rocha, K.L.S.; de Oliveira, D.B.; Kroon, E.G.; Costa, G.B.; de Souza Trindade, G. Twenty years after bovine vaccinia in brazil: Where we are and where are we going? Pathogens 2021, 10, 406. [Google Scholar] [CrossRef]
- Scheffer, M.; Paiva, V.S.F.; Barberia, L.G.; Russo, G. Monkeypox in Brazil between stigma, politics, and structural shortcomings: Have we not been here before? Lancet Reg. Health-Am. 2022, 17, 100394. [Google Scholar] [CrossRef]
- Damaso, C.R.A.; Esposito, J.J.; Condit, R.C.; Moussatché, N. An emergent poxvirus from humans and cattle in Rio de Janeiro state: Cantagalo virus may derive from brazilian smallpox vaccine. Virology 2000, 277, 439–449. [Google Scholar] [CrossRef]
- Borges, I.A.; Reynolds, M.G.; McCollum, A.M.; Figueiredo, P.O.; Ambrosio, L.L.D.; Vieira, F.N.; Costa, G.B.; Matos, A.C.D.; de Andrade Almeida, V.M.; Ferreira, P.C.P.; et al. Serological evidence of Orthopoxvirus circulation among equids, southeast Brazil. Front. Microbiol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Guedes, M.I.M.; Trindade, G.S.; Fonseca, F.G.; Campos, R.K.; Mota, B.F.; Lobato, Z.I.P.; Silva-Fernandes, A.T.; Rodrigues, G.O.L.; Lima, L.S.; et al. One more piece in the VACV ecological puzzle: Could peridomestic rodents be the link between wildlife and bovine vaccinia outbreaks in Brazil? PLoS ONE 2009, 4, e7428. [Google Scholar] [CrossRef]
- Mota, B.E.F.; Trindade, G.S.; Diniz, T.C.; da Silva-Nunes, M.; Braga, E.M.; Urbano-Ferreira, M.; Rodrigues, G.O.L.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Seroprevalence of orthopoxvirus in an Amazonian rural village, Acre, Brazil. Arch. Virol. 2010, 155, 1139–1144. [Google Scholar] [CrossRef]
- Rivetti, A.V.; Guedes, M.I.M.C.; Rehfeld, I.S.; Oliveira, T.M.L.; Matos, A.C.D.; Abrahão, J.S.; Kroon, E.G.; Lobato, Z.I.P. Bovine vaccinia, a systemic infection: Evidence of fecal shedding, viremia and detection in lymphoid organs. Vet. Microbiol. 2013, 162, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, I.S.; Fraiha, A.L.S.; Matos, A.C.D.; Guedes, M.I.M.C.; Costa, E.A.; de Souza, M.R.; Cavalcante, L.F.L.; Lobato, Z.I.P. Short communication: Survival of Vaccinia virus in inoculated cheeses during 60-day ripening. J. Dairy Sci. 2017, 100, 7051–7054. [Google Scholar] [CrossRef] [PubMed]
- Siqueira Ferreira, J.M.; Abrahão, J.S.; Drumond, B.P.; Oliveira, F.M.; Alves, P.A.; Pascoal-Xavier, M.A.; Lobato, Z.I.P.; Bonjardim, C.A.; Peregrino Ferreira, P.C.; Kroon, E.G. Vaccinia virus: Shedding and horizontal transmission in a murine model. J. Gen. Virol. 2008, 89, 2986–2991. [Google Scholar] [CrossRef] [PubMed]
- Goff, A.; Twenhafel, N.; Garrison, A.; Mucker, E.; Lawler, J.; Paragas, J. In vivo imaging of cidofovir treatment of cowpox virus infection. Virus Res. 2007, 128, 88–98. [Google Scholar] [CrossRef]
- Balamurugan, V.; Venkatesan, G.; Bhanuprakash, V.; Singh, R.K. Camelpox, an emerging orthopox viral disease. Indian J. Virol. 2013, 24, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Bera, B.C.; Shanmugasundaram, K.; Barua, S.; Venkatesan, G.; Virmani, N.; Riyesh, T.; Gulati, B.R.; Bhanuprakash, V.; Vaid, R.K.; Kakker, N.K.; et al. Zoonotic cases of camelpox infection in India. Vet. Microbiol. 2011, 152, 29–38. [Google Scholar] [CrossRef]
- Eltom, K.H.; Samy, A.M.; El Wahed, A.A.; Czerny, C.P. Buffalopox virus: An emerging virus in livestock and humans. Pathogens 2020, 9, 676. [Google Scholar] [CrossRef] [PubMed]
- Afrough, B.; Zafar, A.; Hasan, R.; Hewson, R. Complete genome sequence of buffalopox virus. Genome Announc. 2018, 6, e00444-18. [Google Scholar] [CrossRef]
- Yadav, P.D.; Mauldin, M.R.; Nyayanit, D.A.; Albariño, C.G.; Sarkale, P.; Shete, A.; Guerrero, L.W.; Nakazawa, Y.; Nichol, S.T.; Mourya, D.T. Isolation and phylogenomic analysis of buffalopox virus from human and buffaloes in India. Virus Res. 2020, 277, 197836. [Google Scholar] [CrossRef]
- Baxby, D.; Hill, B.J. Characteristics of a new poxvirus isolated from indian buffaloes. Arch. Gesamte Virusforsch. 1971, 35, 70–79. [Google Scholar] [CrossRef]
- Zafar, A.; Swanepoel, R.; Hewson, R.; Nizam, M.; Ahmed, A.; Husain, A.; Grobbelaar, A.; Bewley, K.; Mioulet, V.; Dowsett, B.; et al. Nosocomial buffalopoxvirus infection, Karachi, Pakistan. Emerg. Infect. Dis. 2007, 13, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Najarro, P.; Traktman, P.; Lewis, J.A. Vaccinia Virus Blocks Gamma Interferon Signal Transduction: Viral VH1 Phosphatase Reverses Stat1 Activation. J. Virol. 2001, 75, 3185–3196. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.; Kiss-Toth, E.; Symons, J.A.; Smith, G.L.; Dower, S.K.; O’Neill, L.A.J. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 2000, 97, 10162–10167. [Google Scholar] [CrossRef]
- Alcamí, A.; Smith, G.L. The vaccinia virus soluble interferon-γ receptor is a homodimer. J. Gen. Virol. 2002, 83, 545–549. [Google Scholar] [CrossRef]
- Liptáková, H.; Kontseková, E.; Alcamí, A.; Smith, G.L.; Kontsek, P. Analysis of an interaction between the soluble vaccinia virus-coded type I interferon (IFN)-receptor and human IFN-α1 and IFN-α2. Virology 1997, 232, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun. 2022, 131, 102855. [Google Scholar] [CrossRef]
- Kozlov, M. Monkeypox outbreaks: 4 key questions researchers have. Nature 2022, 606, 238–239. [Google Scholar] [PubMed]
- Von Magnus, P.; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A pox-like disease in cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Arita, I.; Henderson, D.A. Smallpox and monkeypox in non-human primates. Bull. World Health Organ. 1968, 39, 277–283. [Google Scholar] [PubMed]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar] [PubMed]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A tale of two clades: Monkeypox viruses. J. Gen. Virol. 2005, 86, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Totmenin, A.V.; Babkin, I.V.; Safronov, P.F.; Ryazankina, O.I.; Petrov, N.A.; Gutorov, V.V.; Uvarova, E.A.; Mikheev, M.V.; Sisler, J.R.; et al. Human monkeypox and smallpox viruses: Genomic comparison. FEBS Lett. 2001, 509, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Happi, C.; Adetifa, I.; Mbala, P.; Njouom, R.; Nakoune, E.; Happi, A.; Ndodo, N.; Ayansola, O.; Mboowa, G.; Bedford, T.; et al. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. PLoS Biol. 2022, 20, e3001769. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, H.; Ding, K.; Wang, X.-H.; Sun, G.-Y.; Liu, Z.-X.; Luo, Y. The evolving epidemiology of monkeypox virus. Cytokine Growth Factor Rev. 2022, 68, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Luna, N.; Ramírez, A.L.; Muñoz, M.; Ballesteros, N.; Patiño, L.H.; Castañeda, S.A.; Bonilla-Aldana, D.K.; Paniz-Mondolfi, A.; Ramírez, J.D. Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: Emergence of a novel viral lineage? Travel Med. Infect. Dis. 2022, 49, 102402. [Google Scholar] [CrossRef]
- Kmiec, D.; Kirchhoff, F. Monkeypox: A New Threat? Int. J. Mol. Sci. 2022, 23, 7866. [Google Scholar] [CrossRef]
- Ježek, Z.; Szczeniowski, M.; Paluku, K.M.; Mutombo, M. Human Monkeypox: Clinical Features of 282 Patients. J. Infect. Dis. 1987, 156, 293–298. [Google Scholar] [CrossRef]
- Sklenovská, N.; Van Ranst, M. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front. Public Health 2018, 6, 241. [Google Scholar] [CrossRef]
- Khodakevich, L.; Jezek, Z.; Kinzanzka, K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet 1986, 1, 98–99. [Google Scholar] [CrossRef]
- Radonić, A.; Metzger, S.; Dabrowski, P.W.; Couacy-Hymann, E.; Schuenadel, L.; Kurth, A.; Mätz-Rensing, K.; Boesch, C.; Leendertz, F.H.; Nitsche, A. Fatal monkeypox in wild-living sooty mangabey, Côte d’Ivoire, 2012. Emerg. Infect. Dis. 2014, 20, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Cann, J.A.; Jahrling, P.B.; Hensley, L.E.; Wahl-Jensen, V. Comparative Pathology of Smallpox and Monkeypox in Man and Macaques. J. Comp. Pathol. 2013, 148, 6–21. [Google Scholar] [CrossRef]
- Xiang, Y.; White, A. Monkeypox Virus Emerges from The Shadow of Its More Infamous Cousin: Family Biology Matters. Emerg. Microbes Infect. 2022, 11, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.G.; Doty, J.B.; Mccollum, A.M.; Olson, V.A.; Nakazawa, Y. Expert Review of Anti-infective Therapy Monkeypox re-emergence in Africa: A call to expand the concept and practice of One Health Monkeypox re-emergence in Africa: A call to expand the concept and practice of One Health. One Health Expert Rev. Anti-Infect. Ther. 2019, 17, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Guagliardo, S.A.J.; Monroe, B.; Moundjoa, C.; Athanase, A.; Okpu, G.; Burgado, J.; Townsend, M.B.; Satheshkumar, P.S.; Epperson, S.; Doty, J.B.; et al. Asymptomatic orthopoxvirus circulation in humans in the wake of a monkeypox outbreak among chimpanzees in Cameroon. Am. J. Trop. Med. Hyg. 2020, 102, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Patrono, L.V.; Pléh, K.; Samuni, L.; Ulrich, M.; Röthemeier, C.; Sachse, A.; Muschter, S.; Nitsche, A.; Couacy-Hymann, E.; Boesch, C.; et al. Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity. Nat. Microbiol. 2020, 5, 955–965. [Google Scholar] [CrossRef]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar]
- Grant, R.; Nguyen, L.B.L.; Breban, R. Modelling human-to-human transmission of monkeypox. Bull. World Health Organ. 2020, 98, 638–640. [Google Scholar] [CrossRef]
- Oladoye, M.J. Monkeypox: A Neglected Viral Zoonotic Disease. Eur. J. Med. Educ. Technol. 2021, 14, em2108. [Google Scholar] [CrossRef]
- Zhu, M.; Ji, J.; Shi, D.; Lu, X.; Wang, B.; Wu, N.; Wu, J.; Yao, H.; Li, L. Unusual global outbreak of monkeypox: What should we do? Front. Med. 2022, 16, 507–517. [Google Scholar] [CrossRef]
- Kisalu, N.K.; Mokili, J.L. Toward Understanding the Outcomes of Monkeypox Infection in Human Pregnancy. J. Infect. Dis. 2017, 216, 795–797. [Google Scholar]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J.; et al. Outbreak of human monkeypox in Nigeria in 2017–18: A clinical and epidemiological report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef] [PubMed]
- McCollum, A.M.; Damon, I.K. Human Monkeypox. Clin. Infect. Dis. 2014, 58, 206–267. [Google Scholar] [CrossRef]
- Moore, M.J.; Rathish, B.; Zahra, F. Monkeypox. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Breman, J.G.; Ruti, K.; Steniowski, M.V. Human monkeypox, 1970–1979. Bull. World Health Organ. 1980, 58, 165–182. [Google Scholar]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Osadebe, L.; Hughes, C.M.; Shongo Lushima, R.; Kabamba, J.; Nguete, B.; Malekani, J.; Pukuta, E.; Karhemere, S.; Muyembe Tamfum, J.J.; Wemakoy Okitolonda, E.; et al. Enhancing case definitions for surveillance of human monkeypox in the Democratic Republic of Congo. PLoS Negl. Trop. Dis. 2017, 11, e0005857. [Google Scholar] [CrossRef]
- Ogoina, D.; Iroezindu, M.; James, H.I.; Oladokun, R.; Yinka-Ogunleye, A.; Wakama, P.; Otike-Odibi, B.; Usman, L.M.; Obazee, E.; Aruna, O.; et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin. Infect. Dis. 2020, 71, E210–E214. [Google Scholar] [CrossRef] [PubMed]
- Kabuga, A.I.; El Zowalaty, M.E. A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J. Med. Virol. 2019, 91, 533–540. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Reed, K.D.; Melski, J.W.; Beth Graham, M.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- Croft, D.R.; Sotir, M.J.; Williams, C.J.; Kazmierczak, J.J.; Wegner, M.V.; Rausch, D.; Graham, M.B.; Foldy, S.L.; Wolters, M.; Damon, I.K.; et al. Occupational Risks during a Monkeypox Outbreak, Wisconsin, 2003. Emerg. Infect. Dis. 2007, 13, 1150. [Google Scholar] [CrossRef]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical Characteristics of Human Monkeypox, and Risk Factors for Severe Disease. Clin. Infect. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.; Heymann, D.; Brown, C.S.; Edmunds, W.J.; Elsgaard, J.; Fine, P.; Hochrein, H.; Hoff, N.A.; Green, A.; Ihekweazu, C.; et al. Human monkeypox—After 40 years, an unintended consequence of smallpox eradication. Vaccine 2020, 38, 5077–5081. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Guitian, J.; Simons, D.; Asogun, D.; Ansumana, R.; Honeyborne, I.; Velavan, T.P.; Ntoumi, F.; Valdoleiros, S.R.; Petersen, E.; et al. Increased outbreaks of monkeypox highlight gaps in actual disease burden in Sub-Saharan Africa and in animal reservoirs. Int. J. Infect. Dis. 2022, 122, 107–111. [Google Scholar] [CrossRef]
- Jamali, Y.A.; Khan, J.A.; Mangrio, W.M.; Majid, A.; Shaikh, S.A.; Farrukh Memon, S.; Shaikh, N.; Maitlo, M.A.; Ghumro, R.; Shaikh, S.H. Human Monkeypox Outbreaks from 2001 to 2021—A Systematic Review. Pak. J. Med. Health Sci. 2022, 16, 1206. [Google Scholar] [CrossRef]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Meseda, C.A.; Weir, J.P. Third-generation smallpox vaccines: Challenges in the absence of clinical smallpox. Future Microbiol. 2010, 5, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.B.; Ovsyannikova, I.G.; Jacobson, R.M.; Poland, G.A. The immunology of smallpox vaccines. Curr. Opin. Immunol. 2009, 21, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Weltzin, R.; Liu, J.; Pugachev, K.V.; Myers, G.A.; Coughlin, B.; Blum, P.S.; Nichols, R.; Johnson, C.; Cruz, J.; Kennedy, J.S.; et al. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat. Med. 2003, 9, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Kaynarcalidan, O.; Moreno Mascaraque, S.; Drexler, I. Vaccinia virus: From crude smallpox vaccines to elaborate viral vector vaccine design. Biomedicines 2021, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, S.; Suzuki, K.; Uchida, N. Gene Structures of Low-Neurovirulent Vaccinia Virus LC16m0, LC16m8, and Their Lister Original (LO) Strains. Microbiol. Immunol. 1985, 29, 421–428. [Google Scholar] [CrossRef]
- Abdelaal, A.; Reda, A.; Lashin, B.I.; Katamesh, B.E.; Brakat, A.M.; AL-Manaseer, B.M.; Kaur, S.; Asija, A.; Patel, N.K.; Basnyat, S.; et al. Preventing the Next Pandemic: Is Live Vaccine Efficacious Against Monkeypox, or There is a Need for Killed Virus and mRNA Vaccines? Vaccines 2022, 10, 1419. [Google Scholar] [CrossRef]
- Food and Drug Administration. Key Facts About Monkeypox Vaccine. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-about-monkeypox-vaccine (accessed on 17 August 2022).
- Food and Drug Administration. Key Facts about Vaccines to Prevent Monkeypox Disease. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-about-vaccines-prevent-monkeypox-disease (accessed on 30 November 2022).
- Jahrling, P.B.; Hensley, L.E.; Martinez, M.J.; LeDuc, J.W.; Rubins, K.H.; Relman, D.A.; Huggins, J.W. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc. Natl. Acad. Sci. USA 2004, 101, 15196–15200. [Google Scholar] [CrossRef]
- Sergeev, A.A.; Kabanov, A.S.; Bulychev, L.E.; Sergeev, A.A.; Pyankov, O.V.; Bodnev, S.A.; Galahova, D.O.; Zamedyanskaya, A.S.; Titova, K.A.; Glotova, T.I.; et al. Using the Ground Squirrel (Marmota bobak) as an Animal Model to Assess Monkeypox Drug Efficacy. Transbound. Emerg. Dis. 2017, 64, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Garver, J.; Weber, L.; Vela, E.M.; Anderson, M.; Warren, R.; Merchlinsky, M.; Houchens, C.; Rogers, J.V. Ectromelia virus disease characterization in the BALB/c mouse: A surrogate model for assessment of smallpox medical countermeasures. Viruses 2016, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Falendysz, E.A.; Lopera, J.G.; Lorenzsonn, F.; Salzer, J.S.; Hutson, C.L.; Doty, J.; Gallardo-Romero, N.; Carroll, D.S.; Osorio, J.E.; Rocke, T.E. Further Assessment of Monkeypox Virus Infection in Gambian Pouched Rats (Cricetomys gambianus) Using In Vivo Bioluminescent Imaging. PLoS Negl. Trop. Dis. 2015, 9, e0004130. [Google Scholar] [CrossRef] [PubMed]
- Americo, J.L.; Moss, B.; Earl, P.L. Identification of Wild-Derived Inbred Mouse Strains Highly Susceptible to Monkeypox Virus Infection for Use as Small Animal Models. J. Virol. 2010, 84, 8172–8180. [Google Scholar] [CrossRef]
- Esteban, D.; Parker, S.; Schriewer, J.; Hartzler, H.; Buller, R.M. Mousepox, a small animal model of smallpox. Methods Mol. Biol. 2012, 890, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Nalca, A.; Nichols, D.K. Rabbitpox: A model of airborne transmission of smallpox. J. Gen. Virol. 2011, 92, 31–35. [Google Scholar] [CrossRef]
- Kramski, M.; Mätz-Rensing, K.; Stahl-Hennig, C.; Kaup, F.J.; Nitsche, A.; Pauli, G.; Ellerbrok, H. A novel highly reproducible and lethal nonhuman primate model for orthopox virus infection. PLoS ONE 2010, 5, e10412. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.L.; Nichols, D.K.; Martinez, M.J.; Raymond, J.W. Animal models of orthopoxvirus infection. Vet. Pathol. 2010, 47, 852–870. [Google Scholar] [CrossRef]
- Gierynska, M.; Szulc-Dabrowska, L.; Dzieciatkowski, T.; Golke, A.; Schollenberger, A. The generation of CD8+ T-cell population specific for vaccinia virus epitope involved in the antiviral protection against ectromelia virus challenge. Pathog. Dis. 2015, 73, ftv088. [Google Scholar] [CrossRef] [PubMed]
- Keckler, M.S.; Carroll, D.S.; Gallardo-Romero, N.F.; Lash, R.R.; Salzer, J.S.; Weiss, S.L.; Patel, N.; Clemmons, C.J.; Smith, S.K.; Hutson, C.L.; et al. Establishment of the Black-Tailed Prairie Dog (Cynomys ludovicianus) as a Novel Animal Model for Comparing Smallpox Vaccines Administered Preexposure in both High- and Low-Dose Monkeypox Virus Challenges. J. Virol. 2011, 85, 7683–7698. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Sbrana, E.; Watts, D.M.; Siirin, M.; Travassos Da Rosa, A.P.A.; Tesh, R.B. Experimental infection of prairie dogs with monkeypox virus. Emerg. Infect. Dis. 2005, 11, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Olson, V.A.; Carroll, D.D.; Abel, J.A.; Hughes, C.M.; Braden, Z.H.; Weiss, S.; Self, J.; Osorio, J.E.; Hudson, P.N.; et al. A prairie dog animal model of systemic orthopoxvirus disease using west African and Congo Basin strains of Monkeypox virus. J. Gen. Virol. 2009, 90, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, I.; Ami, Y.; Suzaki, Y.; Nagata, N.; Fukushi, S.; Ogata, M.; Morikawa, S.; Hasegawa, H.; Mizuguchi, M.; Kurane, I.; et al. A single vaccination of nonhuman primates with highly attenuated smallpox vaccine, LC16m8, provides long-term protection against monkeypox. Jpn. J. Infect. Dis. 2017, 70, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Shannon Keckler, M.; Salzer, J.S.; Patel, N.; Townsend, M.B.; Akazawa, Y.J.; Doty, J.B.; Gallardo-Romero, N.F.; Satheshkumar, P.S.; Carroll, D.S.; Karem, K.L.; et al. IMVAMUNE ® and ACAM2000 ® Provide Different Protection against Disease When Administered Postexposure in an Intranasal Monkeypox Challenge Prairie Dog Model. Vaccines 2020, 8, 396. [Google Scholar] [CrossRef]
- Townsend, M.B.; Keckler, M.S.; Patel, N.; Davies, D.H.; Felgner, P.; Damon, I.K.; Karem, K.L. Humoral Immunity to Smallpox Vaccines and Monkeypox Virus Challenge: Proteomic Assessment and Clinical Correlations. J. Virol. 2013, 87, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, L. DNA-Sensing Antiviral Innate Immunity in Poxvirus Infection. Front. Immunol. 2020, 11, 1637. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, C.A. Regulating IRFs in IFN driven disease. Front. Immunol. 2019, 10, 325. [Google Scholar] [CrossRef]
- Yu, H.; Bruneau, R.C.; Brennan, G.; Rothenburg, S. Battle Royale: Innate Recognition of Poxviruses and Viral Immune Evasion. Biomedicines 2021, 9, 765. [Google Scholar] [CrossRef]
- Caron, J.; Ridgley, L.A.; Bodman-Smith, M. How to Train Your Dragon: Harnessing Gamma Delta T Cells Antiviral Functions and Trained Immunity in a Pandemic Era. Front. Immunol. 2021, 12, 983. [Google Scholar] [CrossRef]
- Katze, M.G.; He, Y.; Gale, M. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef]
- Szulc-Dąbrowska, L.; Wojtyniak, P.; Struzik, J.; Toka, F.N.; Winnicka, A.; Gieryńska, M. ECTV Abolishes the Ability of GM-BM Cells to Stimulate Allogeneic CD4 T Cells in a Mouse Strain-Independent Manner. Immunol. Investig. 2019, 48, 392–409. [Google Scholar] [CrossRef] [PubMed]
- Struzik, J.; Szulc-Dabrowską, L.; Mielcarska, M.B.; Bossowska-Nowicka, M.; Koper, M.; Gieryńska, M. First Insight into the Modulation of Noncanonical NF-κB Signaling Components by Poxviruses in Established Immune-Derived Cell Lines: An In Vitro Model of Ectromelia Virus Infection. Pathogens 2020, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Rehm, K.E.; Connor, R.F.; Jones, G.J.B.; Yimbu, K.; Mannie, M.D.; Roper, R.L. Vaccinia virus decreases major histocompatibility complex (MHC) class II antigen presentation, T-cell priming, and peptide association with MHC class II. Immunology 2009, 128, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Moulton, E.A.; Atkinson, J.P.; Buller, R.M.L. Surviving Mousepox Infection Requires the Complement System. PLoS Pathog. 2008, 4, 1000249. [Google Scholar] [CrossRef]
- Seet, B.T.; Johnston, J.B.; Brunetti, C.R.; Barrett, J.W.; Everett, H.; Cameron, C.; Sypula, J.; Nazarian, S.H.; Lucas, A.; McFadden, G. Poxviruses and immune evasion. Annu. Rev. Immunol. 2003, 21, 377–423. [Google Scholar] [CrossRef]
- Alcami, A.; Koszinowski, U.H. Viral mechanisms of immune evasion. Mol. Med. Today 2000, 6, 365–372. [Google Scholar] [CrossRef]
- Sakala, I.G.; Chaudhri, G.; Buller, R.M.; Nuara, A.A.; Bai, H.; Chen, N.; Karupiah, G. Poxvirus-Encoded Gamma Interferon Binding Protein Dampens the Host Immune Response to Infection. J. Virol. 2007, 81, 3346–3353. [Google Scholar] [CrossRef]
- Albarnaz, J.D.; Torres, A.A.; Smith, G.L. Modulating vaccinia virus immunomodulators to improve immunological memory. Viruses 2018, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Nuara, A.A.; Bai, H.; Chen, N.; Buller, R.M.L.; Walter, M.R. The Unique C Termini of Orthopoxvirus Gamma Interferon Binding Proteins Are Essential for Ligand Binding. J. Virol. 2006, 80, 10675–10682. [Google Scholar] [CrossRef]
- Colamonici, O.R.; Domanski, P.; Sweitzer, S.M.; Larner, A.; Buller, R.M.L. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon α transmembrane signaling. J. Biol. Chem. 1995, 270, 15974–15978. [Google Scholar] [CrossRef]
- Rice, A.D.; Turner, P.C.; Embury, J.E.; Moldawer, L.L.; Baker, H.V.; Moyer, R.W. Roles of Vaccinia Virus Genes E3L and K3L and Host Genes PKR and RNase L during Intratracheal Infection of C57BL/6 Mice. J. Virol. 2011, 85, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Abaitua, F.; Martínez-Sobrido, L.; Esteban, M.; García-Sastre, A.; Rodríguez, D. Host-range restriction of vaccinia virus E3L deletion mutant can be overcome in vitro, but not in vivo, by expression of the influenza virus NS1 protein. PLoS ONE 2011, 6, e28677. [Google Scholar] [CrossRef] [PubMed]
- Tscharke, D.C.; Karupiah, G.; Zhou, J.; Palmore, T.; Irvine, K.R.; Haeryfar, S.M.M.; Williams, S.; Sidney, J.; Sette, A.; Bennink, J.R.; et al. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J. Exp. Med. 2005, 201, 95–104. [Google Scholar] [CrossRef]
- Tscharke, D.C.; Woo, W.-P.; Sakala, I.G.; Sidney, J.; Sette, A.; Moss, D.J.; Bennink, J.R.; Karupiah, G.; Yewdell, J.W. Poxvirus CD8 + T-Cell Determinants and Cross-Reactivity in BALB/c Mice. J. Virol. 2006, 80, 6318–6323. [Google Scholar] [CrossRef]
- Sette, A.; Grey, H.; Oseroff, C.; Peters, B.; Moutaftsi, M.; Crotty, S.; Assarsson, E.; Greenbaum, J.; Kim, Y.; Kolla, R.; et al. Definition of epitopes and antigens recognized by vaccinia specific immune responses: Their conservation in variola virus sequences, and use as a model system to study complex pathogens. Vaccine 2009, 27, G21–G26. [Google Scholar] [CrossRef]
- Moutaftsi, M.; Tscharke, D.C.; Vaughan, K.; Koelle, D.M.; Stern, L.; Calvo-Calle, M.; Ennis, F.; Terajima, M.; Sutter, G.; Crotty, S.; et al. Uncovering the interplay between CD8, CD4 and antibody responses to complex pathogens. Future Microbiol. 2010, 5, 221–239. [Google Scholar] [CrossRef]
- Amanna, I.J.; Slifka, M.K.; Crotty, S. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 2006, 211, 320–337. [Google Scholar] [CrossRef]
- Goulding, J.; Abboud, G.; Tahiliani, V.; Desai, P.; Hutchinson, T.E.; Salek-Ardakani, S. CD8 T Cells Use IFN-γ To Protect against the Lethal Effects of a Respiratory Poxvirus Infection. J. Immunol. 2014, 192, 5415–5425. [Google Scholar] [CrossRef] [PubMed]
- Goulding, J.; Bogue, R.; Tahiliani, V.; Croft, M.; Salek-Ardakani, S. CD8 T Cells Are Essential for Recovery from a Respiratory Vaccinia Virus Infection. J. Immunol. 2012, 189, 2432–2440. [Google Scholar] [CrossRef] [PubMed]
- Holechek, S.A.; Denzler, K.L.; Heck, M.C.; Schriewer, J.; Buller, R.M.; Legrand, F.A.; Verardi, P.H.; Jones, L.A.; Yilma, T.; Jacobs, B.L. Use of a Recombinant Vaccinia Virus Expressing Interferon Gamma for Post-Exposure Protection against Vaccinia and Ectromelia Viruses. PLoS ONE 2013, 8, e77879. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.H.; Fang, M.; Klein-Szanto, A.; Sigal, L.J. Memory CD8+ T cells are gatekeepers of the lymph node draining the site of viral infection. Proc. Natl. Acad. Sci. USA 2007, 104, 10992–10997. [Google Scholar] [CrossRef] [PubMed]
- Edghill-Smitlh, Y.; Bray, M.; Whitehouse, C.A.; Miller, D.; Mucker, E.; Manischewitz, J.; King, L.R.; Hobart-Guroff, M.; Hryniewicz, A.; Venzom, D.; et al. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J. Infect. Dis. 2005, 191, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Edghill-Smith, Y.; Golding, H.; Manischewitz, J.; King, L.R.; Scott, D.; Bray, M.; Nalca, A.; Hooper, J.W.; Whitehouse, C.A.; Schmitz, J.E.; et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005, 11, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Abate, G.; Eslick, J.; Newman, F.K.; Frey, S.E.; Belshe, R.B.; Monath, T.P.; Hoft, D.F. Flow-cytometric detection of vaccinia-induced memory effector CD4+, CD8+, and γδTCR+ T cells capable of antigen-specific expansion and effector functions. J. Infect. Dis. 2005, 192, 1362–1371. [Google Scholar] [CrossRef]
- Worku, S.; Gorse, G.J.; Belshe, R.B.; Hoft, D.F. Canarypox vaccines induce antigen-specific human γδ T cells capable of interferon-γ production. J. Infect. Dis. 2001, 184, 525–532. [Google Scholar] [CrossRef]
- Johnson, M.D.; Witherden, D.A.; Havran, W.L. The Role of Tissue-resident T Cells in Stress Surveillance and Tissue Maintenance. Cells 2020, 9, 686. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Witherden, D.A.; Havran, W.L. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017, 17, 733–745. [Google Scholar] [CrossRef]
- Muoz-Ruiz, M.; Ribot, J.C.; Grosso, A.R.; Gonalves-Sousa, N.; Pamplona, A.; Pennington, D.J.; Regueiro, J.R.; Fernndez-Malavé, E.; Silva-Santos, B. TCR signal strength controls thymic differentiation of discrete proinflammatory γδT cell subsets. Nat. Immunol. 2016, 17, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Lawand, M.; Déchanet-Merville, J.; Dieu-Nosjean, M.C. Key features of gamma-delta T-cell subsets in human diseases and their immunotherapeutic implications. Front. Immunol. 2017, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Himoudi, N.; Morgenstern, D.A.; Yan, M.; Vernay, B.; Saraiva, L.; Wu, Y.; Cohen, C.J.; Gustafsson, K.; Anderson, J. Human γδ T Lymphocytes Are Licensed for Professional Antigen Presentation by Interaction with Opsonized Target Cells. J. Immunol. 2012, 188, 1708–1716. [Google Scholar] [CrossRef]
- Gieryńska, M.; Pawlak, E.; Schollenberger, A.; Cespedes, I.S. Dendritic epidermal T cells: Their role in the early phase of ectromelia virus infection. Postep. Hig. Med. Dosw. 2009, 63, 369–376. [Google Scholar]
- Selin, L.K.; Santolucito, P.A.; Pinto, A.K.; Szomolanyi-Tsuda, E.; Welsh, R.M. Innate Immunity to Viruses: Control of Vaccinia Virus Infection by γδ T Cells. J. Immunol. 2001, 166, 6784–6794. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Huang, X.; Yang, Y. γδT Cells Are Required for CD8+ T Cell Response to Vaccinia Viral Infection. Front. Immunol. 2021, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Mack, T.M.; Noble, J.; Thomas, D.B. A prospective study of serum antibody and protection against smallpox. Am. J. Trop. Med. Hyg. 1972, 21, 214–218. [Google Scholar] [CrossRef]
- Cherry, J.D.; Connor, J.D.; McIntosh, K.; Benenson, A.S.; Alling, D.W.; Rolfe, U.T.; Schanberger, J.E.; Mattheis, M.J. Standard percutaneous revaccination of children who received primary percutaneous vaccination. J. Infect. Dis. 1977, 135, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Kempe, C.H.; Bowles, C.; Meiklejohn, G.; Berge, T.O.; St Vincent, L.; Babu, B.V.; Govindarajan, S.; Ratnakannan, N.R.; Downie, A.W.; Murthy, V.R. The use of vaccinia hyperimmune gamma-globulin in the prophylaxis of smallpox. Bull. World Health Organ. 1961, 25, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, G.; Panchanathan, V.; Bluethmann, H.; Karupiah, G. Obligatory Requirement for Antibody in Recovery from a Primary Poxvirus Infection. J. Virol. 2006, 80, 6339–6344. [Google Scholar] [CrossRef]
- Panchanathan, V.; Chaudhri, G.; Karupiah, G. Antiviral protection following immunization correlates with humoral but not cell-mediated immunity. Immunol. Cell Biol. 2010, 88, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S.; Felgner, P.; Davies, H.; Glidewell, J.; Villarreal, L.; Ahmed, R. Cutting edge: Long-term B cell memory in humans after smallpox vaccination. J. Immunol. 2003, 171, 4969–4973. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Chung, C.-S.; Heine, H.G.; Chang, W. Vaccinia Virus Envelope H3L Protein Binds to Cell Surface Heparan Sulfate and Is Important for Intracellular Mature Virion Morphogenesis and Virus Infection In Vitro and In Vivo. J. Virol. 2000, 74, 3353–3365. [Google Scholar] [CrossRef]
- Hammarlund, E.; Lewis, M.W.; Hansen, S.G.; Strelow, L.I.; Nelson, J.A.; Sexton, G.J.; Hanifin, J.M.; Slifka, M.K. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003, 9, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Sivapalasingam, S.; Kennedy, J.S.; Borkowsky, W.; Valentine, F.; Zhan, M.X.; Pazoles, P.; Paolino, A.; Ennis, F.A.; Steigbigel, N.H. Immunological memory after exposure to variola virus, monkeypox virus, and vaccinia virus. J. Infect. Dis. 2007, 195, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, K.; Mohamed, M.R.; Zhang, L.; Villa, N.Y.; Werden, S.J.; Liu, J.; McFadden, G. Poxvirus Proteomics and Virus-Host Protein Interactions. Microbiol. Mol. Biol. Rev. 2009, 73, 730–749. [Google Scholar] [CrossRef] [PubMed]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B.; et al. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell 2016, 167, 684–694.e9. [Google Scholar] [CrossRef]
- Combadiere, B.; Boissonnas, A.; Carcelain, G.; Lefranc, E.; Samri, A.; Bricaire, F.; Debre, P.; Autran, B. Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans. J. Exp. Med. 2004, 199, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.S.; Frey, S.E.; Yan, L.; Rothman, A.L.; Cruz, J.; Newman, F.K.; Orphin, L.; Belshe, R.B.; Ennis, F.A. Induction of human T cell-mediated immune responses after primary and secondary smallpox vaccination. J. Infect. Dis. 2004, 190, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gieryńska, M.; Szulc-Dąbrowska, L.; Struzik, J.; Gregorczyk-Zboroch, K.P.; Mielcarska, M.B.; Toka, F.N.; Schollenberger, A.; Biernacka, Z. Orthopoxvirus Zoonoses—Do We Still Remember and Are Ready to Fight? Pathogens 2023, 12, 363. https://doi.org/10.3390/pathogens12030363
Gieryńska M, Szulc-Dąbrowska L, Struzik J, Gregorczyk-Zboroch KP, Mielcarska MB, Toka FN, Schollenberger A, Biernacka Z. Orthopoxvirus Zoonoses—Do We Still Remember and Are Ready to Fight? Pathogens. 2023; 12(3):363. https://doi.org/10.3390/pathogens12030363
Chicago/Turabian StyleGieryńska, Małgorzata, Lidia Szulc-Dąbrowska, Justyna Struzik, Karolina Paulina Gregorczyk-Zboroch, Matylda Barbara Mielcarska, Felix Ngosa Toka, Ada Schollenberger, and Zuzanna Biernacka. 2023. "Orthopoxvirus Zoonoses—Do We Still Remember and Are Ready to Fight?" Pathogens 12, no. 3: 363. https://doi.org/10.3390/pathogens12030363
APA StyleGieryńska, M., Szulc-Dąbrowska, L., Struzik, J., Gregorczyk-Zboroch, K. P., Mielcarska, M. B., Toka, F. N., Schollenberger, A., & Biernacka, Z. (2023). Orthopoxvirus Zoonoses—Do We Still Remember and Are Ready to Fight? Pathogens, 12(3), 363. https://doi.org/10.3390/pathogens12030363





