Intermediate Cluster Disinfection: Which Disinfection Solution Is Most Effective on Milking Liners? A Comparison of Microorganism Reduction on Liner Inner Surfaces Using Quantitative Swab Sampling Technique
Abstract
1. Introduction
2. Materials and Methods
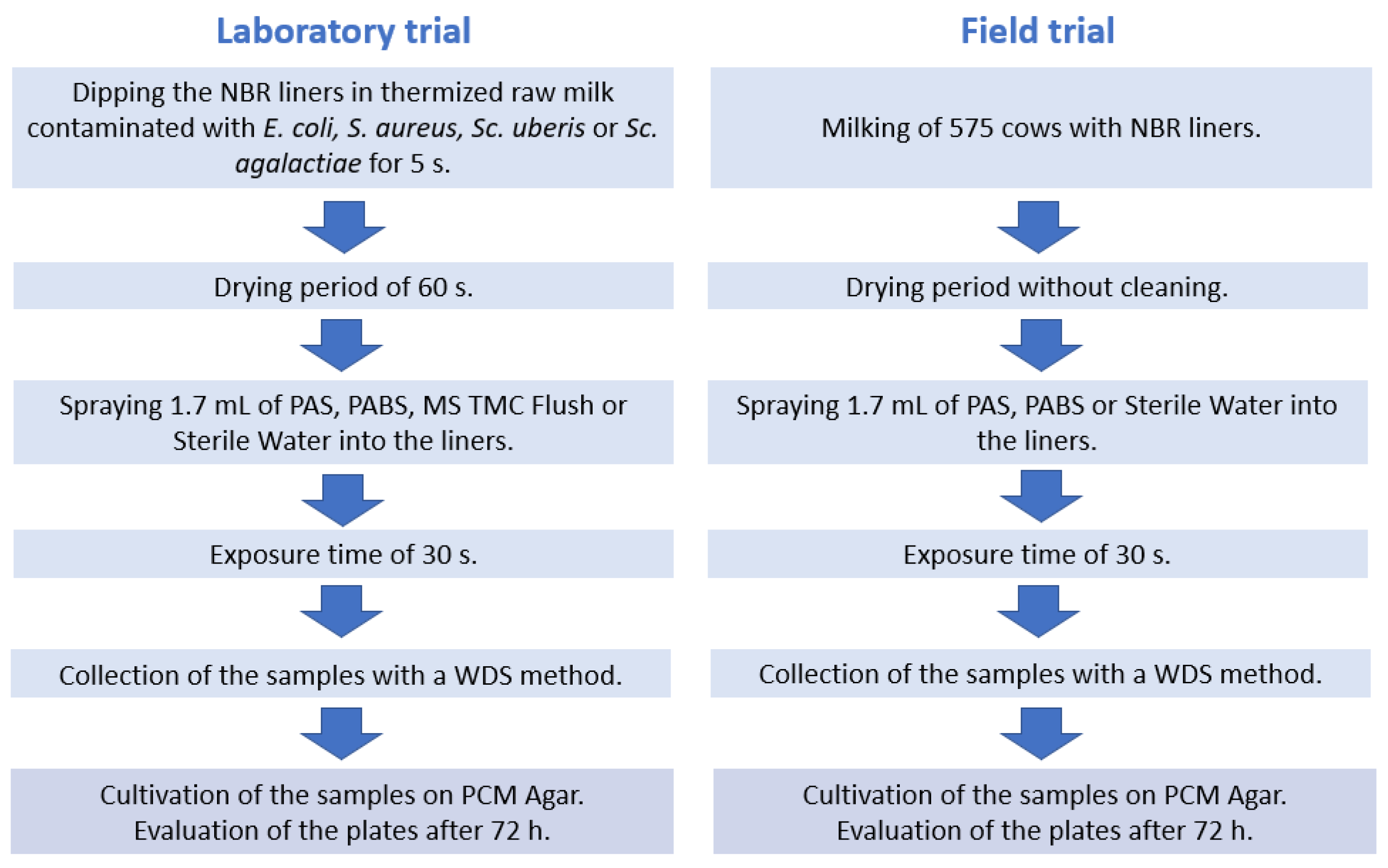
2.1. Laboratory Trial
2.2. Field Trial
2.3. Sampling Technique
2.4. Microbiological Analysis
2.5. Statistical Analysis
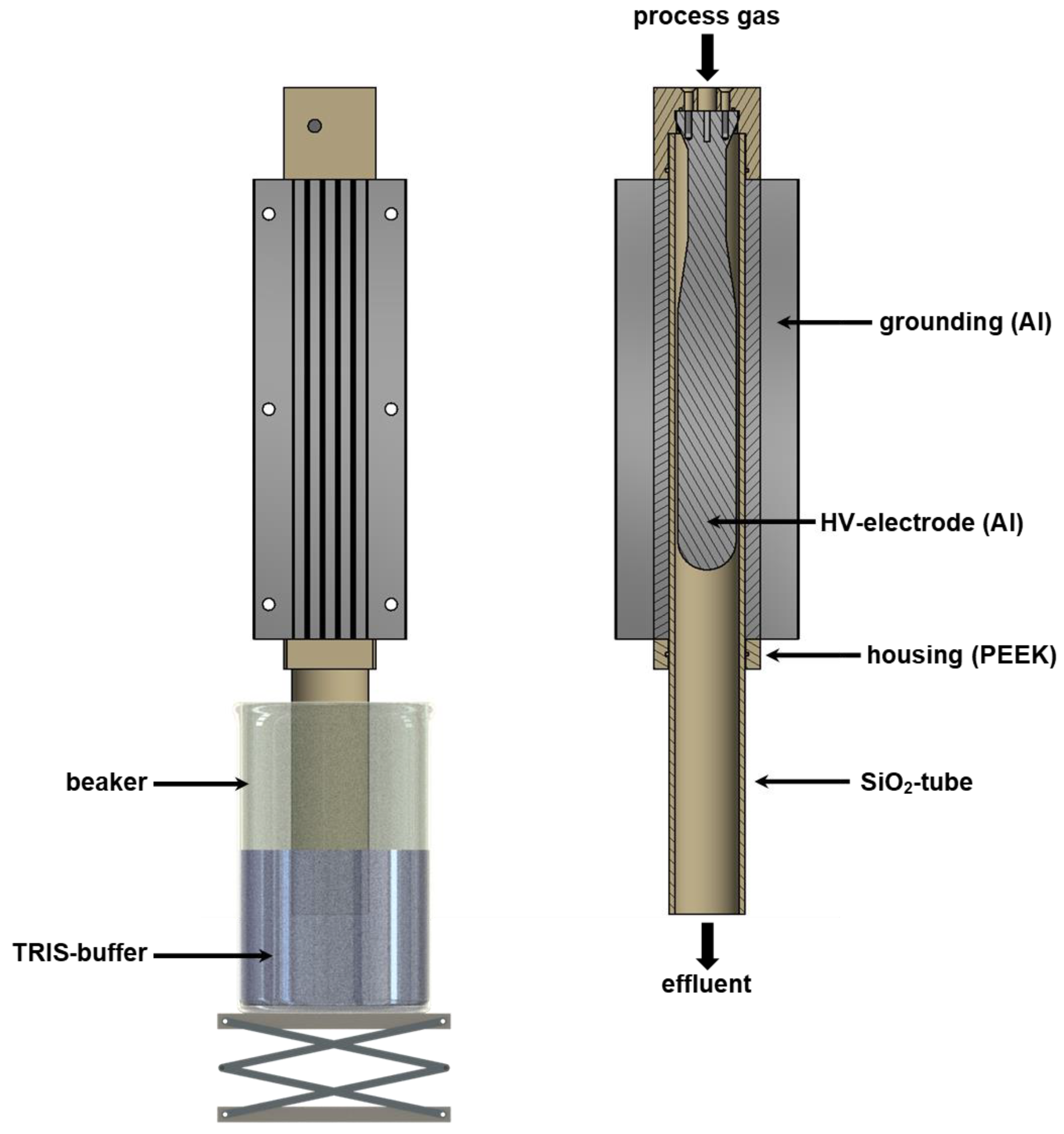
2.6. Plasma Device and Production of PABS
3. Results
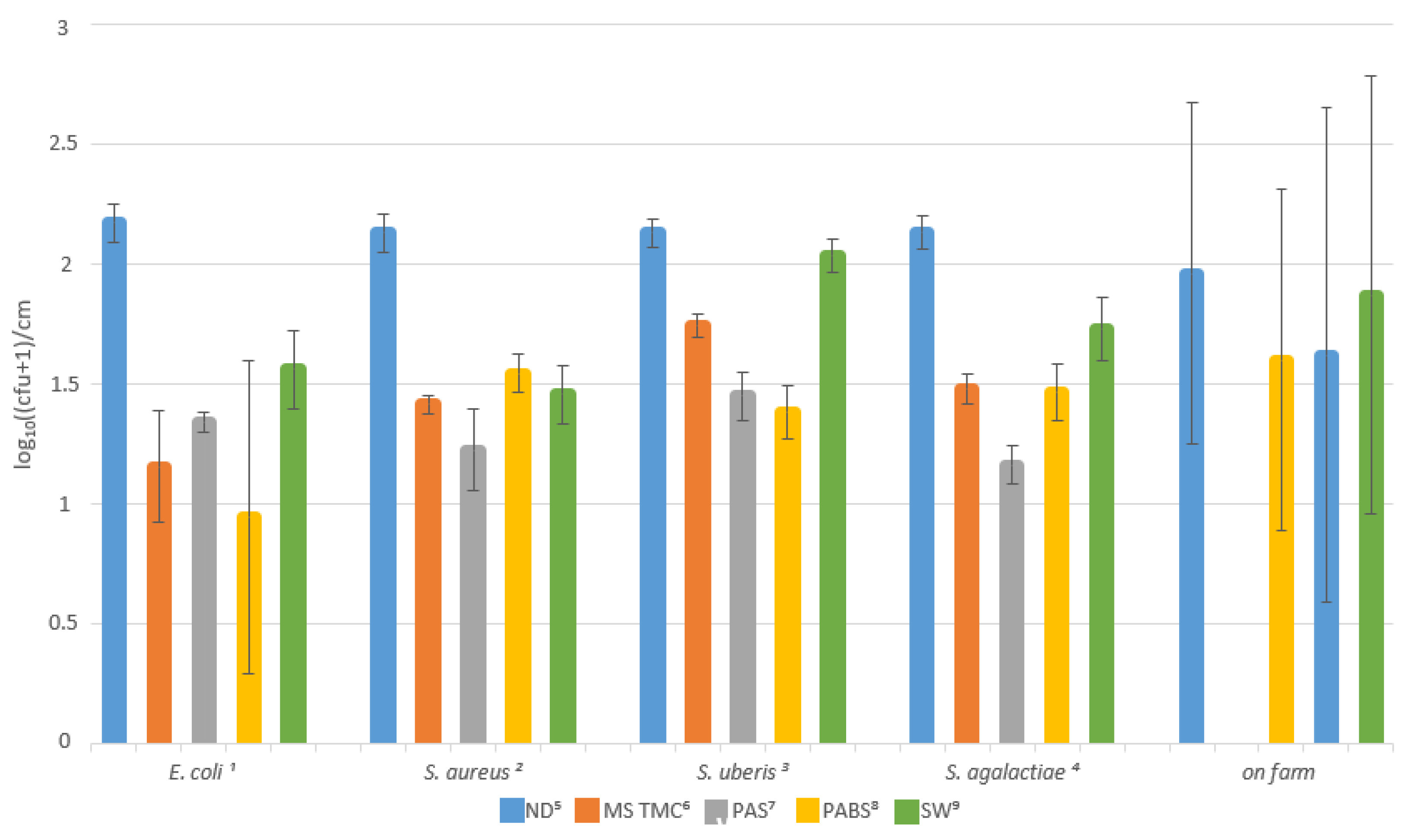
3.1. Lab Trial
3.2. Field Trial
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Le Blanc, S.J.; Lissemore, K.D.; Kelton, D.F.; Duffield, T.F.; Leslie, K.E. Major Advances in Disease Prevention in Dairy Cattle. J. Dairy Sci. 2006, 89, 1267–1279. [Google Scholar] [CrossRef]
- Barkema, H.W.; Schukken, Y.H.; Lam, T.J.G.M.; Beiboer, M.L.; Benedictus, G.; Brand, A. Management Practices Associated with the Incidence Rate of Clinical Mastitis. J. Dairy Sci. 1999, 82, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, S.M. The Role of Bacterial Contamination of Milking Utensils and Disinfecting Solutions in the Pathogenesis of Clinical Mastitis in Dairy Cows. [Bakteriell Kontaminierte Desinfektionsmittel und Gerätschaften Beim Melkakt Als Mögliche Quelle Für Mastitiden]. Ph.D. Thesis, University of Zurich, Vetsuisse Faculty, Zurich, Switzerland, 2010. [Google Scholar]
- Feldmann, M.; Zimmermann, A.; Hoedemaker, M. Influence of milking technique, milking hygiene and environmental hygiene parameters on the microbial contamination of milking machines. Dtsch. Tierarztl. Wochenschr. 2006, 113, 274–281. [Google Scholar]
- Garcia, A. Contagious vs. Environmental Mastitis. SDSU Ext. Extra Arch. 2004, 126, 1–4. [Google Scholar]
- Krömker, V. Udder health and machine milking-pathogen transfer via direct mechanisms. Arch. Lebensmittelhyg. 2014, 64, 95–97. [Google Scholar]
- Besier, J.; Lind, O.; Bruckmaier, R.M. Dynamics of teat-end vacuum during machine milking: Types, causes and impacts on teat condition and udder health—A literature review. J. Appl. Anim. Res. 2016, 44, 263–272. [Google Scholar] [CrossRef]
- Zadoks, R.N.; Allore, H.G.; Barkema, H.W.; Sampimon, O.C.; Gröhn, Y.T.; Schukken, Y.H. Analysis of an Outbreak of Streptococcus uberis Mastitis. J. Dairy Sci. 2001, 84, 590–599. [Google Scholar] [CrossRef]
- Zadoks, R.N.; Gillespie, B.E.; Barkema, H.W.; Sampimon, O.C.; Oliver, S.P.; Schukken, Y.H. Clinical, epidemiological and molecular characteristics of Streptococcus uberis infections in dairy herds. Epidemiol. Infect. 2003, 130, 335–349. [Google Scholar] [CrossRef]
- German Veterinary Association. Leitlinien Bekämpfung der Mastitis des Rindes als Bestandsproblem, 5th ed.; Verlag der DVG: Gießen, Germany, 2012; pp. 24–36. [Google Scholar]
- Newbould, F.H.S.; Barnum, D.A. The reduction of the microflora of milking machine inflations by teat dipping and teat cup pasteurization. J. Milk Food Technol. 1960, 23, 374–376. [Google Scholar] [CrossRef]
- Newbould, F.H.S. Disinfection in the prevention of udder infections: A review. Can. Vet. J. 1965, 6, 29–37. [Google Scholar]
- Hovinen, M.; Pyörälä, S. Invited review: Udder health of dairy cows in automatic milking. J. Dairy Sci. 2011, 94, 547–562. [Google Scholar] [CrossRef]
- Svennesen, L.; Nielsen, S.; Mahmmod, Y.; Krömker, V.; Pederen, K.; Klaas, I. Association between teat skin colonization and intramammary infection with Staphylococcus aureus and Streptococcus agalactiae in herds with automatic milking systems. J. Dairy Sci. 2019, 102, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Krömker, V. Short textbook dairy science and milky hygiene. In Kurzes Lehrbuch Milchkunde und Milchhygiene, 1st ed.; Verlag Parey: Stuttgart, Germany, 2006; pp. 38–41. [Google Scholar]
- Zimmermann, A. Comparison of Different Techniques for the Determination of Bacteriological Contamination of the Milking Equipment and Possible Interactions with Milking Procedures and Udder-Health. [Vergleich Verschiedener Verfahren zur Beurteilung der Mikrobiellen Kontamination der Melkzeuge Bzw. der Melkanlage und Mögliche Beziehungen zur Melktechnik und Eutergesundheit]. Ph.D. Thesis, Stiftung Tierärztliche Hochschule Hannover, Hannover, Germany, 2003. [Google Scholar]
- Köster, G.; Tenhagen, B.-A.; Scheibe, N.; Heuwieser, W. Factors associated with High Milk Test Day Somatic Cell Counts in Large Dairy Herds in Brandenburg. II. Milking Practices. J. Vet. Med. A 2006, 53, 209–214. [Google Scholar] [CrossRef]
- Bützner, P. Peroxyacetic acid: Simple, but effective (Peroxyessigsäure: Einfach, aber wirksam). CLB Chem. Lab. Biotechn. 2012, 62, 96–115. [Google Scholar]
- Kunigk, L.; Almeida, M.C.B. Action of peracetic acid on Escherichia coli and Staphylococcus aureus in suspension or settled on stainless steel surface. Braz. J. Microbiol. 2001, 32, 38–41. [Google Scholar] [CrossRef]
- Robert Koch-Institute (RKI): Anforderungen an die Hygiene bei der Reinigung und Desinfektion von Flächen. Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut. Bundesgesundheitsblatt 2022, 65, 1074–1115. [Google Scholar] [CrossRef]
- Mitsching, M.; Schwabe, H. Anwendungsmöglichkeiten von Peressigsäurepräparaten zur Prophylaxe und Therapie infektiöser Erkrankungen bei landwirtschaftlichen Nutztieren. Prakt. Tierarzt 1999, 80, 547–562. [Google Scholar]
- Fröhling, A.; Wienke, M.; Rose-Meierhöfer, S.; Schlüter, O. Improved Method for Mastitis Detection and Evaluation of Disinfectant Efficiency During Milking Process. Food Bioprocess Technol. 2010, 3, 892–900. [Google Scholar] [CrossRef]
- Ruf, J.; Johler, S.; Merz, A.; Stalder, U.; Hässig, M. Success of interventions in mastitis problems with Staphylococcus aureus after the introduction of an automatic milking system. Schweiz. Arch. Tierheilkd. 2015, 157, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma activated water (PAW): Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Rahman, M.; Hasan, M.S.; Islam, R.; Rana, R.; Sayem, A.; Sad, M.A.A.; Matin, A.; Raposo, A.; Zandonadi, R.P.; Han, H.; et al. Plasma-Activated Water for Food Safety and Quality: A Review of Recent Developments. Int. J. Environ. Res. Public Health 2022, 19, 6630. [Google Scholar] [CrossRef]
- Soni, A.; Choi, J.; Brightwell, G. Plasma-Activated Water (PAW) as a Disinfection Technology for Bacterial Inactivation with a Focus on Fruit and Vegetables. Foods 2021, 10, 166. [Google Scholar] [CrossRef]
- Hoeben, W.F.L.M.; van Ooij, P.P.; Schram, D.C.; Huiskamp, T.; Pemen, A.J.M.; Lukeš, P. On the Possibilities of Straightforward Characterization of Plasma Activated Water. Plasma Chem. Plasma Process. 2019, 39, 597–626. [Google Scholar] [CrossRef]
- Laroussi, M.; Bekeschus, S.; Keidar, M.; Bogaerts, A.; Fridmann, A.; Lu, X.; Ostrikov, K.; Hori, M.; Stapelmann, K.; Miller, V.; et al. Low-Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 127–157. [Google Scholar] [CrossRef]
- Große-Peclum, V.; Siekmann, L.; Krischek, C.; Avramidis, G.; ten Bosch, L.; Harms, M.; Ochs, C.; Ortmann, R.; Hoedemaker, M.; Ahlfeld, B.; et al. An In Vitro Model Using TRIS-Buffered Plasma-Activated Water to Reduce Pathogenic Microorganisms Involved in Digital Dermatitis Infection in Dairy Cattle. Appl. Sci. 2022, 12, 12325. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D Appl. Phys. 2011, 44, 472001. [Google Scholar] [CrossRef]
- Chen, H.; Bai, F.; Xiu, Z. Oxidative Stress Induced in Saccharomyces Cerevisiae Exposed to Dielectric Barrier Discharge Plasma in Air at Atmospheric Pressure. IEEE Trans. Plasma Sci. 2010, 38, 1885–1891. [Google Scholar] [CrossRef]
- Yusupov, M.; Bogaerts, A.; Huygh, S.; Snoeckx, R.; van Duin, A.C.T.; Neyts, E.C. Plasma-Induced Destruction of Bacterial Cell Wall Components: A Reactive Molecular Dynamics Stimulation. J. Phys. Chem. C 2013, 117, 5993–5998. [Google Scholar] [CrossRef]
- Smith, T.W.; Eberhart, R.J.; Spencer, S.B.; Kesler, E.M.; Hargrove, G.L.; Wilson, R.W.; Heald, C.W. Effect of Automatic Backflushing on Number of New Intramammary Infections, Bacteria on Teatcup Liners, and Milk Iodine. J. Dairy Sci. 1985, 68, 424–432. [Google Scholar] [CrossRef]
- Skarbye, A.P.; Thomsen, P.T.; Krogh, M.A.; Svennesen, L.; Østergaard, S. Effect of automatic cluster flushing on the concentration of Staphylococcus aureus in teat cup liners. J. Dairy Sci. 2020, 103, 5431–5439. [Google Scholar] [CrossRef]
- Hay, J.R. The bacterial flora and disinfection of teat cups. Am. J. Vet. Res. 1941, 2, 297–304. [Google Scholar]
- Scheib, S.; Wente, N.; Leimbach, S.; Krömker, V. Comparison of different swab techniques for the quantitative analysis of mastitis relevant pathogens on liner surfaces. Milk Sci. Int. 2023, 76, 1–7. [Google Scholar] [CrossRef]
- De Paoli, P. Bio-banking in microbiology: From sample collection to epidemiology, diagnosis and research. FEMS Microbiol. Rev. 2005, 29, 897–910. [Google Scholar] [CrossRef]
- Hamel, J.; Zhang, Y.; Wente, N.; Krömker, V. Heat stress and cow factors affect bacteria shedding pattern from naturally infected mammary gland quarters in dairy cattle. J. Dairy Sci. 2021, 104, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Pfannenschmidt, F. Qualification of the Wet-Dry-Swab-Technique DIN 10113; 1997-07 for the Determination of the Hygienic Status in Milking Machines. [Eignung des Nass-Trockentupfer Verfahrens (NTT) DIN 10113; 1997-07 zur Bestimmung des Hygienestatus in Melkanlagen]. Ph.D. Thesis, Stiftung Tierärztliche Hochschule Hannover, Hannover, Germany, 2003. [Google Scholar]
- Noorlander, D.O.; Heckmann, R. Scanning Electron Microscopy, A Technique for Evaluating Milking Machine Inflations and Tubes. J. Dairy Sci. 1980, 63, 991–1005. [Google Scholar] [CrossRef]
- Rockhoff, V. Vergleichende Untersuchung von Agarplatten-Oberflächen-Verfahren und Most-Probable-Number-Methode zur Desinfektionsmittelprüfung Gemäß der CEN-Normen EN 1656 und EN 1657. Ph.D. Thesis, Universität Leipzig, Leipzig, Germany, 2008. [Google Scholar]
- Reybrouck, G. The testing of disinfectants. Intern. Biodeterior. Biodegrad. 1998, 41, 269–272. [Google Scholar] [CrossRef]
- Borneff, J.; Werner, H.; Van de Voorde, H.; Reybrouck, G. Critical assessment of methods for testing chemical disinfectants and disinfection procedures. [Kritische Beurteilung der Prüfmethoden für chemische Desinfektionsmittel und -verfahren]. Zentralbl. Bakteriol. Parasitenkd, Infektionskr. Hyg. 1975, 160, 590–600. [Google Scholar]
- Mac Kinnon, I.H. The use of inactivators in the evaluation of disinfectants. J. Hyg. 1974, 73, 189–195. [Google Scholar] [CrossRef]
- Pineau, L.; Desbuquois, C.; Marchetti, B.; Luu Duc, D. Comparison of the fixative properties of five disinfectant solutions. J. Hosp. Infect. 2008, 68, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V.; Patel, D.; Butani, S.; Nema, S.K. Investigation of Physicochemical Properties of Plasma Activated Water and its Bactericidal Efficacy. Plasma Chem. Plasma Process. 2021, 41, 871–902. [Google Scholar] [CrossRef]
- Zhang, J.; Su, P.; Chen, H.; Qiao, M.; Yang, B.; Zhao, X. Impact of reactive oxygen species on cell activity and structural integrity of Gram-positive and Gram-negative bacteria in electrochemical disinfection system. Chem. Eng. J. 2023, 451, 138879. [Google Scholar] [CrossRef]
- Ursache, M.; Moraru, R.; Eugen, H.; Nâstasâ, V.; Mares, M. Comparative assessment of the relation between energy consumption and bacterial burden reduction using plasma activated water. In Proceedings of the 2014 International Conference on Optimization of Electrical and Electronic Equipment (OPTIM), Bran Romania 2014, Piscataway, NJ, USA, 22–24 May 2014; pp. 1036–1041. [Google Scholar] [CrossRef]
- Joshi, I.; Salvi, D.; Schaffner, D.W.; Karwe, M.V. Characterization of Microbial Inactivation Using Plasma-Activated Water and Plasma-Activated Acidified Buffer. J. Food Prot. 2018, 81, 1472–1480. [Google Scholar] [CrossRef]
| Pathogen | Disinfectant | Mean (log10((cfu + 1)/cm2)) 1 | 95% CI 2 Lower Bound | 95% CI 2 Upper Bound |
|---|---|---|---|---|
| E. coli 3 | ND 7 | 2.172 | 1.992 | 2.412 |
| MS TMC 8 | 1.156 a,b | 0.907 | 1.406 | |
| PAS 9 | 1.341 a | 1.092 | 1.591 | |
| PABS 10 | 0.944 a,b,c | 0.695 | 1.194 | |
| SW 11 | 1.559 a | 1.309 | 1.808 | |
| S. aureus 4 | ND 7 | 2.138 | 1.998 | 2.277 |
| MS TMC 8 | 1.414 a | 1.274 | 1.553 | |
| PAS 9 | 1.225 a,b,d | 1.086 | 1.365 | |
| PABS 10 | 1.543 a | 1.403 | 1.683 | |
| SW 11 | 1.456 a | 1.316 | 1.595 | |
| Sc. uberis 5 | ND 7 | 2.129 | 2.032 | 2.236 |
| MS TMC 8 | 1.741 a,b | 1.635 | 1.848 | |
| PAS 9 | 1.450 a,b,e | 1.343 | 1.556 | |
| PABS 10 | 1.383 a,b,e | 1.277 | 1.489 | |
| SW 11 | 2.036 | 1.930 | 2.142 | |
| Sc. agalactiae 6 | ND 7 | 2.132 | 2.007 | 2.258 |
| MS TMC 8 | 1.479 a,b | 1.353 | 1.605 | |
| PAS 9 | 1.162 a,b,d,e | 1.036 | 1.288 | |
| PABS 10 | 1.466 a,b | 1.341 | 1.592 | |
| SW 11 | 1.730 a | 1.604 | 1.856 |
| Pathogen | DM1 1 | DM2 1 | Mean Difference (log10((cfu + 1)/cm2)) 2 (DM1–DM2) | p-Value | 95% CI 3 Lower Bound | 95% CI 3 Upper Bound |
|---|---|---|---|---|---|---|
| E. coli 4 | ND 8 | MS TMC 9 | 1.015 | <0.001 | 0.448 | 1.582 |
| PAS 10 | 0.830 | 0.004 | 0.264 | 1.397 | ||
| PABS 11 | 1.227 | <0.001 | 0.661 | 1.794 | ||
| SW 12 | 0.613 | 0.031 | 0.459 | 1.180 | ||
| SW 12 | PABS 11 | 0.615 | 0.003 | 0.262 | 0.967 | |
| S. aureus 5 | ND 8 | MS TMC 9 | 0.724 | <0.001 | 0.407 | 1.041 |
| PAS 10 | 0.913 | <0.001 | 0.595 | 1.229 | ||
| PABS 11 | 0.595 | <0.001 | 0.277 | 0.912 | ||
| SW 12 | 0.682 | <0.001 | 0.365 | 0.999 | ||
| PABS 11 | PAS 10 | 0.318 | 0.049 | 0.001 | 0.635 | |
| Sc. uberis 6 | ND 8 | MS TMC 9 | 0.388 | 0.002 | 0.146 | 0.630 |
| PAS 10 | 0.680 | <0.001 | 0.438 | 0.921 | ||
| PABS 11 | 0.746 | <0.001 | 0.505 | 0.988 | ||
| MS TMC 9 | PAS 10 | 0.292 | 0.015 | 0.050 | 0.533 | |
| PABS 11 | 0.358 | 0.003 | 0.117 | 0.600 | ||
| SW 12 | MS TMC 9 | 0.295 | 0.014 | 0.053 | 0.536 | |
| PAS 10 | 0.587 | <0.001 | 0.345 | 0.828 | ||
| PABS 11 | 0.653 | <0.001 | 0.412 | 0.895 | ||
| Sc. agalactiae 7 | ND 8 | MS TMC 9 | 0.654 | <0.001 | 0.368 | 0.940 |
| PAS 10 | 0.971 | <0.001 | 0.685 | 1.257 | ||
| PABS 11 | 0.666 | <0.001 | 0.380 | 0.952 | ||
| SW 12 | 0.403 | 0.005 | 0.117 | 0.689 | ||
| MS TMC 9 | PAS 10 | 0.317 | 0.027 | 0.308 | 0.603 | |
| PABS 11 | PAS 10 | 0.305 | 0.034 | 0.185 | 0.591 | |
| SW 12 | PAS 10 | 0.568 | <0.001 | 0.282 | 0.854 |
| Disinfectant | Mean (log10((cfu + 1)/cm2)) 1 | 95% CI 2 Lower Bound | 95% CI 2 Upper Bound |
|---|---|---|---|
| ND 3 | 1.927 | 1.639 | 2.214 |
| PAS 4 | 1.574 | 1.301 | 1.847 |
| PABS 5 | 1.774 | 1.482 | 2.066 |
| SW 6 | 1.863 | 1.575 | 2.150 |
| DM1 1 | DM2 1 | Mean Difference (log10((cfu + 1)/cm2)) 2 (DM1–DM2) | p-Value | 95 % CI 3 Lower Bound | 95 % CI 3 Upper Bound |
|---|---|---|---|---|---|
| ND 4 | PAS 5 | 0.353 | 0.483 | −0.185 | 0.891 |
| PABS 6 | 0.153 | 1.000 | −0.403 | 0.709 | |
| SW 7 | 0.064 | 1.000 | −0.487 | 0.615 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheib, S.; Leimbach, S.; Avramidis, G.; Bellmann, M.; Nitz, J.; Ochs, C.; Tellen, A.; Wente, N.; Zhang, Y.; Viöl, W.; et al. Intermediate Cluster Disinfection: Which Disinfection Solution Is Most Effective on Milking Liners? A Comparison of Microorganism Reduction on Liner Inner Surfaces Using Quantitative Swab Sampling Technique. Pathogens 2023, 12, 560. https://doi.org/10.3390/pathogens12040560
Scheib S, Leimbach S, Avramidis G, Bellmann M, Nitz J, Ochs C, Tellen A, Wente N, Zhang Y, Viöl W, et al. Intermediate Cluster Disinfection: Which Disinfection Solution Is Most Effective on Milking Liners? A Comparison of Microorganism Reduction on Liner Inner Surfaces Using Quantitative Swab Sampling Technique. Pathogens. 2023; 12(4):560. https://doi.org/10.3390/pathogens12040560
Chicago/Turabian StyleScheib, Sabrina, Stefanie Leimbach, Georg Avramidis, Martin Bellmann, Julia Nitz, Christian Ochs, Anne Tellen, Nicole Wente, Yanchao Zhang, Wolfgang Viöl, and et al. 2023. "Intermediate Cluster Disinfection: Which Disinfection Solution Is Most Effective on Milking Liners? A Comparison of Microorganism Reduction on Liner Inner Surfaces Using Quantitative Swab Sampling Technique" Pathogens 12, no. 4: 560. https://doi.org/10.3390/pathogens12040560
APA StyleScheib, S., Leimbach, S., Avramidis, G., Bellmann, M., Nitz, J., Ochs, C., Tellen, A., Wente, N., Zhang, Y., Viöl, W., & Krömker, V. (2023). Intermediate Cluster Disinfection: Which Disinfection Solution Is Most Effective on Milking Liners? A Comparison of Microorganism Reduction on Liner Inner Surfaces Using Quantitative Swab Sampling Technique. Pathogens, 12(4), 560. https://doi.org/10.3390/pathogens12040560







