Limnotrachelobdella hypophthalmichthysa n. sp. (Hirudinida: Piscicolidae) on Gills of Bighead Carp Hypophthalmichthys nobilis in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preservation
2.2. Morphological Identification
2.3. Histopathological Observation
2.4. DNA Extraction, Amplification, and Sequencing
2.5. Molecular Identification and Phylogenetic Analyses
3. Results
3.1. Infection by Fish Leeches
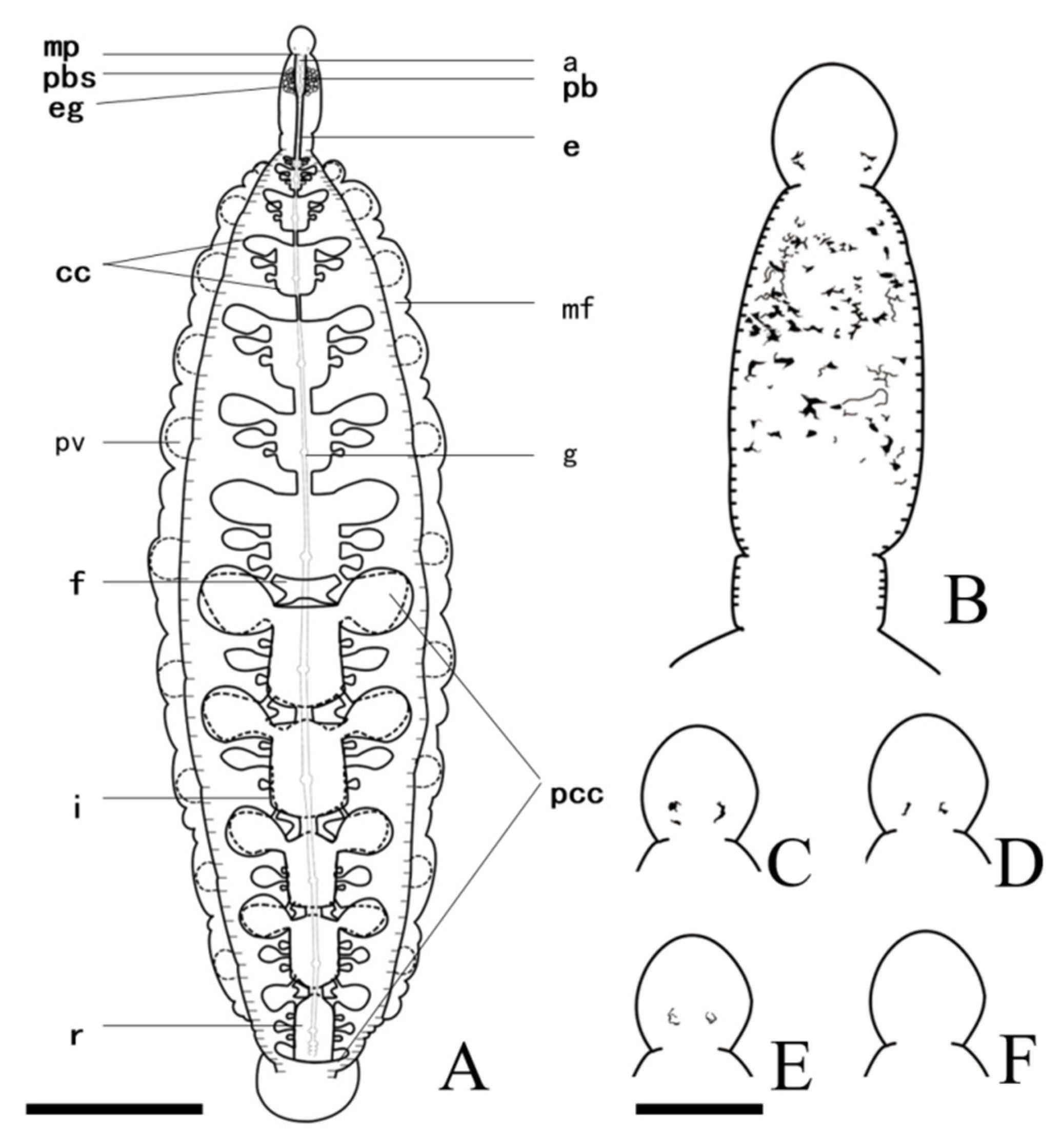
3.2. Description
3.3. Molecular Identification and Phylogenetic Analyses
3.4. Taxonomic Summary
3.5. Histopathological Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bottari, T.; Profeta, A.; Rinelli, P.; Gaglio, G.; La Spada, G.; Smedile, F.; Giordano, D. On the presence of Pontobdella muricata (Hirudinea: Piscicolidae) on some elasmobranchs of the Tyrrhenian Sea (Central Mediterranean). Acta Adriat. 2017, 58, 225–234. [Google Scholar] [CrossRef]
- SIDDALL, M.E. Phylogeny of adeleid blood parasites with a partial systematic revision of the haemogregarine complex. J. Eukaryot. Microbiol. 1995, 42, 116–125. [Google Scholar] [CrossRef]
- Karlsbakk, E. A trypanosome of Atlantic cod, Gadus morhua L., transmitted by the marine leech Calliobdella nodulifera (Malm, 1863) (Piscicolidae). Parasitol. Res. 2004, 93, 155–158. [Google Scholar] [CrossRef]
- Siddall, M.E.; Desser, S.S. Ultrastructure of merogonic development of Haemogregarina (sensu lato) myoxocephali (Apicomplexa: Adeleina) in the marine leech Malmiana scorpii and localization of infective stages in the salivary cells. Eur. J. Protistol. 1993, 29, 191–201. [Google Scholar] [CrossRef]
- Karlsbakk, E.; Haugen, E.; Nylund, A. Morphology and aspects of growth of a trypanosome transmitted by the marine leech Johanssonia arctica (Piscicolidae) from Northern Norway. Folia Parasitol. 2005, 52, 209. [Google Scholar] [CrossRef]
- Burreson, E.M. Phylum Annelida: Hirudinea as vectors and disease agents. In Fish Diseases and Disorders. Volume 1: Protozoan and Metazoan Infections; CABI: Wallingford, UK, 2006. [Google Scholar]
- Kazuo, O.; Olga, R.; Masaharu, T. New Record of the Leech Limnotrachelobdella sinensis Infecting Freshwater Fish from Japanese Waters. Fish Pathol. 2007, 42, 85–89. [Google Scholar] [CrossRef]
- Kua, B. Marine leech isolated from cage-cultured sea bass (Lates calcarifer) fingerlings: A parasite or vector? Malay. Fish. J. 2009. Available online: https://cir.nii.ac.jp/crid/1571417126167425280 (accessed on 1 January 2020).
- Cruz-Lacierda, E.R.; Toledo, J.D.; Tan-Fermin, J.D.; Burreson, E.M. Marine leech (Zeylanicobdella arugamensis) infestation in cultured orange-spotted grouper, Epinephelus coioides. Aquaculture 2000, 185, 191–196. [Google Scholar] [CrossRef]
- Utevsky, S.Y.; Trontelj, P. Phylogenetic relationships of fish leeches (Hirudinea, Piscicolidae) based on mitochondrial DNA sequences and morphological data. Zool. Scr. 2004, 33, 375–385. [Google Scholar] [CrossRef]
- Williams, J.I.; Burreson, E.M. Phylogeny of the fish leeches (Oligochaeta, Hirudinida, Piscicolidae) based on nuclear and mitochondrial genes and morphology. Zool. Scr. 2006, 35, 627–639. [Google Scholar] [CrossRef]
- Blanchard, R. Description de quelques Hirudinees asiatiques. Mem. Soc. Zool. Fr. 1896, 9, 316–330. [Google Scholar]
- Moore, J.P. Notes on some Asiatic leeches (Hirudinea) principally from China, Kashmir, and British India. Proc. Acad. Nat. Sci. USA 1924, 76, 343–388. Available online: https://www.jstor.org/stable/4063928 (accessed on 14 February 2023).
- Epshtein, V. A new species of leech from the Amur Basin. Zool. Zhurnal 1957, 36, 1414-141. [Google Scholar]
- Yang, T. On the genus Limnotrachelobdella Epstein, 1968 and a new species from South China Sea. Acta Hydrobiol. Sin. 1987, 11, 268–273. Available online: http://ir.ihb.ac.cn/handle/152342/6212 (accessed on 14 February 2023).
- Epstein, V. From morphology to phylogeny (on the example of study of the fish leeches of Palearctic). Вісник Харківськoгo Націoнальнoгo Університету Імені Вн Каразіна. Серія Біoлoгія 2013, 18, 144–150. Available online: https://periodicals.karazin.ua/biology/article/view/13754 (accessed on 14 February 2023).
- Yang, T. Fauna Sinica, Annelida: Hirudinea; Science Press: Beijing, China, 1996. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovli, I.; Zou, H.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.; Huelsenbeck, J. MrBayes version 3.0: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Park, M.A.; Kim, S.R.; Kim, M.S.; Kim, J.H.; Park, J.J. Histopathological observation of the gill of the crucian carp, Carassius auratus by the leech, Limnotrachelobdella sinensis. J. Fish Pathol. 2010, 23, 399–407. [Google Scholar]
- Rhee, J.K. Trachelobdella sinensis Blanchard, 1896 found from Cyprinus carpio nudus in Korea. Kisaengchunghak Chapchi 1986, 24, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K.; Park, S.-W.; Kim, Y.-G.; Kim, H.J. Limnotrachelobdella sinensis, a leech associated with mortality in a wild population of Japaneses curcian carp Carassius cuvieri in Korea. J. Grad. Sch. Biosph. Sci. 2009, 48, 49–53. [Google Scholar] [CrossRef]
- Nagasawa, K.; Tanaka, M. The fish leech Limnotrachelobdella sinensis (Hirudinida, Piscicolidae) invaded Kyoto Prefecture, central Japan. Biogeography 2009, 11, 17–21. Available online: http://ir.lib.hiroshima-u.ac.jp/00030959 (accessed on 14 February 2023).
- Bauer, O.N. Key to the parasites of freshwater fish of the USSR, Volume 3, Parasitic Metazoa (part 2). In Opredelitel’ Parezitov Presnovodnykh Ryb Fauny SSSR, Tom 3, Paraziticheskie Mnogokletochnye (Vtoraya Chast’); Nauka: Leningrad, Russia, 1987. [Google Scholar]
- Nagasawa, K.; Tanaka, M. Common carp (Cyprinus carpio carpio) as a host for the fish leech Limnotrachelobdella sinensis (Hirudinida: Piscicolidae) in Japan <Short Communication>. Biogeography 2011, 13, 111–113. Available online: https://ir.lib.hiroshima-u.ac.jp/00031800 (accessed on 14 February 2023).
- Nagasawa, K.; Sasaki, M.; Matsubara, H. Limnotrachelobdella okae (Hirudinida: Piscicolidae) Parasitic on Big-scaled Redfin, Pseudaspius hakonensis (Cypriniformes: Leuciscidae), in Two Brackish Water Lakes, Hokkaido, Japan. Species Divers. 2022, 27, 279–284. [Google Scholar] [CrossRef]
- Nagasawa, K.; Izumikawa, K.; Yamanoi, H.; Umino, T. New Hosts, Including Marine Fishes Cultured in Japan, of Limnotrachelobdella okae (Hirudinida: Piscicolidae). Comp. Parasitol. 2009, 76, 127–129. [Google Scholar] [CrossRef]
- Bielecki, A.; Kapusta, A.; Cichocka, J. Atlantic sturgeon, Acipenser oxyrinchus Mitchill, infected by the parasitic leech, Caspiobdella fadejewi (Epshtein) (Hirudinea; Piscicolidae), in the Drwęca River. Arch. Pol. Fish. 2011, 19, 87–93. [Google Scholar] [CrossRef]
- Bauer, O.; Pugachev, O.; Voronin, V. Study of parasites and diseases of sturgeons in Russia: A review. J. Appl. Ichthyol. 2002, 18, 420–429. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Wr, H.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial Cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Kaygorodova, I.; Matveenko, E. Diversity of the Piscicola Species (Hirudinea, Piscicolidae) in the Eastern Palaearctic with a Description of Three New Species and Notes on Their Biogeography. Diversity 2023, 15, 98. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Beresic-Perrins, R.K.; Govedich, F.R.; Banister, K.; Bain, B.A.; Rose, D.; Shuster, S.M. Helobdella blinni sp. n. (Hirudinida, Glossiphoniidae) a new species inhabiting Montezuma Well, Arizona, USA. Zookeys 2017, 661, 137–155. [Google Scholar] [CrossRef] [PubMed]




| Gene or Region | Primer Name | Sequence (5′-3′) | Accession Number (Gene Length) | ||
|---|---|---|---|---|---|
| Limnotrachelobdella hypophthalmichthysa n. sp. | |||||
| Z1 | H1 | J1 | |||
| 18S | 18SF | 5′-GATTAAGCCATGCATGTCTA-3′ | OP595730 (1712 bp) | OP709950 (1712 bp) | OP709959 (1713 bp) |
| 18SR | 5′-ACTTCCTCTAGATGATCAAG-3′ | ||||
| ITS | ITSF | 5′-TCGCGTTGATTACGTCCCTG-3′ | OP723914 (1495 bp) | OP723913 (1490 bp) | OP723912 (1489 bp) |
| ITSR | 5′-GCATTCTCAAACAACCCGAC-3′ | ||||
| COX1 | COX1F | 5′-GCTTCTAACTTTTAGTRGRTAG-3′ | OP711958 (1537 bp) | OP712182 (1537 bp) | OP712183 (1537 bp) |
| COX1R | 5′-CTTCTARTTAACAGTTAGRTGCA-3′ | ||||
| Species | Environment | GenBank Accession Number | |
|---|---|---|---|
| 18S | COX1 | ||
| Ingroup | |||
| Piscicolidae | |||
| Stibarobdella macrothela | Brackish to marine | DQ414295 | DQ414340 |
| Pontobdella muricata | Marine | AF099945 | AY336029 |
| Aestabdella abditovesiculata | Marine | DQ414254 | DQ414300 |
| Austrobdella bilobata | Marine | DQ414255 | DQ414301 |
| Bathybdella sawyeri | Marine | DQ414265 | DQ414311 |
| Beringobdella rectangulata | Marine | DQ414264 | DQ414310 |
| Heptacyclus virgatus | Brackish to marine | DQ414273 | DQ414319 |
| Janusion virida | Marine | DQ414281 | N/A |
| Myzobdella lugubris | Freshwater to marine | DQ414278 | DQ414324 |
| Notobdella nototheniae | Marine | DQ414283 | DQ414328 |
| Oceanobdella khani | Marine | DQ414286 | DQ414331 |
| Oxytonostoma typica | Marine | DQ414288 | DQ414333 |
| Piscicolaria reducta | Freshwater | DQ414294 | DQ414339 |
| Platybdella anarrhichae | Marine | DQ414290 | DQ414335 |
| Pterobdella amara | Brackish | DQ414289 | DQ414334 |
| Trulliobdella capitis | Marine | N/A | AY336030 |
| Zeylanicobdella arugamensis | Brackish to marine | DQ414299 | DQ414344 |
| Alexandrobdella makhrovi | Freshwater | MN312187 | MN295413 |
| Baicalobdella torquata | Freshwater | N/A | AY336018 |
| Branchellion lobata | Marine | DQ414261 | DQ414307 |
| Calliobdella lophii | Marine | DQ414268 | DQ414314 |
| Caspiobdella fadejewi | Freshwater | N/A | AY336019 |
| Cystobranchus meyeri | Freshwater | DQ414269 | DQ414315 |
| Gonimosobdella vivida | Freshwater | AF115992 | AF003260 |
| Johanssonia arctica | Marine | DQ414274 | DQ414320 |
| Limnotrachelobdella hypophthalmichthysa n. sp. Z1 | Freshwater | OP595730 | OP711958 |
| Limnotrachelobdella hypophthalmichthysa n. sp. H1 | OP709950 | OP712182 | |
| Limnotrachelobdella hypophthalmichthysa n. sp. J1 | OP709959 | OP712183 | |
| Limnotrachelobdella okae | Freshwater to marine | N/A | AY336022 |
| Limnotrachelobdella sinensis | Freshwater to brackish | LC275139 | LC275140 |
| Piscicola geometra | Freshwater to marine | AF115995 | AF003280 |
| Trachelobdellina glabra | Marine | N/A | EF405597 |
| Outgroup | |||
| Ozobranchidae | |||
| Ozobranchus branchiatus | Marine | KF728214 | KF728213 |
| Ozobranchus margoi | Marine | AF115991 | AF003268 |
| L. hypophthalmichthysa n. sp. | L. sinensis | L. okae | L. taimeni | L. fujianensis | L. turkestanica | |
|---|---|---|---|---|---|---|
| Eyes | 0–2 | 2 | 2 | 0 * | 2 | N/A |
| Pulsatile vesicles | 10 | 11 | 11–13 | 10 | 12 | 11 |
| Testisacs | 5 | 6 | 6 | N/A | 6 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Cheng, H.; Li, W.; Li, M.; Zou, H.; Wang, G. Limnotrachelobdella hypophthalmichthysa n. sp. (Hirudinida: Piscicolidae) on Gills of Bighead Carp Hypophthalmichthys nobilis in China. Pathogens 2023, 12, 562. https://doi.org/10.3390/pathogens12040562
Lin L, Cheng H, Li W, Li M, Zou H, Wang G. Limnotrachelobdella hypophthalmichthysa n. sp. (Hirudinida: Piscicolidae) on Gills of Bighead Carp Hypophthalmichthys nobilis in China. Pathogens. 2023; 12(4):562. https://doi.org/10.3390/pathogens12040562
Chicago/Turabian StyleLin, Lin, Houda Cheng, Wenxiang Li, Ming Li, Hong Zou, and Guitang Wang. 2023. "Limnotrachelobdella hypophthalmichthysa n. sp. (Hirudinida: Piscicolidae) on Gills of Bighead Carp Hypophthalmichthys nobilis in China" Pathogens 12, no. 4: 562. https://doi.org/10.3390/pathogens12040562
APA StyleLin, L., Cheng, H., Li, W., Li, M., Zou, H., & Wang, G. (2023). Limnotrachelobdella hypophthalmichthysa n. sp. (Hirudinida: Piscicolidae) on Gills of Bighead Carp Hypophthalmichthys nobilis in China. Pathogens, 12(4), 562. https://doi.org/10.3390/pathogens12040562








