Development of In Vitro Assays with the Canine Hookworm Uncinaria stenocephala and Assessment of Natural Plant Products for Anti-Parasitic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Extracts
2.2. Parasites
2.3. Egg Hatch Assay
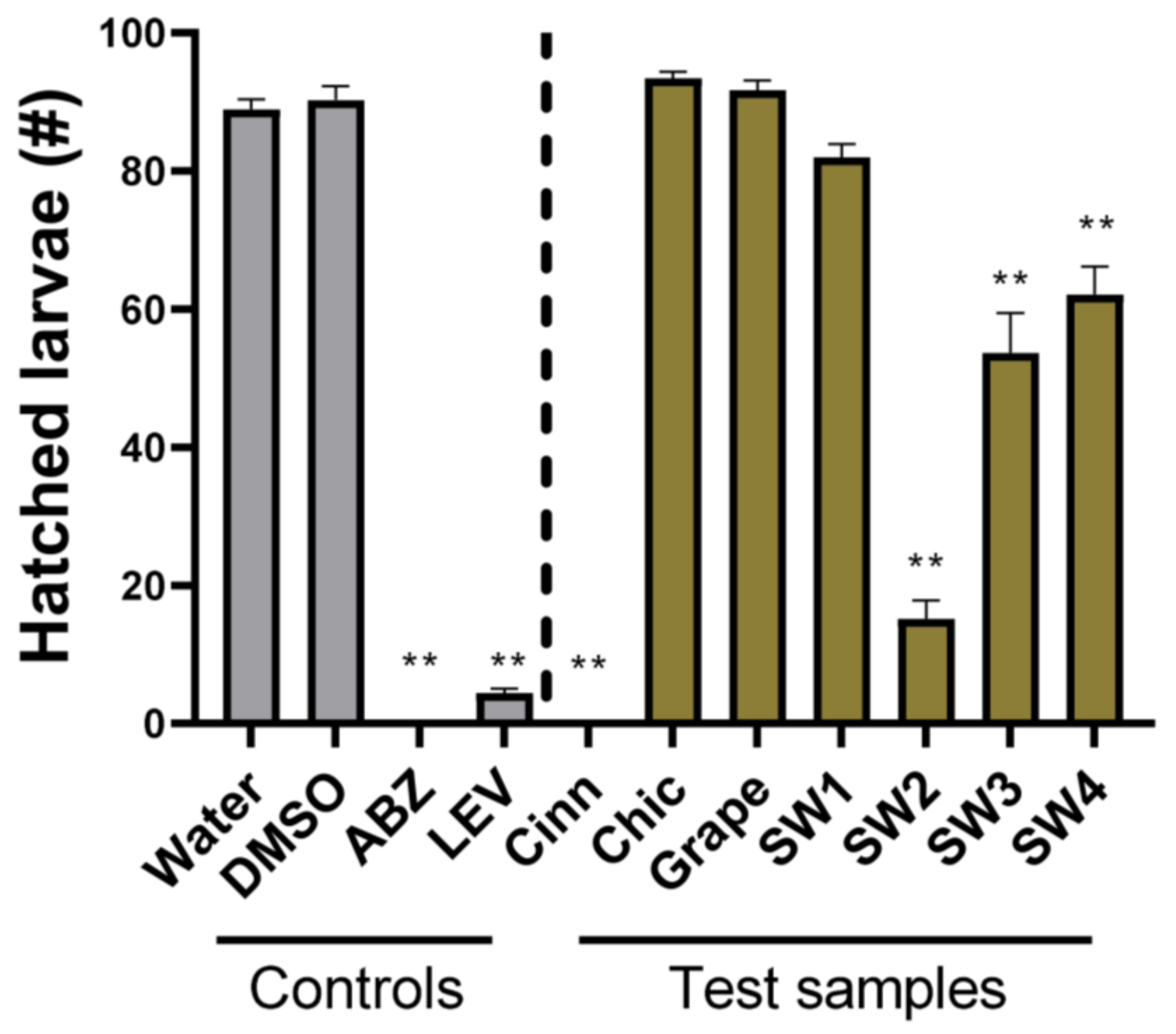
2.4. Larval Development Assay
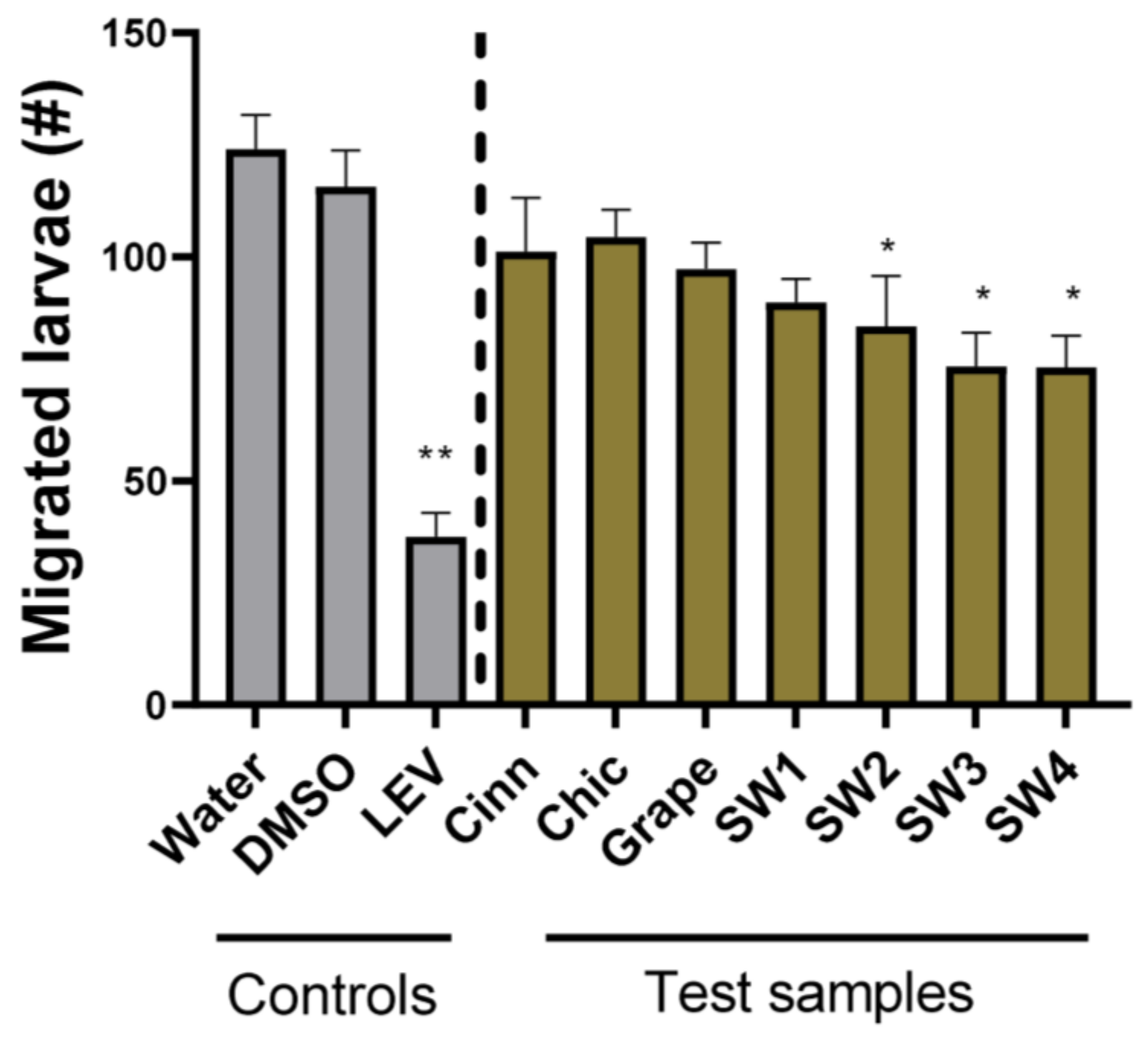
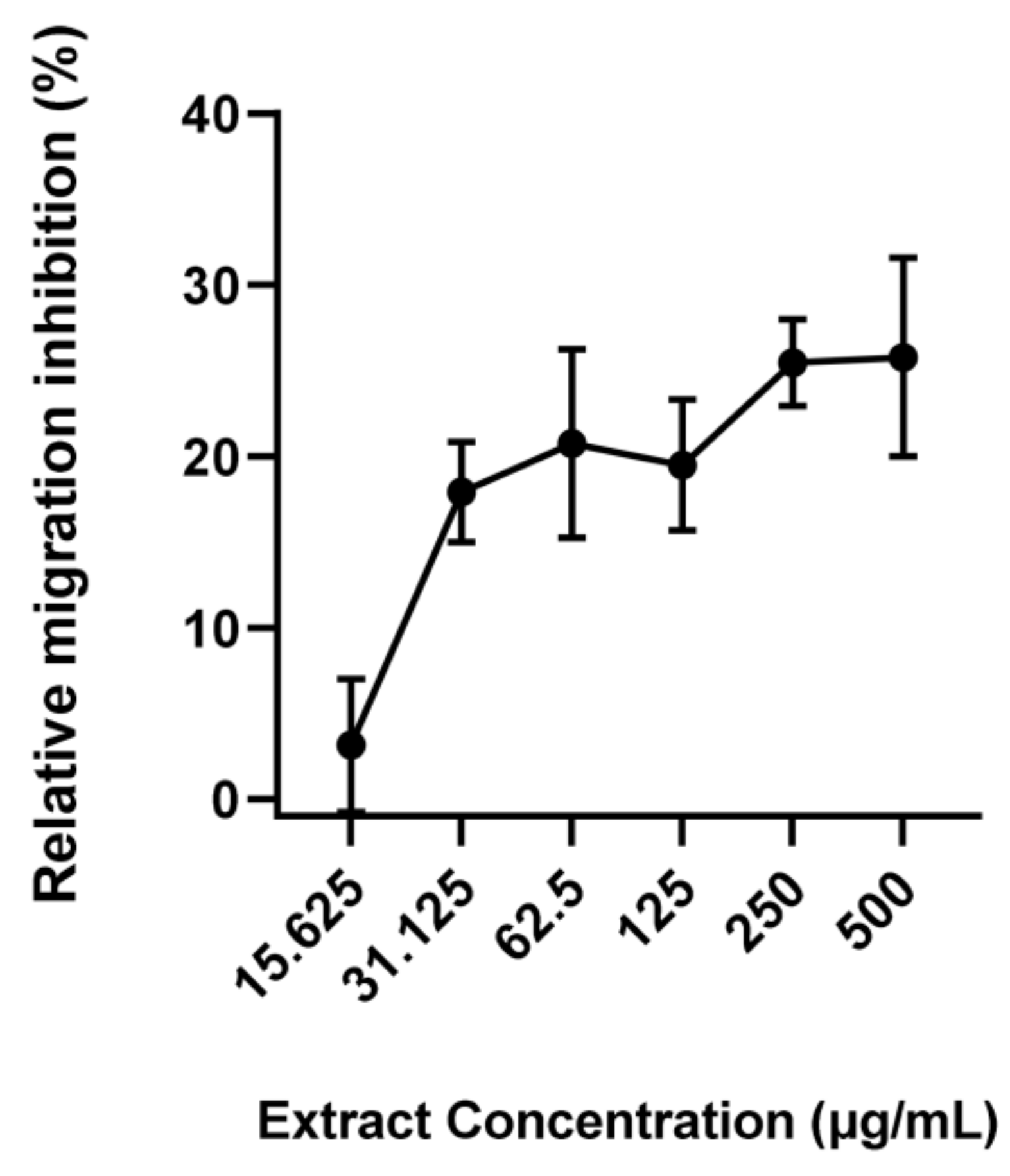
2.5. Larval Migration Assay
2.6. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dantas-Torres, F.; Otranto, D. Dogs, cats, parasites, and humans in Brazil: Opening the black box. Parasites Vectors 2014, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, E.R.; Aziz, N.A.A.; Blanchard, A.; Charlier, J.; Charvet, C.; Claerebout, E.; Geldhof, P.; Greer, A.W.; Hertzberg, H.; Hodgkinson, J.; et al. 100 questions in livestock helminthology research. Trends Parasitol. 2019, 35, 52–71. [Google Scholar] [CrossRef] [Green Version]
- Else, K.J.; Keiser, J.; Holland, C.V.; Grencis, R.K.; Sattelle, D.B.; Fujiwara, R.T.; Bueno, L.L.; Asaolu, S.O.; Sowemimo, O.A.; Cooper, P.J. Whipworm and roundworm infections. Nat. Rev. Dis. Prim. 2020, 6, 44. [Google Scholar] [CrossRef]
- Stehr-Green, J.K.; Schantz, P.M. The impact of zoonotic diseases transmitted by pets on human health and the economy. Vet. Clin. N. Am. Small Anim. Pract. 1987, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.J.; Zendejas-Heredia, P.A.; Massetti, L.; Colella, V. Zoonotic hookworms of dogs and cats—Lessons from the past to inform current knowledge and future directions of research. Int. J. Parasitol. 2021, 51, 1233–1241. [Google Scholar] [CrossRef]
- Traversa, D. Pet roundworms and hookworms: A continuing need for global worming. Parasites Vectors 2012, 5, 91. [Google Scholar] [CrossRef] [Green Version]
- Charlier, J.; Bartley, D.J.; Sotiraki, S.; Martinez-Valladares, M.; Claerebout, E.; von Samson-Himmelstjerna, G.; Thamsborg, S.M.; Hoste, H.; Morgan, E.R.; Rinaldi, L. Anthelmintic resistance in ruminants: Challenges and solutions. Adv. Parasitol. 2022, 115, 171–227. [Google Scholar] [PubMed]
- Raza, A.; Qamar, A.G.; Hayat, K.; Ashraf, S.; Williams, A.R. Anthelmintic resistance and novel control options in equine gastrointestinal nematodes. Parasitology 2019, 146, 425–437. [Google Scholar] [CrossRef]
- Jimenez Castro, P.D.; Howell, S.B.; Schaefer, J.J.; Avramenko, R.W.; Gilleard, J.S.; Kaplan, R.M. Multiple drug resistance in the canine hookworm Ancylostoma caninum: An emerging threat? Parasites Vectors 2019, 12, 576. [Google Scholar] [CrossRef] [Green Version]
- Von Samson-Himmelstjerna, G.; Thompson, R.A.; Krücken, J.; Grant, W.; Bowman, D.D.; Schnyder, M.; Deplazes, P. Spread of anthelmintic resistance in intestinal helminths of dogs and cats is currently less pronounced than in ruminants and horses—Yet it is of major concern. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 36–45. [Google Scholar] [CrossRef]
- Castro, P.D.J.; Venkatesan, A.; Redman, E.; Chen, R.; Malatesta, A.; Huff, H.; Zuluaga Salazar, D.A.; Avramenko, R.; Gilleard, J.S.; Kaplan, R.M. Multiple drug resistance in hookworms infecting greyhound dogs in the USA. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 107–117. [Google Scholar] [CrossRef]
- Bethony, J.M.; Cole, R.N.; Guo, X.; Kamhawi, S.; Lightowlers, M.W.; Loukas, A.; Petri, W.; Reed, S.; Valenzuela, J.G.; Hotez, P.J. Vaccines to combat the neglected tropical diseases. Immunol. Rev. 2011, 239, 237–270. [Google Scholar] [CrossRef] [Green Version]
- Loukas, A.; Hotez, P.J.; Diemert, D.; Yazdanbakhsh, M.; McCarthy, J.S.; Correa-Oliveira, R.; Croese, J.; Bethony, J.M. Hookworm infection. Nat. Rev. Dis. Prim. 2016, 2, 16088. [Google Scholar] [CrossRef]
- Bartsch, S.M.; Hotez, P.J.; Asti, L.; Zapf, K.M.; Bottazzi, M.E.; Diemert, D.J.; Lee, B.Y. The global economic and health burden of human hookworm infection. PLoS Negl. Trop. Dis. 2016, 10, e0004922. [Google Scholar] [CrossRef] [Green Version]
- Bowman, D.D. 4-Helminths. In Georgis’ Parasitology for Veterinarians, 11th ed.; Bowman, D.D., Ed.; W.B. Saunders: St. Louis, MO, USA, 2021; pp. 135–260. [Google Scholar]
- Bowman, D.D.; Lucio-Forster, A.; Janeczko, S. Internal parasites. Infect. Dis. Manag. Anim. Shelter. 2021, 393–418. [Google Scholar] [CrossRef]
- Tu, C.H.; Liao, W.C.; Chiang, T.H.; Wang, H.P. Pet parasites infesting the human colon. Gastrointest. Endosc. 2008, 67, 159–160. [Google Scholar] [CrossRef]
- Štrkolcová, G.; Mravcová, K.; Mucha, R.; Mulinge, E.; Schreiberová, A. Occurrence of hookworm and the first molecular and morphometric identification of Uncinaria stenocephala in dogs in Central Europe. Acta Parasitol. 2022, 67, 764–772. [Google Scholar] [CrossRef]
- Idrissi, H.; Khatat, S.E.H.; Duchateau, L.; Kachani, M.; Daminet, S.; El Asatey, S.; Tazi, N.; Azrib, R.; Sahibi, H. Prevalence, risk factors and zoonotic potential of intestinal parasites in dogs from four locations in Morocco. Veter. Parasitol. Reg. Stud. Rep. 2022, 34, 100775. [Google Scholar] [CrossRef]
- Bourgoin, G.; Callait-Cardinal, M.P.; Bouhsira, E.; Polack, B.; Bourdeau, P.; Roussel Ariza, C.; Carassou, L.; Lienard, E.; Drake, J. Prevalence of major digestive and respiratory helminths in dogs and cats in France: Results of a multicenter study. Parasites Vectors 2022, 15, 314. [Google Scholar] [CrossRef]
- Niamatali, S.; Bhopale, V.; Schad, G.A. Efficacy of milbemycin oxime against experimentally induced Ancylostoma caninum and Uncinaria stenocephala infections in dogs. J. Am. Vet. Med. Assoc. 1992, 201, 1385–1387. [Google Scholar]
- Bowman, D.D.; Lin, D.S.; Johnson, R.C.; Hepler, D.I. Effects of milbemycin oxime on adult Ancylostoma caninum and Uncinaria stenocephala in dogs with experimentally induced infections. Am. J. Vet. Res. 1991, 52, 64–67. [Google Scholar]
- Williams, A.R.; Krych, L.; Fauzan Ahmad, H.; Nejsum, P.; Skovgaard, K.; Nielsen, D.S.; Thamsborg, S.M. A polyphenol-enriched diet and Ascaris suum infection modulate mucosal immune responses and gut microbiota composition in pigs. PLoS ONE 2017, 12, e0186546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonde, C.S.; Bornancin, L.; Lu, Y.; Simonsen, H.T.; Martínez-Valladares, M.; Peña-Espinoza, M.; Mejer, H.; Williams, A.R.; Thamsborg, S.M. Bio-guided fractionation and molecular networking reveal fatty acids to be principal anti-parasitic compounds in nordic seaweeds. Front. Pharmacol. 2021, 12, 674520. [Google Scholar] [CrossRef] [PubMed]
- Pena-Espinoza, M.; Boas, U.; Williams, A.R.; Thamsborg, S.M.; Simonsen, H.T.; Enemark, H.L. Sesquiterpene lactone containing extracts from two cultivars of forage chicory (Cichorium intybus) show distinctive chemical profiles and in vitro activity against Ostertagia ostertagi. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Katiki, L.M.; Barbieri, A.M.E.; Araujo, R.C.; Veríssimo, C.J.; Louvandini, H.; Ferreira, J.F.S. Synergistic interaction of ten essential oils against Haemonchus contortus in vitro. Vet. Parasitol. 2017, 243, 47–51. [Google Scholar] [CrossRef]
- Oliveira, M.; Lima, C.S.; Llorent-Martínez, E.J.; Hoste, H.; Custódio, L. Impact of Seasonal and Organ-Related Fluctuations on the Anthelmintic Properties and Chemical Profile of Cladium mariscus (L.) Pohl Extracts. Front. Plant Sci. 2022, 13, 934644. [Google Scholar] [CrossRef]
- Hoste, H.; Jackson, F.; Athanasiadou, S.; Thamsborg, S.M.; Hoskin, S.O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006, 22, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Taki, A.C.; Brkljača, R.; Wang, T.; Koehler, A.V.; Ma, G.; Danne, J.; Ellis, S.; Hofmann, A.; Chang, B.C.H.; Jabbar, A.; et al. Natural compounds from the marine brown alga Caulocystis cephalornithos with potent in vitro-activity against the parasitic nematode Haemonchus contortus. Pathogens 2020, 9, 550. [Google Scholar] [CrossRef]
- Fritsch, D.A.; Jackson, M.I.; Wernimont, S.M.; Feld, G.K.; Badri, D.V.; Brejda, J.J.; Cochrane, C.Y.; Gross, K.L. Adding a polyphenol-rich fiber bundle to food impacts the gastrointestinal microbiome and metabolome in dogs. Front. Vet. Sci. 2022, 9, 1039032. [Google Scholar] [CrossRef]
- Ruiz-Cano, D.; Sánchez-Carrasco, G.; El-Mihyaoui, A.; Arnao, M.B. Essential Oils and Melatonin as Functional Ingredients in Dogs. Animals 2022, 12, 2089. [Google Scholar] [CrossRef]
- Williams, A.R.; Fryganas, C.; Ramsay, A.; Mueller-Harvey, I.; Thamsborg, S.M. Direct anthelmintic effects of condensed tannins from diverse plant sources against Ascaris suum. PLoS ONE 2014, 9, e97053. [Google Scholar] [CrossRef] [Green Version]
- Coles, G.C.; Jackson, F.; Pomroy, W.E.; Prichard, R.K.; von Samson-Himmelstjerna, G.; Silvestre, A.; Taylor, M.A.; Vercruysse, J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006, 136, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Ropiak, H.M.; Fryganas, C.; Desrues, O.; Mueller-Harvey, I.; Thamsborg, S.M. Assessment of the anthelmintic activity of medicinal plant extracts and purified condensed tannins against free-living and parasitic stages of Oesophagostomum dentatum. Parasites Vectors 2014, 7, 518. [Google Scholar] [CrossRef] [PubMed]
- Andersen-Civil, A.I.S.; Myhill, L.J.; Büdeyri Gökgöz, N.; Engström, M.T.; Mejer, H.; Zhu, L.; Zeller, W.E.; Salminen, J.P.; Krych, L.; Lauridsen, C.; et al. Dietary proanthocyanidins promote localized antioxidant responses in porcine pulmonary and gastrointestinal tissues during Ascaris suum-induced type 2 inflammation. FASEB J. 2022, 36, e22256. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.H.; de Roode, M.; Ernst, M.; Pena-Espinoza, M.; Bornancin, L.; Bonde, C.S.; Martínez-Valladares, M.; Ramünke, S.; Krücken, J.; Simonsen, H.T.; et al. Identification of compounds responsible for the anthelmintic effects of chicory (Cichorium intybus) by molecular networking and bio-guided fractionation. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Roepstorff, A.; Nansen, P. Epidemiology, Diagnosis and Control of Helminth Parasites of Swine; Food and Agriculture Organization (FAO): Rome, Italy, 1998. [Google Scholar]
- Kalkofen, U.P. Hookworms of dogs and cats. Vet. Clin. N. Am. Small. Anim. Pract. 1987, 17, 1341–1354. [Google Scholar] [CrossRef]
- David, E.D.; Lindquist, W.D. Determination of the specific gravity of certain helminth eggs using sucrose density gradient centrifugation. J. Parasitol. 1982, 68, 916–919. [Google Scholar] [CrossRef]
- Coles, G.C.; Bauer, C.; Borgsteede, F.H.M.; Geerts, S.; Klei, T.R.; Taylor, M.A.; Waller, P.J. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992, 44, 35–44. [Google Scholar] [CrossRef]
- Boyko, A.A.; Brygadyrenko, V.V. Changes in the viability of Strongyloides ransomi larvae (Nematoda, Rhabditida) under the influence of synthetic flavourings. Regul. Mech. Biosyst. 2017, 1, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.R.; Ramsay, A.; Hansen, T.V.; Ropiak, H.M.; Mejer, H.; Nejsum, P.; Mueller-Harvey, I.; Thamsborg, S.M. Anthelmintic activity of trans-cinnamaldehyde and A-and B-type proanthocyanidins derived from cinnamon (Cinnamomum verum). Sci. Rep. 2015, 5, 14791. [Google Scholar] [CrossRef] [Green Version]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.-P.; et al. Benefits of condensed tannins in forage legumes fed to ruminants: Importance of structure, concentration, and diet composition. Crop Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef] [Green Version]
- Gawor, J.; Jank, M.; Jodkowska, K.; Klim, E.; Svensson, U.K. Effects of edible treats containing Ascophyllum nodosum on the oral health of dogs: A double-blind, randomized, placebo-controlled single-center study. Front. Vet. Sci. 2018, 5, 168. [Google Scholar] [CrossRef] [PubMed]
- Gawor, J.P.; Wilczak, J.; Svensson, U.K.; Jank, M. Influence of Dietary Supplementation with a Powder Containing AN ProDen™ (Ascophyllum Nodosum) Algae on Dog Saliva Metabolome. Front. Vet. Sci. 2021, 8, 681951. [Google Scholar] [CrossRef] [PubMed]
- Dahms, I.; Bailey-Hall, E.; Sylvester, E.; Parenteau, A.; Yu, S.; Karagiannis, A.; Roos, F.; Wilson, J. Safety of a novel feed ingredient, Algal Oil containing EPA and DHA, in a gestation-lactation-growth feeding study in Beagle dogs. PLoS ONE 2019, 14, e0217794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinna, C.; Vecchiato, C.G.; Grandi, M.; Stefanelli, C.; Zannoni, A.; Biagi, G. Seaweed Supplementation Failed to Affect Fecal Microbiota and Metabolome as Well as Fecal IgA and Apparent Nutrient Digestibility in Adult Dogs. Animals 2021, 11, 2234. [Google Scholar] [CrossRef]
- Isidori, M.; Rueca, F.; Trabalza-Marinucci, M. Palatability of extruded dog diets supplemented with Ascophyllum nodosum L. (Fucaceae, Phaeophyceae). J. Appl. Phycol. 2019, 31, 3275–3281. [Google Scholar] [CrossRef]

| Sample | Source | Concentration |
|---|---|---|
| trans-cinnamaldehyde | Sigma-Aldrich | 10 µg/mL |
| Chicory extract | [36] | 1 mg/mL |
| Grape seed extract | [35] | 1 mg/mL |
| Seaweed SW1 | [24] | 1 mg/mL |
| Seaweed SW2 * | [24] | ≤1 mg/mL |
| Seaweed SW3 | [24] | 1 mg/mL |
| Seaweed SW4 | [24] | 1 mg/mL |
| α-linolenic acid ** | Sigma-Aldrich | ≤500 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geisshirt, H.A.; Bonde, C.S.; Marcussen, C.; Mejer, H.; Williams, A.R. Development of In Vitro Assays with the Canine Hookworm Uncinaria stenocephala and Assessment of Natural Plant Products for Anti-Parasitic Activity. Pathogens 2023, 12, 536. https://doi.org/10.3390/pathogens12040536
Geisshirt HA, Bonde CS, Marcussen C, Mejer H, Williams AR. Development of In Vitro Assays with the Canine Hookworm Uncinaria stenocephala and Assessment of Natural Plant Products for Anti-Parasitic Activity. Pathogens. 2023; 12(4):536. https://doi.org/10.3390/pathogens12040536
Chicago/Turabian StyleGeisshirt, Heidi A., Charlotte S. Bonde, Caroline Marcussen, Helena Mejer, and Andrew R. Williams. 2023. "Development of In Vitro Assays with the Canine Hookworm Uncinaria stenocephala and Assessment of Natural Plant Products for Anti-Parasitic Activity" Pathogens 12, no. 4: 536. https://doi.org/10.3390/pathogens12040536
APA StyleGeisshirt, H. A., Bonde, C. S., Marcussen, C., Mejer, H., & Williams, A. R. (2023). Development of In Vitro Assays with the Canine Hookworm Uncinaria stenocephala and Assessment of Natural Plant Products for Anti-Parasitic Activity. Pathogens, 12(4), 536. https://doi.org/10.3390/pathogens12040536







