Study of the Genetic Expression of Antiretroviral Restriction Factors and Acute Phase Proteins in Cattle Infected with Bovine Leukemia Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals
2.2. Obtaining PBLs and Plasma
2.3. Detection of BLV Serological Infection
2.4. Lymphocyte Count
2.5. Nucleic Acids
2.6. cDNA
2.7. Multiplex RT-PCR to Identify Bovine Respiratory Complex Viruses
2.8. PCR Detection of BLV Provirus
2.9. Sequencing and Phylogeny
2.10. Sample Classification
2.11. Design of Primers and Positive Controls to Amplify ARF and APP
2.12. Evaluation of Proviral and Viral Loads
2.13. Determination of ARF and APP Expression
2.14. Housekeeping
2.15. Statistical Analysis
3. Results
3.1. Study Group
3.2. BLV Viral and Proviral Load Determination
3.3. Genotyping
3.4. Housekeeping
3.5. ARF and APP Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, A.M.Q.; Lefkowitz, E.J.; Adams, M.J.; Carstens, E.B. International Committee on Taxonomy of Viruses (ICTV). Available online: https://talk.ictvonline.org/taxonomy/ (accessed on 18 October 2018).
- Nieto, M.; Lendez, P.; Marin, M.; Quintana, S.; Martínez-Cuesta, L.; Ceriani, M.C.; Dolcini, G.L. Toll-like Receptors, IFN- γ and IL-12 Expression in Bovine Leukemia Virus-Infected Animals with Low or High Proviral Load. YRVSC 2016, 107, 190–195. [Google Scholar] [CrossRef]
- Burny, A.; Bex, F.; Chantrenng, H.; Cleuter, Y.; Dekegel, D.; Ghysdael, J.; Kettmann, R.; Leclercq, M.; Leunen, J.; Mammerickx, M.; et al. Bovine Leukemia Virus Involvement in Enzootic Bovine Leukosis. Adv. Cancer Res. 1978, 28, 251–311. [Google Scholar] [CrossRef]
- Alvarez, I.; Gutiérrez, G.; Gammella, M.; Martínez, C.; Politzki, R.; González, C.; Caviglia, L.; Carignano, H.; Fondevila, N.; Poli, M.; et al. Evaluation of Total White Blood Cell Count as a Marker for Proviral Load of Bovine Leukemia Virus in Dairy Cattle from Herds with a High Seroprevalence of Antibodies against Bovine Leukemia Virus. Am. J. Vet. Res. 2013, 74, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Mekata, H.; Sekiguchi, S.; Kirino, Y.; Mitoma, S.; Honkawa, K.; Horii, Y.; Norimine, J. Cattle with the BoLA Class II DRB3 * 0902 Allele Have Significantly Lower Bovine Leukemia Proviral Loads. J. Vet. Med. Sci. 2017, 79, 1552–1555. [Google Scholar] [CrossRef]
- Frie, M.C.; Coussens, P.M. Bovine Leukemia Virus: A Major Silent Threat to Proper Immune Responses in Cattle. Vet. Immunol. Immunopathol. 2015, 163, 103–114. [Google Scholar] [CrossRef]
- Passucci, J.; Ceriani, C. A Novel Association of BoLA DRB3 Alleles in BLV Infected Cattle with Different Proviral Loads. J. Veter- Res. Anim. Sci. 2017, 54, 215. [Google Scholar] [CrossRef]
- Cattle, H. Association of BoLA-DRB3. 2 Alleles with BLV Infection Profiles ( Persistent Lymphocytosis / Lymphosarcoma ) and Lymphocyte Subsets in Iranian. Biochem. Genet. 2016, 54, 194–207. [Google Scholar] [CrossRef]
- Xu, A.; Van Eijk, M.J.; Park, C.; Lewin, H.A. Polymorphism in BOLA-DRB3 Exon 2 Correlates with Resistance to Persistent Lymphocytosis Caused by Bovine Leukemia Virus. J. Immunol. 1993, 151, 6977–6985. [Google Scholar] [CrossRef]
- de Pablo-Maiso, L.; Doménech, A.; Echeverría, I.; Gómez-Arrebola, C.; de Andrés, D.; Rosati, S.; Reina, R.; Gómez-Lucia, E. Prospects in Innate Immune Responses as Potential Control Strategies against Non-Primate Lentiviruses. Viruses 2018, 33, 435. [Google Scholar] [CrossRef]
- Yan, N.; Chen, Z.J. Intrinsic Antiviral Immunity. Nat. Immunol. 2013, 13, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.S.; Dudley, J.P. APOBECs and Virus Restriction. Virology 2015, 479–480, 131–145. [Google Scholar] [CrossRef]
- Yamada, E.; Yoshikaw, R.; Nakano, Y.; Misawa, N.; Kobayashi, T. A Naturally Occurring Bovine APOBEC3 Confers Resistance to Bovine Lentiviruses: Implication for the Co-Evolution of Bovids and Their Lentiviruses. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Guo, H.; Ma, Y.; Gai, Y.; Liang, Z.; Ma, J.; Su, Y.; Zhang, Q.; Chen, Q.; Tan, J. Bovine HEXIM1 Inhibits Bovine Immunodeficiency Virus Replication through Regulating BTat-Mediated Transactivation. Veter-Res. 2013, 1, 1–8. [Google Scholar] [CrossRef]
- Reczyńska, D.; Zalewska, M.; Czopowicz, M.; Kaba, J.; Zwierzchowski, L.; Bagnicka, E. Acute Phase Protein Levels as An Auxiliary Tool in Diagnosing Viral Diseases in Ruminants—A Review. Viruses 2018, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Tjùrnehùj, K.; Larsen, L.E.; Viuff, B.; Rùnsholt, L. The Acute Phase Response of Haptoglobin and Serum Amyloid A ( SAA ) in Cattle Undergoing Experimental Infection with Bovine Respiratory Syncytial Virus. Vet. Immunol. Immunopathol. 2000, 77, 151–159. [Google Scholar]
- Cray, C.; Zaias, J.; Altman, N.H. Acute Phase Response in Animals: A Review. Comp. Med. 2009, 59, 517–526. [Google Scholar] [PubMed]
- Montero Machuca, N.; Tórtora Pérez, J.L.; González Méndez, A.S.; García-Camacho, A.L.; Marín Flamand, E.; Ramírez Álvarez, H. Genetic Analysis of the PX Region of Bovine Leukemia Virus Genotype 1 in Holstein Friesian Cattle with Different Stages of Infection. Arch. Virol. 2022, 167, 45–56. [Google Scholar] [CrossRef]
- Cerón, F.; González, A.S.; Tórtora, J.L.; Loza-Rubio, E.; Ramírez, H. Lack of Association between Amino Acid Sequences of the Bovine Leukemia Virus Envelope and Varying Stages of Infection in Dairy Cattle. Virus Res. 2020, 278. [Google Scholar] [CrossRef]
- Ochoa, L.N. Patología Clínica Veterinaria, 2nd ed.; Rodríguez, A.A., Ed.; UNAM: Mexico City, Mexico, 2007. [Google Scholar]
- Tolle, A.; Jahnke, H.-D.; Hasse, G. Zur Diagnostik Der Rinderleukose Und Ihrer Bekämpfung in Südniedersachsen. Zent. Für Veterinärmedizin R. B 2010, 12, 435–443. [Google Scholar] [CrossRef]
- Contreras-Luna, M.J.; Ramírez-Martínez, L.A.; Sarmiento Silva, R.E.; Cruz Lazo, C.; Pérez Torres, A.; Sánchez-Betancourt, J.I. Evidence of Respiratory Syncytial Virus and Parainfluenza-3 Virus in Mexican Sheep. VirusDisease 2017, 28, 102–110. [Google Scholar] [CrossRef]
- Sarmiento-Silva, R.E.; Nakamura-Lopez, Y.; Vaughan, G. Epidemiology, Molecular Epidemiology and Evolution of Bovine Respiratory Syncytial Virus. Viruses 2012, 4, 3452–3467. [Google Scholar] [CrossRef]
- Goodwin, C.; Higgins, D.; Tobe, S.S.; Austin, J.; Wotherspoon, A.; Gahan, M.E.; McNevin, D. Singleplex Quantitative Real-Time PCR for the Assessment of Human Mitochondrial DNA Quantity and Quality. Forensic Sci. Med. Pathol. 2018, 14, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Conte, J.; Potoczniak, M.J.; Tobe, S.S. Using Synthetic Oligonucleotides as Standards in Probe-Based QPCR. Biotechniques 2018, 64, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Patil, I. Visualizations with Statistical Details: The “ggstatsplot” Approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Cumming, G. The New Statistics. Psychol. Sci. 2014, 25, 7–29. [Google Scholar] [CrossRef]
- González-Fernández, V.D.; Tórtora Pérez, J.L.; García Flores, M.M.; Aguilar Setién, J.Á.; Ramírez Álvarez, H. First Evidence of Bovine Immunodeficiency Virus Infection in Mexican Cattle. Transbound. Emerg. Dis. 2020, 67, 1768–1775. [Google Scholar] [CrossRef]
- Su, X.; Wang, H.; Zhou, X.; Li, Z.; Zheng, B.; Zhang, W. Jembrana Disease Virus Vif Antagonizes the Inhibition of Bovine APOBEC3 Proteins through Ubiquitin-Mediate Protein Degradation. Virology 2018, 519, 53–63. [Google Scholar] [CrossRef]
- Xu, W.K.; Byun, H.; Dudley, J.P. The Role of APOBECs in Viral Replication. Microorganisms 2020, 8, 1–46. [Google Scholar] [CrossRef]
- LaRue, R.S.; Andrésdóttir, V.; Blanchard, Y.; Conticello, S.G.; Derse, D.; Emerman, M.; Greene, W.C.; Jónsson, S.R.; Landau, N.R.; Löchelt, M.; et al. Guidelines for Naming Nonprimate APOBEC3 Genes and Proteins. J. Virol. 2009, 83, 494–497. [Google Scholar] [CrossRef]
- Leonard, B.; Starrett, G.J.; Maurer, M.J.; Oberg, A.L.; Van Bockstal, M.; Van Dorpe, J.; De Wever, O.; Helleman, J.; Sieuwerts, A.M.; Berns, E.M.J.J.; et al. APOBEC3G Expression Correlates with T-Cell Infiltration and Improved Clinical Outcomes in High-Grade Serous Ovarian Carcinoma. Clin. Cancer Res. 2016, 22, 4746–4755. [Google Scholar] [CrossRef]
- Aida, Y.; Murakami, H.; Takahashi, M.; Takeshima, S.-N. Mechanisms of Pathogenesis Induced by Bovine Leukemia Virus as a Model for Human T-Cell Leukemia Virus. Front. Microbiol. 2013, 4, 328. [Google Scholar] [CrossRef] [PubMed]
- Gillet, N.; Florins, A.; Boxus, M.; Burteau, C.; Nigro, A.; Vandermeers, F.; Balon, H.; Bouzar, A.-B.; Defoiche, J.; Burny, A.; et al. Mechanisms of Leukemogenesis Induced by Bovine Leukemia Virus: Prospects for Novel Anti-Retroviral Therapies in Human. Retrovirology 2007, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Lew, Q.J.; Chu, K.L.; Chia, Y.L.; Cheong, N.; Chao, S.H. Hexim1, a New Player in the P53 Pathway. Cancers 2013, 5, 838–856. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, M.; August, A.; Henderson, A.J. Combinatorial Signals from CD28 Differentially Regulate Human Immunodeficiency Virus Transcription in T Cells. J. Biol. Chem. 2010, 285, 17338–17347. [Google Scholar] [CrossRef]
- Lau, J.; Qiao, J.L.; Diribarne, G.; Michels, A.A.; Dey, A.; Bensaude, O.; Lane, D.P.; Chao, S.H. Ubiquitination of HEXIM1 by HDM2. Cell Cycle 2009, 8, 2247–2254. [Google Scholar] [CrossRef]
- Yik, J.H.N.; Chen, R.; Pezda, A.C.; Zhou, Q. Compensatory Contributions of HEXIM1 and HEXIM2 in Maintaining the Balance of Active and Inactive Positive Transcription Elongation Factor b Complexes for Control of Transcription. J. Biol. Chem. 2005, 280, 16368–16376. [Google Scholar] [CrossRef]
- Takeda, E.; Nakagawa, S.; Nakaya, Y.; Tanaka, A.; Miyazawa, T.; Yasuda, J. Identification and Functional Analysis of Three Isoforms of Bovine BST-2. PLoS ONE 2012, 7, e41483. [Google Scholar] [CrossRef]
- Pluta, A.; Jaworski, J.P.; Douville, R.N. Regulation of Expression and Latency in BLV and HTLV. Viruses 2020, 12, 1079. [Google Scholar] [CrossRef]
- Schnell, A.P.; Kohrt, S.; Thoma-Kress, A.K. Latency Reversing Agents: Kick and Kill of Htlv-1? Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Bangham, C.R.M. Human T Cell Leukemia Virus Type 1: Persistence and Pathogenesis. Annu. Rev. Immunol. 2018, 36, 43–71. [Google Scholar] [CrossRef]
- Nishi, K.; Yamasaki, K.; Otagiri, M. Serum Albumin, Lipid and Drug Binding. Subcell Biochem. 2020, 94, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Reczyńska, D.; Zalewska, M.; Czopowicz, M.; Kaba, J.; Zwierzchowski, L.; Bagnicka, E. Small Ruminant Lentivirus Infection Influences Expression of Acute Phase Proteins and Cathelicidin Genes in Milk Somatic Cells and Peripheral Blood Leukocytes of Dairy Goats. Vet. Res. 2018, 49, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Gupta, V.K.; Bhanuprakash, A.G.; Mandal, R.S.K.; Dimri, U.; Ajith, Y. Haptoglobin and Serum Amyloid A as Putative Biomarker Candidates of Naturally Occurring Bovine Respiratory Disease in Dairy Calves. Microb. Pathog. 2018, 116, 33–37. [Google Scholar] [CrossRef]
- Eckersall, P.D.; Bell, R. Acute Phase Proteins: Biomarkers of Infection and Inflammation in Veterinary Medicine. Vet. J. 2010, 185, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Czopowicz, M.; Szaluś-Jordanow, O.; Mickiewicz, M.; Moroz, A.; Witkowski, L.; Markowska-Daniel, I.; Stefaniak, T.; Bagnicka, E.; Kaba, J. Haptoglobin and Serum Amyloid A in Goats with Clinical Form of Caprine Arthritis-Encephalitis. Small Rumin. Res. 2017, 156, 73–77. [Google Scholar] [CrossRef]
- Horadagoda, N.U.; Knox, K.M.; Gibbs, H.A.; Reid, S.W.; Horadagoda, A.; Edwards, S.E.; Eckersall, P.D. Acute Phase Proteins in Cattle: Discrimination between Acute and Chronic Inflammation. Vet. Rec. 1999, 144, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Dalal, K.; Dalal, B.; Bhatia, S.; Shukla, A.; Shankarkumar, A. Analysis of Serum Haptoglobin Using Glycoproteomics and Lectin Immunoassay in Liver Diseases in Hepatitis B Virus Infection. Clin. Chim. Acta 2019, 495, 309–317. [Google Scholar] [CrossRef]
- Höfner, M.C.; Fosbery, M.W.; Eckersall, P.D.; Donaldson, A.I. Haptoglobin Response of Cattle Infected with Foot-and-Mouth Disease Virus. Res. Vet. Sci. 1994, 57, 125–128. [Google Scholar] [CrossRef]
- Brym, P.; Ruść, A.; Kamiński, S. Evaluation of Reference Genes for QRT-PCR Gene Expression Studies in Whole Blood Samples from Healthy and Leukemia-Virus Infected Cattle. Vet. Immunol. Immunopathol. 2013, 153, 302–307. [Google Scholar] [CrossRef]
- Chapman, J.R.; Waldenström, J. With Reference to Reference Genes: A Systematic Review of Endogenous Controls in Gene Expression Studies. PLoS ONE 2015, 10, e0141853. [Google Scholar] [CrossRef]
- Juliarena, M.A.; Gutierrez, S.E.; Ceriani, C. Determination of Proviral Load in Bovine Leukemia Virus–Infected Cattle with and without Lymphocytosis. Am. J. Vet. Res. 2007, 68, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Jimba, M.; Takeshima, S.; Matoba, K.; Endoh, D.; Aida, Y. BLV-CoCoMo-QPCR: Quantitation of Bovine Leukemia Virus Proviral Load Using the CoCoMo Algorithm. Retrovirology 2010, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, S.; Kobayashi, S.; Matsunaga, T.; Tonosaki, K.; Leng, D.; Sakai, Y.; Yamada, S.; Kimura, A.; Ichijo, T.; Hikono, H.; et al. Comparative Evaluation of Three Commercial Quantitative Real-Time PCRs Used in Japan for Bovine Leukemia Virus. Viruses 2022, 14, 1182. [Google Scholar] [CrossRef] [PubMed]
- John, E.E.; Droscha, C.; Cameron, M.; Stryhn, H.; Keefe, G.; McClure, J.T. Development of a Predictive Model for Bovine Leukemia Virus Proviral Load. J. Vet. Intern. Med. 2022, 36, 1827–1836. [Google Scholar] [CrossRef]
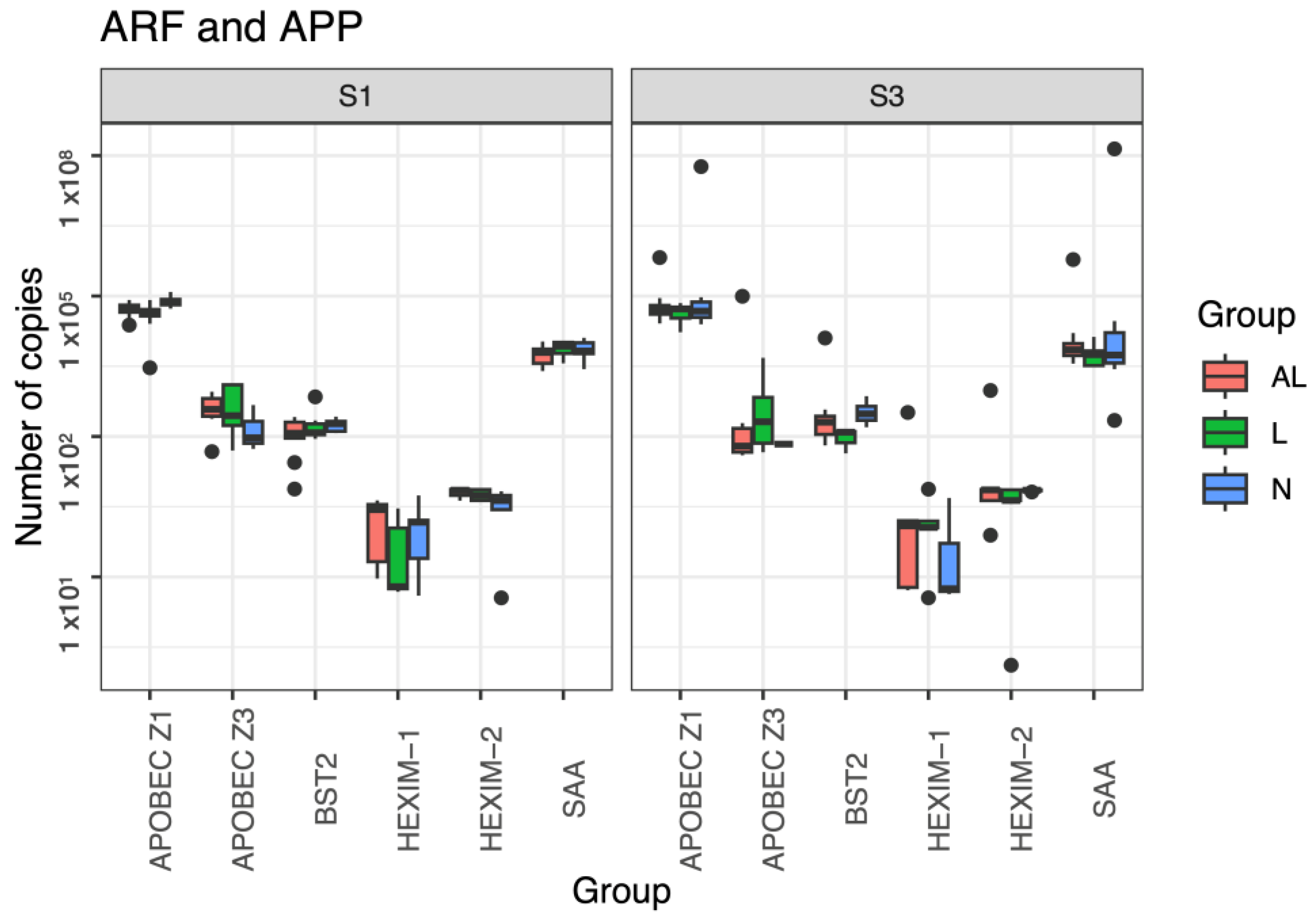
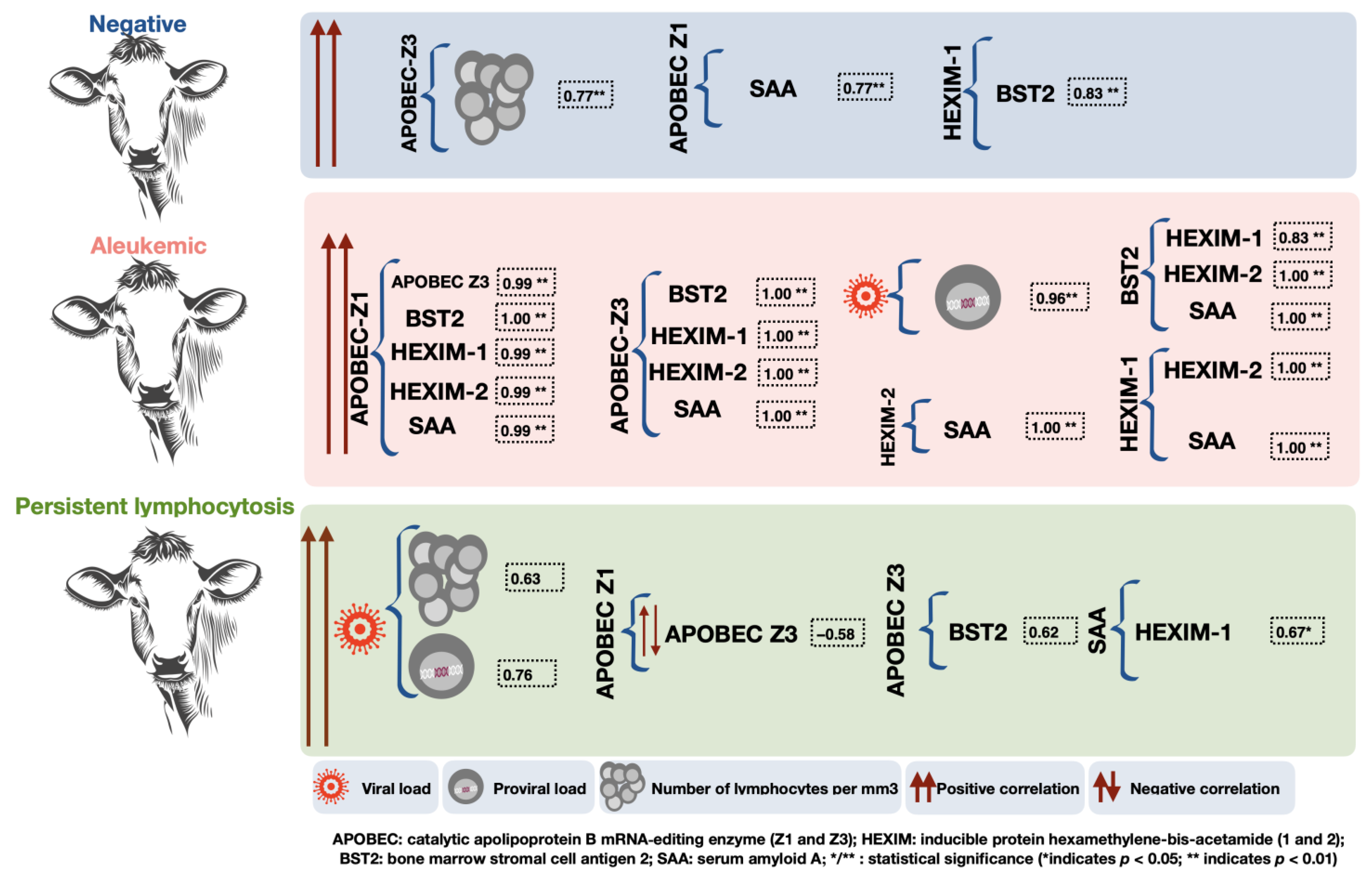
| Variable | Group | M | SD | L⌀’s | Proviral | APOBECZ1 | APOBECZ3 | BST2 | HEXIM1 | HEXIM2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Lymphocytes (L⌀’s) | N | 5359.67 | 1223.49 | |||||||
| AL | 7566.19 | 2596.64 | ||||||||
| PL | 18,931.60 | 6338.98 | ||||||||
| Proviral | N | 0.00 | 0.00 | |||||||
| AL | 402,277.00 | 696,725.51 | ||||||||
| PL | 2,905,680.00 | 5,060,288.29 | ||||||||
| Viral | N | 0.00 | 0.00 | |||||||
| AL | 8123.06 | 14,127.92 | 0.96 ** [0.90, 0.99] | |||||||
| PL | 48,980.50 | 47,596.10 | 0.63 [−0.00, 0.90] | 0.76 * [0.25, 0.94] | ||||||
| APOBECZ1 | N | 6,504,834.44 | 19,381,955.65 | |||||||
| AL | 91,033.12 | 153,826.40 | ||||||||
| PL | 43,547.00 | 22,684.39 | ||||||||
| APOBECZ3 | N | 7.64 | 22.92 | 0.77 * [0.23, 0.95] | ||||||
| AL | 6197.56 | 24,683.37 | 0.99 ** [0.98, 1.00] | |||||||
| PL | 713.56 | 1478.42 | −0.58 [−0.89, 0.07] | |||||||
| BST2 | N | 167.41 | 250.72 | |||||||
| AL | 874.32 | 3155.61 | 1.00 ** [0.99, 1.00] | 1.00 ** [1.0, 1.0] | ||||||
| PL | 29.84 | 53.21 | 0.62 [−0.02, 0.90] | |||||||
| HEXIM1 | N | 0.55 | 1.62 | 0.83 ** [0.38, 0.96] | ||||||
| AL | 20.53 | 81.35 | 0.99 ** [0.98, 1.00] | 1.00 ** [1.0, 1.0] | 1.00 ** [1.0, 1.0] | |||||
| PL | 1.14 | 2.32 | ||||||||
| HEXIM2 | N | 3.14 | 3.73 | |||||||
| AL | 63.66 | 239.35 | 0.99 ** [0.98, 1.00] | 1.00 ** [1.0, 1.0] | 1.00 ** [1.0, 1.0] | 1.00 ** [1.0, 1.0] | ||||
| PL | 3.48 | 3.24 | ||||||||
| SAA | N | 15,472,451.06 | 46,397,831.76 | 1.00 ** [1.0, 1.0] | ||||||
| AL | 42,266.75 | 149,198.29 | 0.99 ** [0.98, 1.00] | 1.00 ** [1.0, 1.0] | 1.00 ** [1.0, 1.0] | 1.00 ** [1.0, 1.0] | 1.00 ** [1.0, 1.0] | |||
| PL | 4232.30 | 4169.27 | 0.67 * [0.06, 0.91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Méndez, A.S.; Tórtora Pérez, J.L.; Rojas-Anaya, E.; Ramírez Álvarez, H. Study of the Genetic Expression of Antiretroviral Restriction Factors and Acute Phase Proteins in Cattle Infected with Bovine Leukemia Virus. Pathogens 2023, 12, 529. https://doi.org/10.3390/pathogens12040529
González-Méndez AS, Tórtora Pérez JL, Rojas-Anaya E, Ramírez Álvarez H. Study of the Genetic Expression of Antiretroviral Restriction Factors and Acute Phase Proteins in Cattle Infected with Bovine Leukemia Virus. Pathogens. 2023; 12(4):529. https://doi.org/10.3390/pathogens12040529
Chicago/Turabian StyleGonzález-Méndez, Ana S., Jorge L. Tórtora Pérez, Edith Rojas-Anaya, and Hugo Ramírez Álvarez. 2023. "Study of the Genetic Expression of Antiretroviral Restriction Factors and Acute Phase Proteins in Cattle Infected with Bovine Leukemia Virus" Pathogens 12, no. 4: 529. https://doi.org/10.3390/pathogens12040529
APA StyleGonzález-Méndez, A. S., Tórtora Pérez, J. L., Rojas-Anaya, E., & Ramírez Álvarez, H. (2023). Study of the Genetic Expression of Antiretroviral Restriction Factors and Acute Phase Proteins in Cattle Infected with Bovine Leukemia Virus. Pathogens, 12(4), 529. https://doi.org/10.3390/pathogens12040529








