Epidemiological and Clinical Features of Streptococcus dysgalactiae ssp. equisimilis stG62647 and Other emm Types in Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Data Preparation
2.3. Microbiological Analyses
2.4. Antibiotic Sensitivity Pattern
2.5. Single Center Microbiological Analyses
2.6. Clinical Chemistry
2.7. Clinical Cohort Description
2.8. Statistical Analysis
3. Results
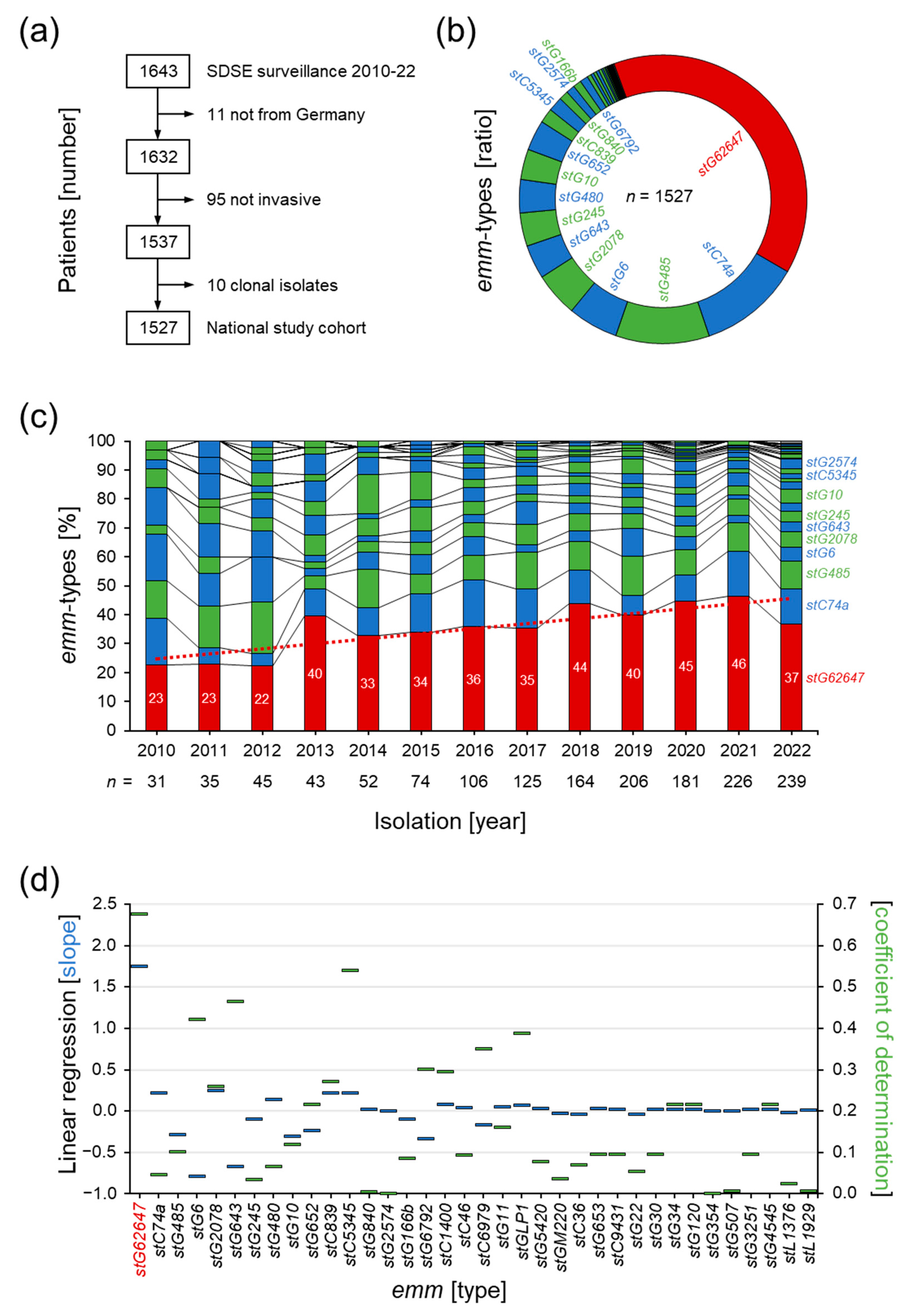
3.1. Invasive SDSE Infections in Germany
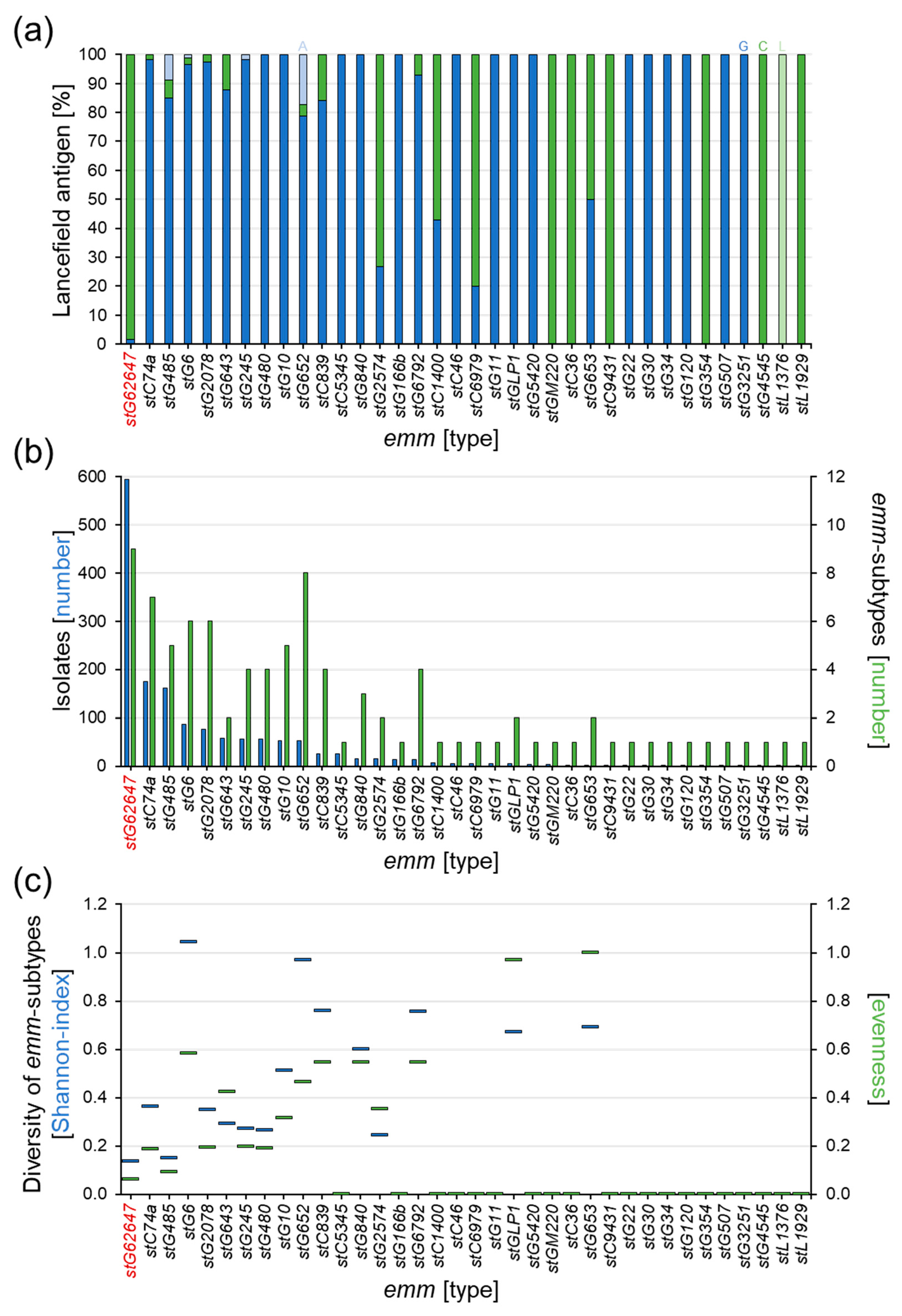
3.2. Dissection of Epidemiological Markers
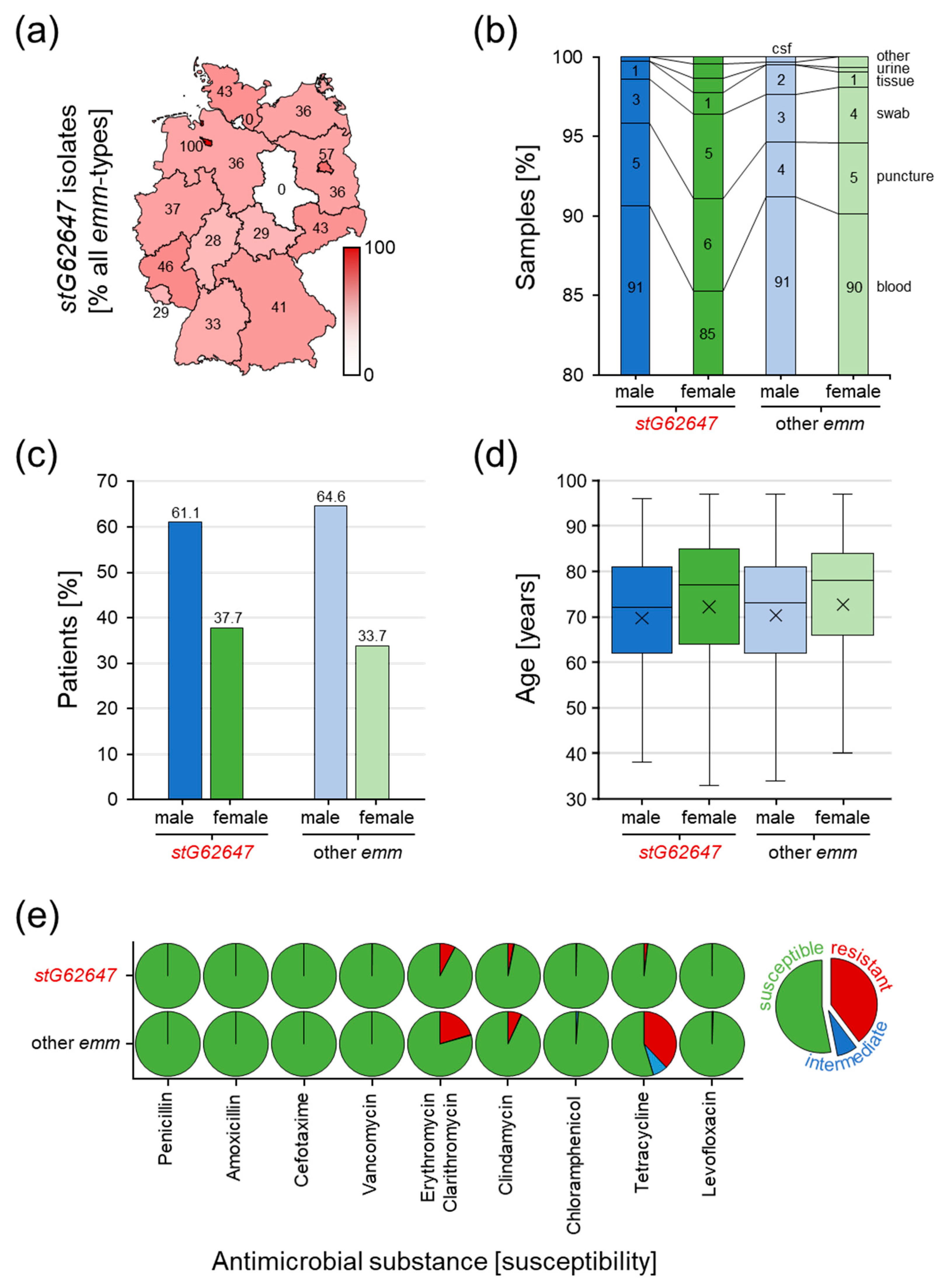
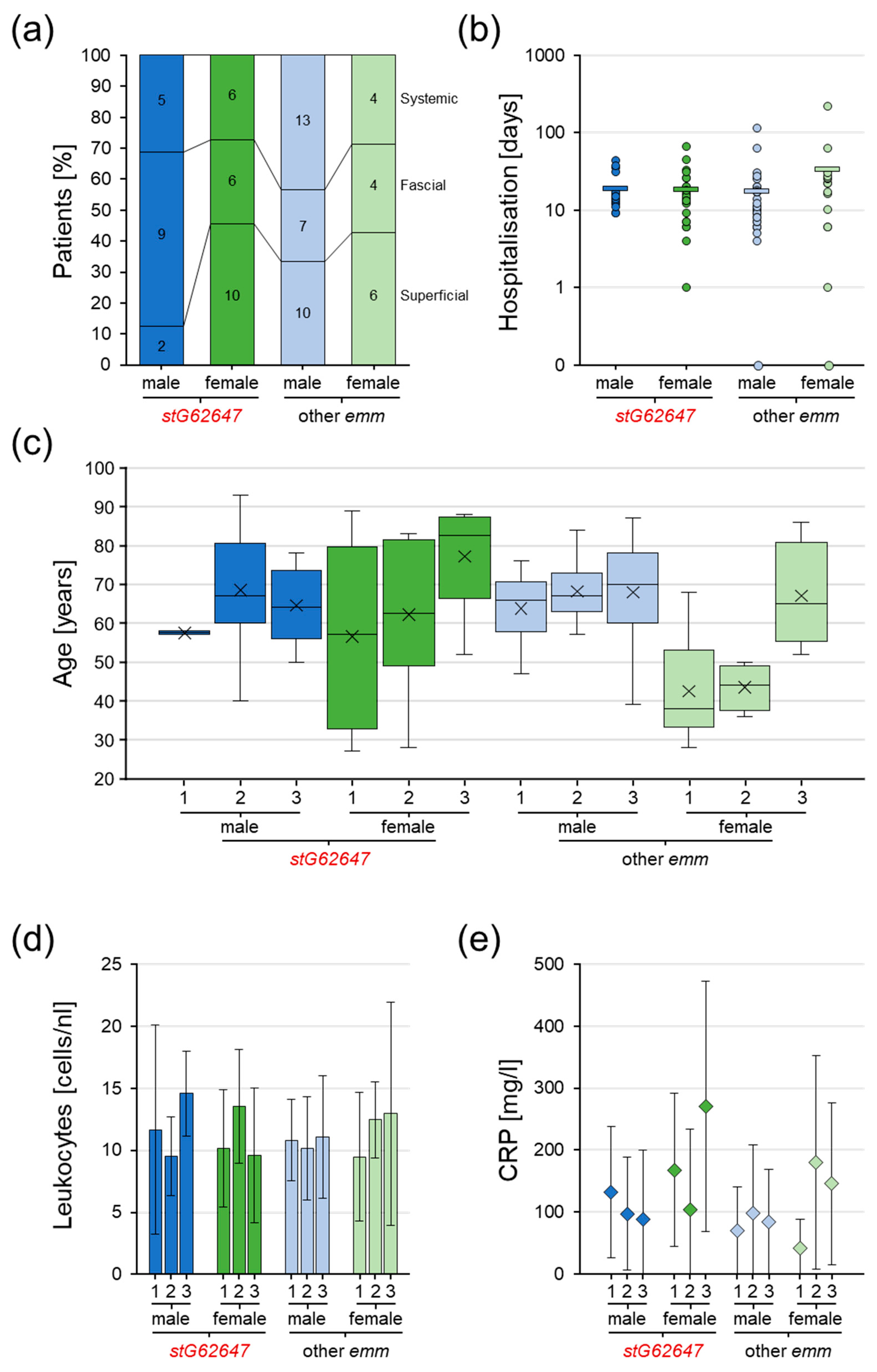
3.3. Comparison of stG62647 with Other emm Types
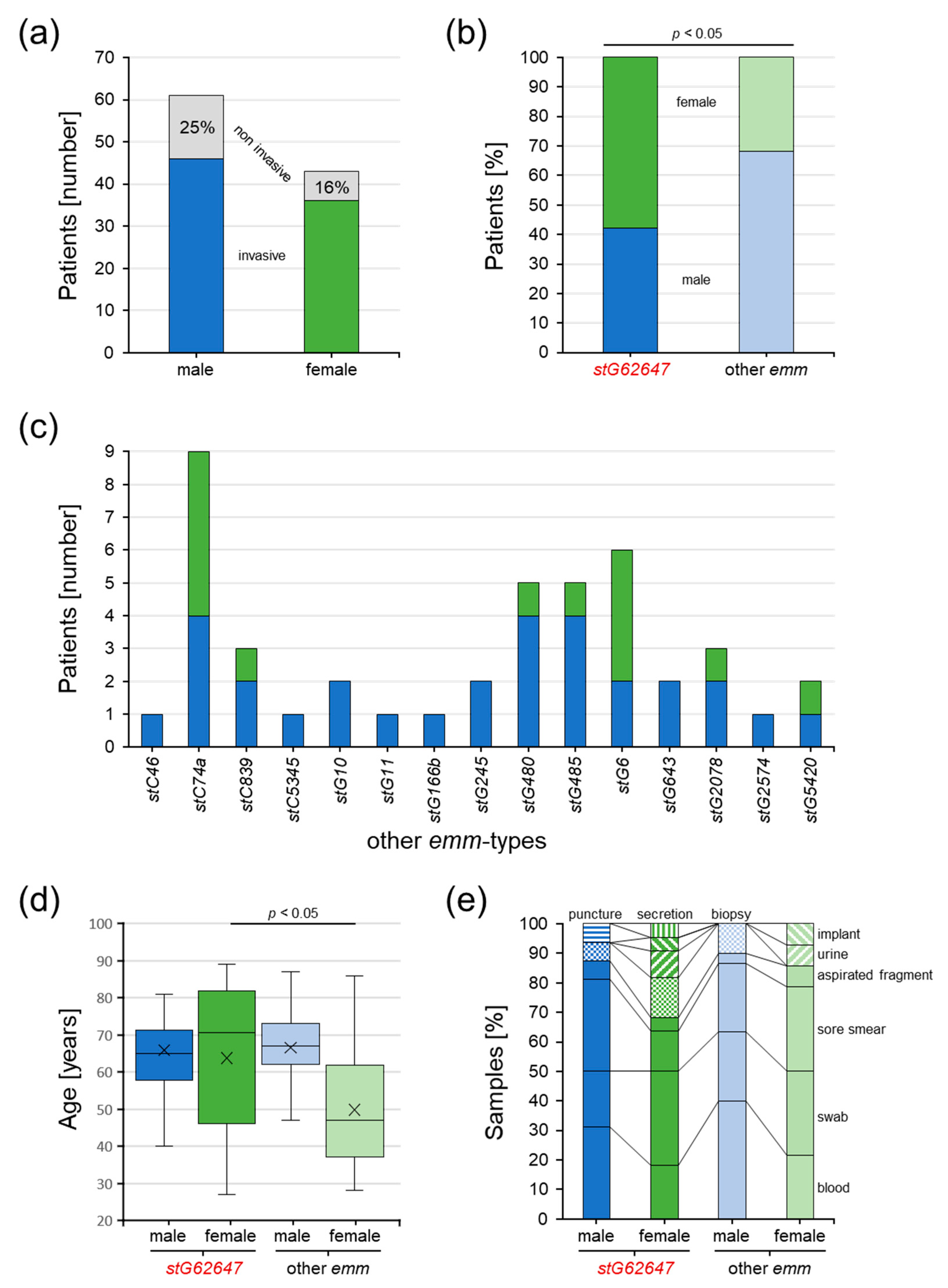
3.4. Description of the Clinical Cohort at Ingolstadt Hospital
3.5. Impact of Bacterial emm Type, Patient Sex, and Age on Clinical Courses
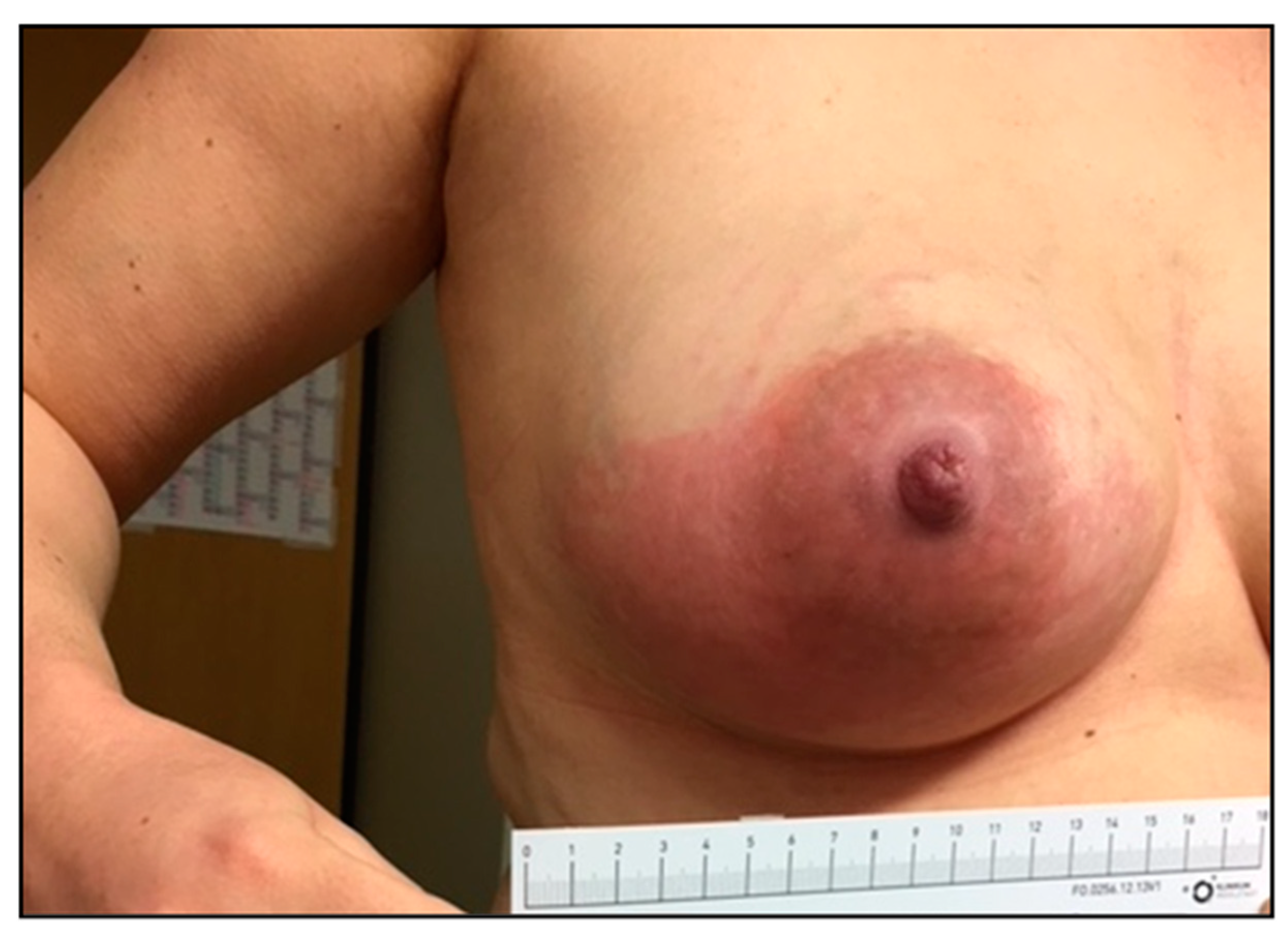
3.6. S. dysgalactiae Infections Resembling S. pyogenes and S. agalactiae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baracco, G.J. Infections Caused by Group C and G Streptococcus (Streptococcus dysgalactiae subsp. equisimilis and Others): Epidemiological and Clinical Aspects. Microbiol. Spectr. 2019, 7. Part HISTORY AND TAXONOMY; HUMAN INFECTIONS. [Google Scholar] [CrossRef]
- Frost, W.D.; Engelbrecht, M.A. The Streptococci. Their Descriptions, Classification, and Distribution, with Special Reference to Those in Milk; Willdof Book Company: Madison, WI, USA, 1940; Volume 1. [Google Scholar]
- Vandamme, P.; Pot, B.; Falsen, E.; Kersters, K.; Devriese, L.A. Taxonomic Study of Lancefield Streptococcal Groups C, G, and L (Streptococcus dysgalactiae) and Proposal of S. dysgalactiae subsp. equisimilis subsp. nov. Int. J. Syst. Evol. Microbiol. 1996, 46, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Vieira, V.V.; Teixeira, L.M.; Zahner, V.; Momen, H.; Facklam, R.R.; Steigerwalt, A.G.; Brenner, D.J.; Castro, A.C.D. Genetic relationships among the different phenotypes of Streptococcus dysgalactiae strains. Int. J. Syst. Evol. Microbiol. 1998, 48, 1231–1243. [Google Scholar] [CrossRef] [Green Version]
- Garvie, E.I.; Farrow, J.A.E.; Bramley, A.J. Streptococcus dysgalactiae (Diernhofer) nom. rev. Int. J. Syst. Evol. Microbiol. 1983, 33, 404–405. [Google Scholar] [CrossRef] [Green Version]
- Ciszewski, M.; Zegarski, K.; Szewczyk, E.M. Streptococcus dysgalactiae subsp. equisimilis Isolated From Infections in Dogs and Humans: Are Current Subspecies Identification Criteria accurate? Curr. Microbiol. 2016, 73, 684–688. [Google Scholar] [CrossRef] [Green Version]
- Porcellato, D.; Smistad, M.; Skeie, S.B.; Jørgensen, H.J.; Austbø, L.; Oppegaard, O. Whole genome sequencing reveals possible host species adaptation of Streptococcus dysgalactiae. Sci. Rep. 2021, 11, 17350. [Google Scholar] [CrossRef]
- Oppegaared, O. Streptococcus Dysgalactiae—Just One Small Strep for Mankind? (#55). In Proceedings of the 21st Lancefield International Symposium for Streptococci and Streptococcal Diseases, Stockholm, Sweden, 7–10 June 2022. [Google Scholar]
- Bruun, T.; Kittang, B.R.; de Hoog, B.; Aardal, S.; Flaatten, H.K.; Langeland, N.; Mylvaganam, H.; Vindenes, H.A.; Skrede, S. Necrotizing soft tissue infections caused by Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis of groups C and G in western Norway. Clin. Microbiol. Infect. 2013, 19, E545–E550. [Google Scholar] [CrossRef] [Green Version]
- Izumi, M.; Hiraiwa, T.; Tomioka, H.; Komura, M.; Hayashi, Y. Fatal necrotizing myositis from fulminant Streptococcus dysgalactiae subspecies equisimilis (SDSE) infection: A case report of autopsy images. J. Gen. Fam. Med. 2018, 19, 50–52. [Google Scholar] [CrossRef] [Green Version]
- Saintot, C.; André, J.; Hentgen, C.; Jacques, L.; Barrans, A. Meningitis and septic shock caused by Streptococcus dysgalactiae subsp. equisimilis. Med. Mal. Infect. 2018, 48, 221–222. [Google Scholar] [CrossRef]
- Trell, K.; Sendi, P.; Rasmussen, M. Recurrent bacteremia with Streptococcus dysgalactiae: A case–control study. Diagn. Microbiol. Infect. Dis. 2016, 85, 121–124. [Google Scholar] [CrossRef]
- Hashikawa, S.; Iinuma, Y.; Furushita, M.; Ohkura, T.; Nada, T.; Torii, K.; Hasegawa, T.; Ohta, M. Characterization of Group C and G Streptococcal Strains That Cause Streptococcal Toxic Shock Syndrome. J. Clin. Microbiol. 2004, 42, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Muñoz, L.; García-Galván, M.Á.; González-Soto, M.A.; Echaniz-Aviles, G.; Solórzano-Santos, F. Toxic shock syndrome caused by Streptococcus dysgalactiae subsp. equisimilis in a Mexican preschool patient. Bol. Med. Hosp. Infant Mex. 2019, 76, 237–240. [Google Scholar] [CrossRef]
- Waltereit, R.; Herrlinger, U.; Stark, M.; Borgmann, S. Meningitis in a pregnant woman caused by Streptococcus dysgalactiae subspecies equisimilis. Pol. J. Microbiol. 2013, 62, 217–219. [Google Scholar] [CrossRef]
- Barnham, M.R.D.; Weightman, N.C. Changing incidence of detected streptococcal bacteraemia in North Yorkshire, England. Indian J. Med. Res. 2004, 119, 160–163. [Google Scholar]
- Ekelund, K.; Skinhøj, P.; Madsen, J.; Konradsen, H. Invasive group A, B, C and G streptococcal infections in Denmark 1999–2002: Epidemiological and clinical aspects. Clin. Microbiol. Infect. 2005, 11, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Beta-Haemolytic Group A, C and G Streptococcal Infections in Southern Hungary: A 10-Year Population-Based Retrospective Survey (2008–2017) and a Review of the Literature. Infect. Drug Resist. 2020, 13, 4739–4749. [Google Scholar] [CrossRef]
- Lambertsen, L.M.; Ingels, H.; Schønheyder, H.C.; Hoffmann, S. Nationwide laboratory-based surveillance of invasive beta-haemolytic streptococci in Denmark from 2005 to 2011. Clin. Microbiol. Infect. 2014, 20, O216–O223. [Google Scholar] [CrossRef] [Green Version]
- Rantala, S.; Vuopio-Varkila, J.; Vuento, R.; Huhtala, H.; Syrjänen, J. Clinical presentations and epidemiology of β-haemolytic streptococcal bacteraemia: A population-based study. Clin. Microbiol. Infect. 2009, 15, 286–288. [Google Scholar] [CrossRef] [Green Version]
- Broyles, L.N.; Van Beneden, C.; Beall, B.; Facklam, R.; Shewmaker, P.L.; Malpiedi, P.; Daily, P.; Reingold, A.; Farley, M.M. Population-Based Study of Invasive Disease Due to β-Hemolytic Streptococci of Groups Other than A and B. Clin. Infect. Dis. 2009, 48, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Oppegaard, O.; Mylvaganam, H.; Kittang, B. Beta-haemolytic group A, C and G streptococcal infections in Western Norway: A 15-year retrospective survey. Clin. Microbiol. Infect. 2015, 21, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Wajima, T.; Morozumi, M.; Hanada, S.; Sunaoshi, K.; Chiba, N.; Iwata, S.; Ubukata, K. Molecular Characterization of Invasive Streptococcus dysgalactiae subsp. equisimilis, Japan. Emerg. Infect. Dis. 2016, 22, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Rantala, S. Streptococcus dysgalactiae subsp. equisimilis bacteremia: An emerging infection. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1303–1310. [Google Scholar] [CrossRef]
- Ferretti, J.J.; Stevens, D.L.; Fischetti, V.A. (Eds.) Streptococcus Pyogenes: Basic Biology to Clinical Manifestations, 2nd ed.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2022. [Google Scholar]
- Collins, C.M.; Kimura, A.; Bisno, A.L. Group G streptococcal M protein exhibits structural features analogous to those of class I M protein of group A streptococci. Infect. Immun. 1992, 60, 3689–3696. [Google Scholar] [CrossRef] [Green Version]
- Vasi, J.; Frykberg, L.; Carlsson, L.E.; Lindberg, M.; Guss, B. M-Like Proteins of Streptococcus dysgalactiae. Infect. Immun. 2000, 68, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, G.N.; Flicker, P.F.; Cohen, C.; Manjula, B.N.; Fischetti, V.A. Streptococcal M protein: Alpha-helical coiled-coil structure and arrangement on the cell surface. Proc. Natl. Acad. Sci. USA 1981, 78, 4689–4693. [Google Scholar] [CrossRef] [Green Version]
- Fischetti, V.; Pancholi, V.; Schneewind, O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from Gram-positive cocci. Mol. Microbiol. 1990, 4, 1603–1605. [Google Scholar] [CrossRef]
- Swanson, J.; Hsu, K.C.; Gotschlich, E.C. Electron microscopic studies on streptococci: I. M antigen. J. Exp. Med. 1969, 130, 1063–1091. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.L.; Cole, J.N.; Olson, J.; Ryba, B.; Ghosh, P.; Nizet, V. The Fibrinogen-binding M1 Protein Reduces Pharyngeal Cell Adherence and Colonization Phenotypes of M1T1 Group A Streptococcus. J. Biol. Chem. 2014, 289, 3539–3546. [Google Scholar] [CrossRef] [Green Version]
- Laabei, M.; Ermert, D. Catch Me if You Can: Streptococcus pyogenes Complement Evasion Strategies. J. Innate Immun. 2018, 11, 3–12. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Terao, Y.; Kawabata, S. Pleiotropic virulence factor -Streptococcus pyogenes fibronectin-binding proteins. Cell. Microbiol. 2012, 15, 503–511. [Google Scholar] [CrossRef]
- Horstmann, R.D.; Sievertsen, H.J.; Knobloch, J.; Fischetti, V.A. Antiphagocytic activity of streptococcal M protein: Selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. USA 1988, 85, 1657–1661. [Google Scholar] [CrossRef] [Green Version]
- McArthur, J.D.; Walker, M.J. Domains of group A streptococcal M protein that confer resistance to phagocytosis, opsonization and protection: Implications for vaccine development. Mol. Microbiol. 2006, 59, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Cue, D.; Dombek, P.E.; Lam, H.; Cleary, P.P. Streptococcus pyogenes Serotype M1 Encodes Multiple Pathways for Entry into Human Epithelial Cells. Infect. Immun. 1998, 66, 4593–4601. [Google Scholar] [CrossRef] [Green Version]
- Cue, D.; Lam, H.; Cleary, P. Genetic dissection of the Streptococcus pyogenes M1 protein: Regions involved in fibronectin binding and intracellular invasion. Microb. Pathog. 2001, 31, 231–242. [Google Scholar] [CrossRef]
- Nerlich, A.; Rohde, M.; Talay, S.R.; Genth, H.; Just, I.; Chhatwal, G.S. Invasion of Endothelial Cells by Tissue-invasive M3 Type Group A Streptococci Requires Src Kinase and Activation of Rac1 by a Phosphatidylinositol 3-Kinase-independent Mechanism. J. Biol. Chem. 2009, 284, 20319–20328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, N.; Liszewski, M.K.; Atkinson, J.P.; Caparon, M. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc. Natl. Acad. Sci. USA 1995, 92, 2489–2493. [Google Scholar] [CrossRef] [Green Version]
- Beachey, E.H.; Seyer, J.M.; Dale, J.B.; Simpson, W.A.; Kang, A.H. Type-specific protective immunity evoked by synthetic peptide of Streptococcus pyogenes M protein. Nature 1981, 292, 457–459. [Google Scholar] [CrossRef]
- Campbell, P.T.; Tong, S.Y.C.; Geard, N.; Davies, M.; Worthing, K.A.; Lacey, J.A.; Smeesters, P.; Batzloff, M.R.; Kado, J.; Jenney, A.W.J.; et al. Longitudinal Analysis of Group A Streptococcus emm Types and emm Clusters in a High-Prevalence Setting: Relationship between Past and Future Infections. J. Infect. Dis. 2020, 221, 1429–1437. [Google Scholar] [CrossRef]
- Lancefield, R.C. Current knowledge of type-specific M antigens of group A streptococci. J. Immunol. 1962, 89, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Beall, B.; Facklam, R.; Thompson, T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J. Clin. Microbiol. 1996, 34, 953–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facklam, R.; Beall, B.; Efstratiou, A.; Fischetti, V.; Johnson, D.; Kaplan, E.; Kriz, P.; Lovgren, M.; Martin, D.; Schwartz, B.; et al. Emm Typing and Validation of Provisional M Types for Group A Streptococci1. Emerg. Infect. Dis. 1999, 5, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Streptococci Group a Subtyping Database. Available online: https://ftp.cdc.gov/pub/infectious_diseases/biotech/tsemm/ (accessed on 1 February 2023).
- Rößler, S.; Berner, R.; Jacobs, E.; Toepfner, N. Prevalence and molecular diversity of invasive Streptococcus dysgalactiae and Streptococcus pyogenes in a German tertiary care medical centre. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1325–1332. [Google Scholar] [CrossRef]
- German National Center for Streptococci. Streptococcal Surveillance. Available online: https://www.ukaachen.de/kliniken-institute/institut-fuer-medizinische-mikrobiologie/forschung/nationales-referenzzentrum-fuer-streptokokken/publikationen/surveillance/ (accessed on 1 February 2023).
- Traverso, F.; Blanco, A.; Villalón, P.; Beratz, N.; Nieto, J.A.S.; Lopardo, H. Molecular characterization of invasive Streptococcus dysgalactiae subsp. equisimilis. Multicenter study: Argentina 2011–2012. Rev. Argent. Microbiol. 2016, 48, 279–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruppen, C.; Rasmussen, M.; Casanova, C.; Sendi, P. A 10-year observational study of Streptococcus dysgalactiae bacteraemia in adults: Frequent occurrence among female intravenous drug users. Swiss Med. Wkly. 2017, 147, w14469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppegaard, O.; Mylvaganam, H.; Skrede, S.; Lindemann, P.C.; Kittang, B.R. Emergence of a Streptococcus dysgalactiae subspecies equisimilis stG62647-lineage associated with severe clinical manifestations. Sci. Rep. 2017, 7, 7589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bläckberg, A.; Nilson, B.; Özenci, V.; Olaison, L.; Rasmussen, M. Infective endocarditis due to Streptococcus dysgalactiae: Clinical presentation and microbiological features. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2261–2272. [Google Scholar] [CrossRef] [Green Version]
- Bruun, T.; Rath, E.; Madsen, M.B.; Oppegaard, O.; Nekludov, M.; Arnell, P.; Karlsson, Y.; Babbar, A.; Bergey, F.; Itzek, A.; et al. Risk Factors and Predictors of Mortality in Streptococcal Necrotizing Soft-tissue Infections: A Multicenter Prospective Study. Clin. Infect. Dis. 2021, 72, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Rojo-Bezares, B.; Toca, L.; Azcona-Gutiérrez, J.M.; Ortega-Unanue, N.; Toledano, P.; Sáenz, Y. Streptococcus dysgalactiae subsp. equisimilis from invasive and non-invasive infections in Spain: Combining epidemiology, molecular characterization, and genetic diversity. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1013–1021. [Google Scholar] [CrossRef]
- Yajko, D.M.; Lawrence, J.; Nassos, P.; Young, J.; Hadley, W.K. Clinical trial comparing bacitracin with Strep-A-Chek for accuracy and turnaround time in the presumptive identification of Streptococcus pyogenes. J. Clin. Microbiol. 1986, 24, 431–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellstood, S.A. Rapid, cost-effective identification of group A streptococci and enterococci by pyrrolidonyl-beta-naphthylamide hydrolysis. J. Clin. Microbiol. 1987, 25, 1805–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podbielski, A.; Melzer, B.; Lütticken, R. Application of the polymerase chain reaction to study the M protein(-like) gene family in beta-hemolytic streptococci. Med. Microbiol. Immunol. 1991, 180, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Protocol for Emm Typing. Available online: https://www.cdc.gov/streplab/groupa-strep/emm-typing-protocol.html (accessed on 1 February 2023).
- Fan, W.-T.; Qin, T.-T.; Bi, R.-R.; Kang, H.-Q.; Ma, P.; Gu, B. Performance of the matrix-assisted laser desorption ionization time-of-flight mass spectrometry system for rapid identification of streptococci: A review. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1005–1012. [Google Scholar] [CrossRef]
- Borgmann, S.; Pfeifer, Y.; Becker, L.; Rieß, B.; Siegmund, R.; Sagel, U. Findings from an outbreak of carbapenem-resistant Klebsiella pneumoniae emphasize the role of antibiotic treatment for cross transmission. Infection 2017, 46, 103–112. [Google Scholar] [CrossRef]
- Destatis. Statistisches Bundesamt. Bevölkerung Im Wandel—Annahmen Und Ergebnisse Der 14. Koordinierten Bevölkerungsvorausberechnung. Available online: https://www.destatis.de/DE/Presse/Pressekonferenzen/2019/Bevoelkerung/pressebroschuere-bevoelkerung.pdf?__blob=publicationFile (accessed on 1 February 2023).
- Kline, K.A.; Bowdish, D.M. Infection in an aging population. Curr. Opin. Microbiol. 2016, 29, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Leitner, E.; Zollner-Schwetz, I.; Zarfel, G.; Masoud-Landgraf, L.; Gehrer, M.; Wagner-Eibel, U.; Grisold, A.J.; Feierl, G. Prevalence of emm types and antimicrobial susceptibility of Streptococcus dysgalactiae subsp. equisimilis in Austria. Int. J. Med. Microbiol. 2015, 305, 918–924. [Google Scholar] [CrossRef]
- Madalli, S.; Beyrau, M.; Whiteford, J.; Duchene, J.; Nandhra, I.S.; Patel, N.S.A.; Motwani, M.P.; Gilroy, D.W.; Thiemermann, C.; Nourshargh, S.; et al. Sex-specific regulation of chemokine Cxcl5/6 controls neutrophil recruitment and tissue injury in acute inflammatory states. Biol. Sex Differ. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, C.; Cascella, F.; Mellai, M.; Barizzone, N.; Mignone, F.; Massa, N.; Nobile, V.; Bona, E. Influence of Sex on the Microbiota of the Human Face. Microorganisms 2022, 10, 2470. [Google Scholar] [CrossRef]
- Steglińska, A.; Jachowicz, A.; Szulc, J.; Adamiak, J.; Otlewska, A.; Pielech-Przybylska, K.; Gutarowska, B. Factors Influencing Microbiological Biodiversity of Human Foot Skin. Int. J. Environ. Res. Public Health 2019, 16, 3503. [Google Scholar] [CrossRef] [Green Version]
- Miselli, F.; Frabboni, I.; Di Martino, M.; Zinani, I.; Buttera, M.; Insalaco, A.; Stefanelli, F.; Lugli, L.; Berardi, A. Transmission of Group B Streptococcus in late-onset neonatal disease: A narrative review of current evidence. Ther. Adv. Infect. Dis. 2022, 9, 20499361221142732. [Google Scholar] [CrossRef]
- Shabayek, S.; Spellerberg, B. Group B Streptococcal Colonization, Molecular Characteristics, and Epidemiology. Front. Microbiol. 2018, 9, 437. [Google Scholar] [CrossRef]
| Sex | Male 46 (56.1%) | Female 36 (43.9%) | ||
|---|---|---|---|---|
| Age [years] | Median 65 | Range 27–93 | ||
| Material [sampling] | Blood 24 (29.3%) | Swab 21 (25.6%) | Sore smear 19 (23.2%) | Other 18 (21.9%) |
| Infection [classification] | Superficial 28 (34.1%) | Fascial 26 (31.7%) | Systemic 28 (34.1%) | |
| Sampling [days] | Average 3.7 | Range −5–175 | ||
| Hospital stay [days] | Average 20.8 | Range 0–217 | ||
| Lancefield [antigen] | C 40 (48.8%) | G 42 (51.2%) | ||
| Genotype [emm type] | stG62647 38 (46.3%) | Other 44 (53.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itzek, A.; Weißbach, V.; Meintrup, D.; Rieß, B.; van der Linden, M.; Borgmann, S. Epidemiological and Clinical Features of Streptococcus dysgalactiae ssp. equisimilis stG62647 and Other emm Types in Germany. Pathogens 2023, 12, 589. https://doi.org/10.3390/pathogens12040589
Itzek A, Weißbach V, Meintrup D, Rieß B, van der Linden M, Borgmann S. Epidemiological and Clinical Features of Streptococcus dysgalactiae ssp. equisimilis stG62647 and Other emm Types in Germany. Pathogens. 2023; 12(4):589. https://doi.org/10.3390/pathogens12040589
Chicago/Turabian StyleItzek, Andreas, Victoria Weißbach, David Meintrup, Beate Rieß, Mark van der Linden, and Stefan Borgmann. 2023. "Epidemiological and Clinical Features of Streptococcus dysgalactiae ssp. equisimilis stG62647 and Other emm Types in Germany" Pathogens 12, no. 4: 589. https://doi.org/10.3390/pathogens12040589
APA StyleItzek, A., Weißbach, V., Meintrup, D., Rieß, B., van der Linden, M., & Borgmann, S. (2023). Epidemiological and Clinical Features of Streptococcus dysgalactiae ssp. equisimilis stG62647 and Other emm Types in Germany. Pathogens, 12(4), 589. https://doi.org/10.3390/pathogens12040589






