A Review of the Current Status of G6PD Deficiency Testing to Guide Radical Cure Treatment for Vivax Malaria
Abstract
1. Introduction
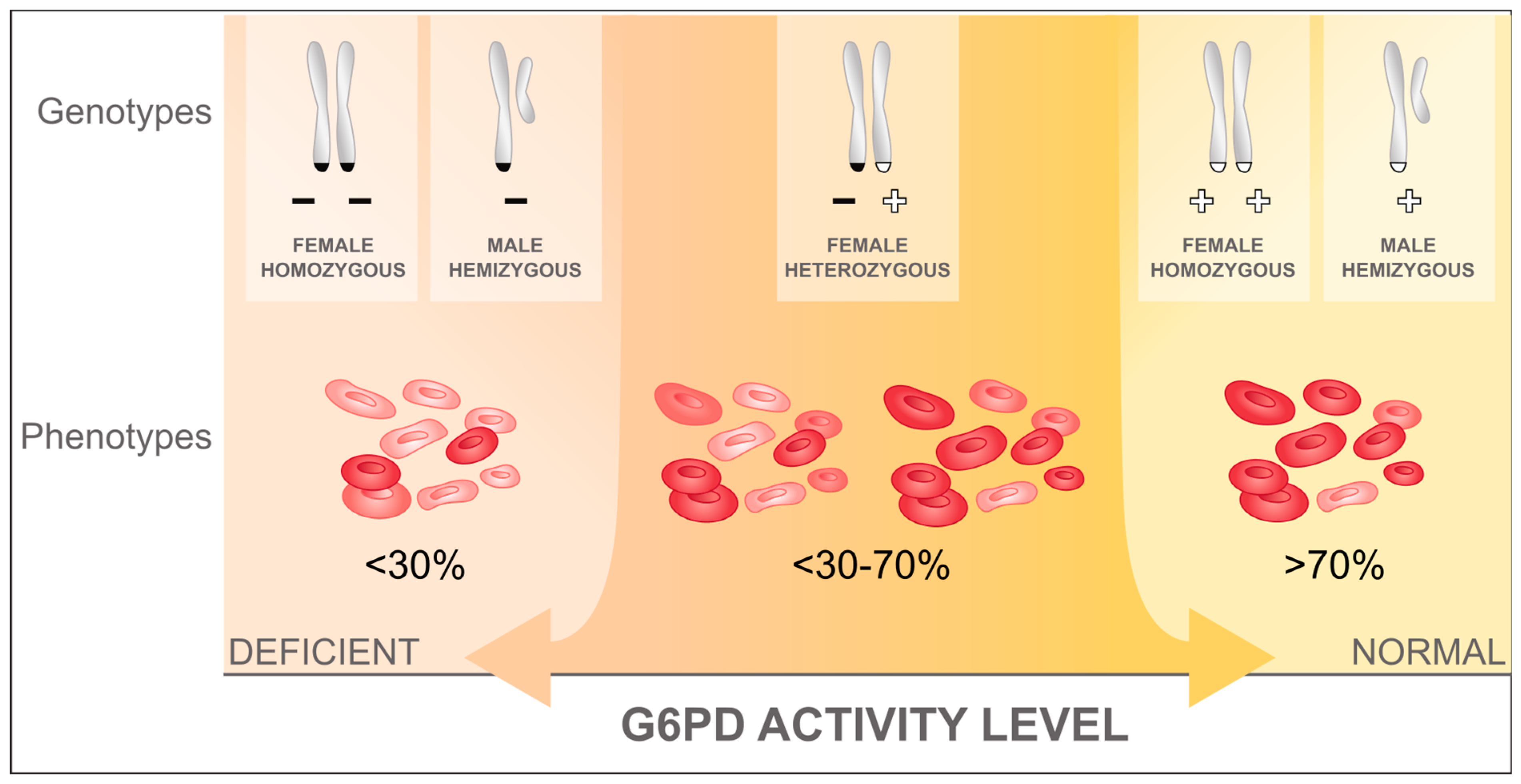
2. G6PD Deficiency
3. Overview of G6PD Test Formats and Products
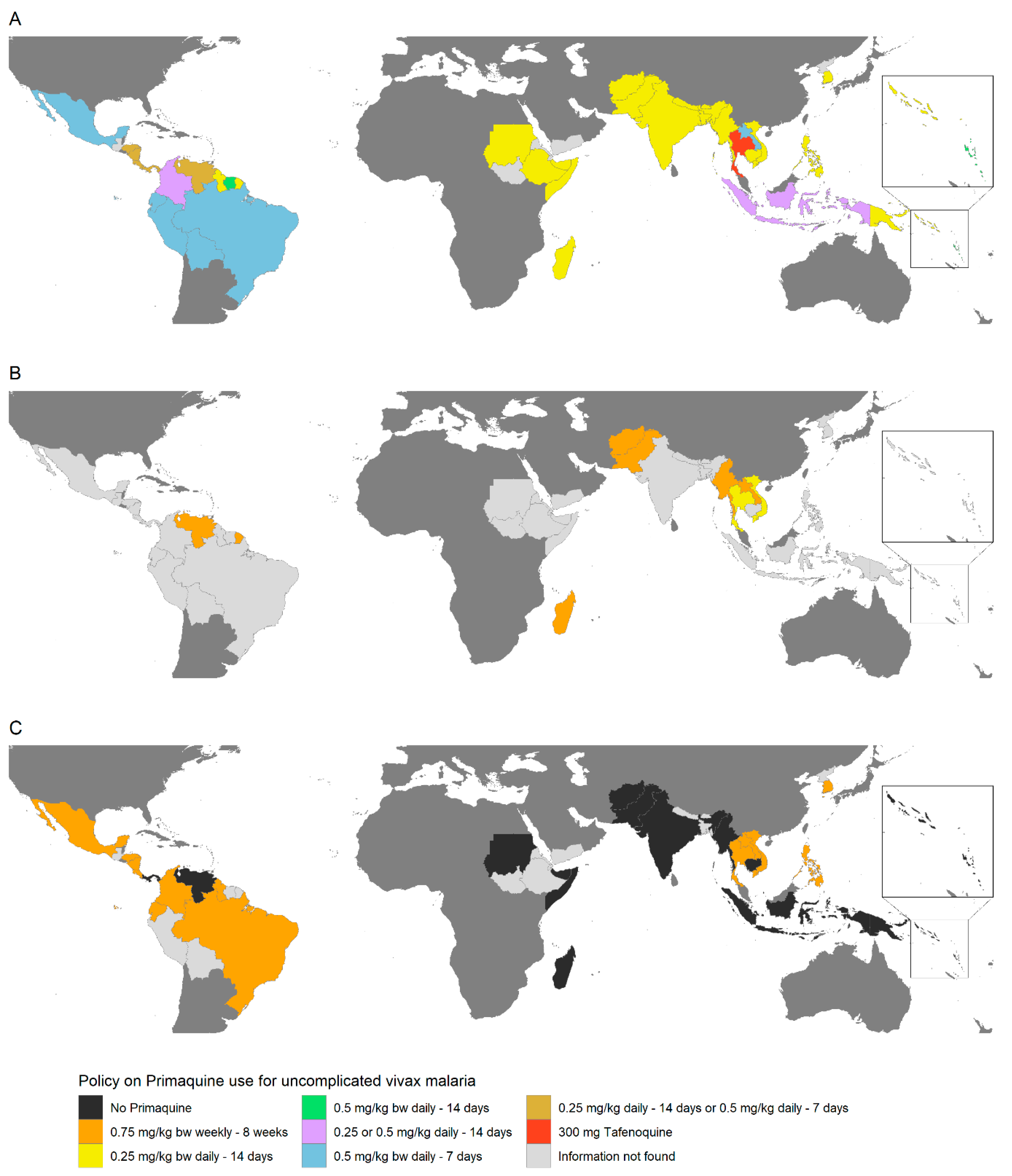
4. Policy and Practice of G6PD Testing
5. Early Experiences with the Implementation of PoC G6PD Testing
5.1. Technical Challenges
5.2. Logistical Considerations
5.3. Training and Supervision
5.4. Considerations about Level of Health Care System
6. Other Considerations for the Update of Novel Diagnostics
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howes, R.E.; Battle, K.E.; Mendis, K.N.; Smith, D.L.; Cibulskis, R.E.; Baird, J.K.; Hay, S.I. Global Epidemiology of Plasmodium vivax. Am. J. Trop. Med. Hyg. 2016, 95, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Battle, K.E.; Lucas, T.C.D.; Nguyen, M.; Howes, R.E.; Nandi, A.K.; Twohig, K.A.; Pfeffer, D.A.; Cameron, E.; Rao, P.C.; Casey, D.; et al. Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000–2017: A spatial and temporal modelling study. Lancet 2019, 394, 332–343. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-006489-8.
- Nguitragool, W.; Mueller, I.; Kumpitak, C.; Saeseu, T.; Bantuchai, S.; Yorsaeng, R.; Yimsamran, S.; Maneeboonyang, W.; Sa-angchai, P.; Chaimungkun, W.; et al. Very high carriage of gametocytes in asymptomatic low-density Plasmodium falciparum and P. vivax infections in western Thailand. Parasites Vectors 2017, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Rovira-Vallbona, E.; Contreras-Mancilla, J.J.; Ramirez, R.; Guzman-Guzman, M.; Carrasco-Escobar, G.; Llanos-Cuentas, A.; Vinetz, J.M.; Gamboa, D.; Rosanas-Urgell, A. Predominance of asymptomatic and sub-microscopic infections characterizes the Plasmodium gametocyte reservoir in the Peruvian Amazon. PLoS Negl. Trop. Dis. 2017, 11, e0005674. [Google Scholar] [CrossRef] [PubMed]
- Motshoge, T.; Ababio, G.K.; Aleksenko, L.; Read, J.; Peloewetse, E.; Loeto, M.; Mosweunyane, T.; Moakofhi, K.; Ntebele, D.S.; Chihanga, S.; et al. Molecular evidence of high rates of asymptomatic P. vivax infection and very low P. falciparum malaria in Botswana. BMC Infect. Dis. 2016, 16, 520. [Google Scholar] [CrossRef]
- Almeida, A.C.G.; Kuehn, A.; Castro, A.J.M.; Vitor-Silva, S.; Figueiredo, E.F.G.; Brasil, L.W.; Brito, M.A.M.; Sampaio, V.S.; Bassat, Q.; Felger, I.; et al. High proportions of asymptomatic and submicroscopic Plasmodium vivax infections in a peri-urban area of low transmission in the Brazilian Amazon. Parasites Vectors 2018, 11, 194. [Google Scholar] [CrossRef]
- Cheng, Q.; Cunningham, J.; Gatton, M.L. Systematic Review of Sub-microscopic P. vivax Infections: Prevalence and Determining Factors. PLoS Negl. Trop. Dis. 2015, 9, e3413. [Google Scholar] [CrossRef]
- Ding, X.C.; Ade, M.P.; Baird, J.K.; Cheng, Q.; Cunningham, J.; Dhorda, M.; Drakeley, C.; Felger, I.; Gamboa, D.; Harbers, M.; et al. Defining the next generation of Plasmodium vivax diagnostic tests for control and elimination: Target product profiles. PLoS Negl. Trop. Dis. 2017, 11, e0005516. [Google Scholar] [CrossRef]
- Battle, K.E.; Karhunen, M.S.; Bhatt, S.; Gething, P.W.; Howes, R.E.; Golding, N.; Van Boeckel, T.P.; Messina, J.P.; Shanks, G.D.; Smith, D.L.; et al. Geographical variation in Plasmodium vivax relapse. Malar. J. 2014, 13, 144. [Google Scholar] [CrossRef]
- Mueller, I.; Galinski, M.R.; Baird, J.K.; Carlton, J.M.; Kochar, D.K.; Alonso, P.L.; del Portillo, H.A. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect. Dis. 2009, 9, 555–566. [Google Scholar] [CrossRef]
- Commons, R.J.; Simpson, J.A.; Watson, J.; White, N.J.; Price, R.N. Estimating the Proportion of Plasmodium vivax Recurrences Caused by Relapse: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2020, 103, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; White, N.J. Management of relapsing Plasmodium vivax malaria. Expert Rev. Anti-Infect. Ther. 2016, 14, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Edgcomb, J.H.; Arnold, J.; Yount, E.H.; Alving, A.S.; Eichelberger, L.; Jeffery, G.M.; Eyles, D.; Young, M.D. Primaquine, SN 13272, a new curative agent in vivax malaria; a preliminary report. J. Natl. Malar. Soc. 1950, 9, 285–292. [Google Scholar] [PubMed]
- World Health Organization. WHO Guidelines for Malaria, 13 July 2021; World Health Organization: Geneva, Switzerland, 2021; (WHO/UCN/GMP/2021.01 Rev.1); License: CC BY-NC-SA 3.0 IGO.
- World Health Organization. WHO Guidelines for Malaria, 14 March 2023; World Health Organization: Geneva, Switzerland, 2023; (WHO/UCN/GMP/2023.01); License: CC BY-NC-SA 3.0 IGO.
- Baird, J.K. 8-Aminoquinoline Therapy for Latent Malaria. Clin. Microbiol. Rev. 2019, 32, e00011-19. [Google Scholar] [CrossRef]
- Douglas, N.M.; Poespoprodjo, J.R.; Patriani, D.; Malloy, M.J.; Kenangalem, E.; Sugiarto, P.; Simpson, J.A.; Soenarto, Y.; Anstey, N.M.; Price, R.N. Unsupervised primaquine for the treatment of Plasmodium vivax malaria relapses in southern Papua: A hospital-based cohort study. PLoS Med. 2017, 14, e1002379. [Google Scholar] [CrossRef]
- Thriemer, K.; Bobogare, A.; Ley, B.; Gudo, C.S.; Alam, M.S.; Anstey, N.M.; Ashley, E.; Baird, J.K.; Gryseels, C.; Jambert, E.; et al. Quantifying primaquine effectiveness and improving adherence: A round table discussion of the APMEN Vivax Working Group. Malar. J. 2018, 17, 241. [Google Scholar] [CrossRef]
- Taylor, W.R.J.; Thriemer, K.; Seidlein, L.v.; Yuentrakul, P.; Assawariyathipat, T.; Assefa, A.; Auburn, S.; Chand, K.; Chau, N.H.; Cheah, P.Y.; et al. Short-course primaquine for the radical cure of Plasmodium vivax malaria: A multicentre, randomised, placebo-controlled non-inferiority trial. Lancet 2019, 394, 929–938. [Google Scholar] [CrossRef]
- Shanks, G.D.; Oloo, A.J.; Aleman, G.M.; Ohrt, C.; Klotz, F.W.; Braitman, D.; Horton, J.; Brueckner, R. A New Primaquine Analogue, Tafenoquine (WR 238605), for Prophylaxis against Plasmodium falciparum Malaria. Clin. Infect. Dis. 2001, 33, 1968–1974. [Google Scholar] [CrossRef]
- Brueckner, R.P.; Lasseter, K.C.; Lin, E.T.; Schuster, B.G. First-time-in-humans safety and pharmacokinetics of WR 238605, a new antimalarial. Am. J. Trop. Med. Hyg. 1998, 58, 645–649. [Google Scholar] [CrossRef]
- Cappellini, M.; Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008, 371, 64–74. [Google Scholar] [CrossRef]
- Mason, P.J.; Bautista, J.M.; Gilsanz, F. G6PD deficiency: The genotype-phenotype association. Blood Rev. 2007, 21, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Luzzatto, L.; Ally, M.; Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Blood 2020, 136, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Control and Elimination of Plasmodium vivax Malaria: A Technical Brief; World Health Organization: Geneva, Switzerland, 2015.
- Howes, R.E.; Piel, F.B.; Patil, A.P.; Nyangiri, O.A.; Gething, P.W.; Dewi, M.; Hogg, M.M.; Battle, K.E.; Padilla, C.D.; Baird, J.K.; et al. G6PD Deficiency Prevalence and Estimates of Affected Populations in Malaria Endemic Countries: A Geostatistical Model-Based Map. PLoS Med. 2012, 9, e1001339. [Google Scholar] [CrossRef] [PubMed]
- Howes, R.E.; Battle, K.E.; Satyagraha, A.W.; Baird, J.K.; Hay, S.I. Chapter Four—G6PD Deficiency: Global Distribution, Genetic Variants and Primaquine Therapy. In Advances in Parasitology; Hay, S.I., Price, R.N., Baird, J.K., Eds.; The Epidemiology of Plasmodium vivax; Academic Press: Cambridge, MA, USA, 2013; Volume 81, pp. 133–201. [Google Scholar]
- Howes, R.E.; Dewi, M.; Piel, F.B.; Monteiro, W.M.; Battle, K.E.; Messina, J.P.; Sakuntabhai, A.; Satyagraha, A.W.; Williams, T.N.; Baird, J.K.; et al. Spatial distribution of G6PD deficiency variants across malaria-endemic regions. Malar. J. 2013, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- Mbanefo, E.C.; Ahmed, A.M.; Titouna, A.; Elmaraezy, A.; Trang, N.T.H.; Phuoc Long, N.; Hoang Anh, N.; Diem Nghi, T.; The Hung, B.; Van Hieu, M.; et al. Association of glucose-6-phosphate dehydrogenase deficiency and malaria: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 45963. [Google Scholar] [CrossRef]
- Awab, G.R.; Aaram, F.; Jamornthanyawat, N.; Suwannasin, K.; Pagornrat, W.; Watson, J.A.; Woodrow, C.J.; Dondorp, A.M.; Day, N.P.; Imwong, M.; et al. Protective effect of Mediterranean-type glucose-6-phosphate dehydrogenase deficiency against Plasmodium vivax malaria. eLife 2021, 10, e62448. [Google Scholar] [CrossRef]
- Beutler, E.; Yeh, M.; Fairbanks, V.F. The normal human female as a mosaic of X-chromosome activity: Studies using the gene for G-6-PD-deficiency as a marker. Proc. Natl. Acad. Sci. USA 1962, 48, 9–16. [Google Scholar] [CrossRef]
- Domingo, G.J.; Advani, N.; Satyagraha, A.W.; Sibley, C.H.; Rowley, E.; Kalnoky, M.; Cohen, J.; Parker, M.; Kelley, M. Addressing the gender-knowledge gap in glucose-6-phosphate dehydrogenase deficiency: Challenges and opportunities. Int. Health 2019, 11, 7–14. [Google Scholar] [CrossRef]
- Pfeffer, D.A.; Ley, B.; Howes, R.E.; Adu, P.; Alam, M.S.; Bansil, P.; Boum, Y.; Brito, M.; Charoenkwan, P.; Clements, A.; et al. Quantification of glucose-6-phosphate dehydrogenase activity by spectrophotometry: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003084. [Google Scholar] [CrossRef]
- Domingo, G.J.; Satyagraha, A.W.; Anvikar, A.; Baird, K.; Bancone, G.; Bansil, P.; Carter, N.; Cheng, Q.; Culpepper, J.; Eziefula, C.; et al. G6PD testing in support of treatment and elimination of malaria: Recommendations for evaluation of G6PD tests. Malar. J. 2013, 12, 391. [Google Scholar] [CrossRef]
- Pfeffer, D.A.; Satyagraha, A.W.; Sadhewa, A.; Alam, M.S.; Bancone, G.; Boum, Y.; Brito, M.; Cui, L.; Deng, Z.; Domingo, G.J.; et al. Genetic Variants of Glucose-6-Phosphate Dehydrogenase and Their Associated Enzyme Activity: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Satyagraha, A.W.; Sadhewa, A.; Panggalo, L.V.; Subekti, D.; Elyazar, I.; Soebianto, S.; Mahpud, N.; Harahap, A.R.; Baird, J.K. Genotypes and phenotypes of G6PD deficiency among Indonesian females across diagnostic thresholds of G6PD activity guiding safe primaquine therapy of latent malaria. PLoS Negl. Trop. Dis. 2021, 15, e0009610. [Google Scholar] [CrossRef] [PubMed]
- von Seidlein, L.; Auburn, S.; Espino, F.; Shanks, D.; Cheng, Q.; McCarthy, J.; Baird, K.; Moyes, C.; Howes, R.; Ménard, D.; et al. Review of key knowledge gaps in glucose-6-phosphate dehydrogenase deficiency detection with regard to the safe clinical deployment of 8-aminoquinoline treatment regimens: A workshop report. Malar. J. 2013, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guide to G6PD Deficiency Rapid Diagnostic Testing to Support P. vivax Radical Cure; World Health Organization: Geneva, Switzerland, 2018; p. 26.
- Shah, S.S.; Diakite, S.A.S.; Traore, K.; Diakite, M.; Kwiatkowski, D.P.; Rockett, K.A.; Wellems, T.E.; Fairhurst, R.M. A novel cytofluorometric assay for the detection and quantification of glucose-6-phosphate dehydrogenase deficiency. Sci. Rep. 2012, 2, 299. [Google Scholar] [CrossRef]
- Peters, A.L.; Noorden, C.J.F.V. Glucose-6-phosphate Dehydrogenase Deficiency and Malaria: Cytochemical Detection of Heterozygous G6PD Deficiency in Women. J. Histochem. Cytochem. 2009, 57, 1003–1011. [Google Scholar] [CrossRef]
- Minucci, A.; Giardina, B.; Zuppi, C.; Capoluongo, E. Glucose-6-phosphate dehydrogenase laboratory assay: How, when, and why? IUBMB Life 2009, 61, 27–34. [Google Scholar] [CrossRef]
- Kaplan, M.; Hammerman, C. Neonatal Screening for Glucose-6-Phosphate Dehydrogenase Deficiency: Biochemical Versus Genetic Technologies. Semin. Perinatol. 2011, 35, 155–161. [Google Scholar] [CrossRef]
- Rumaseb, A.; Marfurt, J.; Kho, S.; Kahn, M.; Price, R.N.; Ley, B. A fluorometric assay to determine the protective effect of glucose-6-phosphate dehydrogenase (G6PD) against a Plasmodium spp. infection in females heterozygous for the G6PD gene: Proof of concept in Plasmodium falciparum. BMC Res. Notes 2022, 15, 76. [Google Scholar] [CrossRef]
- Ley, B.; Alam, M.S.; Kibria, M.G.; Marfurt, J.; Phru, C.S.; Ami, J.Q.; Thriemer, K.; Auburn, S.; Jahan, N.; Johora, F.T.; et al. Glucose-6-phosphate dehydrogenase activity in individuals with and without malaria: Analysis of clinical trial, cross-sectional and case–control data from Bangladesh. PLoS Med. 2021, 18, e1003576. [Google Scholar] [CrossRef]
- Ley, B.; Alam, M.S.; Satyagraha, A.W.; Phru, C.S.; Thriemer, K.; Tadesse, D.; Shibiru, T.; Hailu, A.; Kibria, M.G.; Hossain, M.S.; et al. Variation in Glucose-6-Phosphate Dehydrogenase activity following acute malaria. PLoS Negl. Trop. Dis. 2022, 16, e0010406. [Google Scholar] [CrossRef]
- Taylor, W.R.J.; Kim, S.; Kheng, S.; Muth, S.; Tor, P.; Christophel, E.; Mukaka, M.; Kerleguer, A.; Luzzatto, L.; Baird, J.K.; et al. Dynamics of G6PD activity in patients receiving weekly primaquine for therapy of Plasmodium vivax malaria. PLoS Negl. Trop. Dis. 2021, 15, e0009690. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Standardization of Procedures for the Study of Glucose-6-Phosphate Dehydrogenase; Report of a WHO Scientific Group. World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 1967; Volume 366, pp. 1–53.
- Alam, M.S.; Kibria, M.G.; Jahan, N.; Price, R.N.; Ley, B. Spectrophotometry assays to determine G6PD activity from Trinity Biotech and Pointe Scientific G6PD show good correlation. BMC Res. Notes 2018, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Mitchell, M. Brief Report: Special Modifications of the Fluorescent Screening Method for Glucose-6-Phosphate Dehydrogenase Deficiency. Blood 1968, 32, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Halasz, A. A Series of New Screening Procedures for Pyruvate Kinase Deficiency, Glucose-6-Phosphate Dehydrogenase Deficiency, and Glutathione Reductase Deficiency. Blood 1966, 28, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Henriques, G.; Phommasone, K.; Tripura, R.; Peto, T.J.; Raut, S.; Snethlage, C.; Sambo, I.; Sanann, N.; Nguon, C.; Adhikari, B.; et al. Comparison of glucose-6 phosphate dehydrogenase status by fluorescent spot test and rapid diagnostic test in Lao PDR and Cambodia. Malar. J. 2018, 17, 243. [Google Scholar] [CrossRef] [PubMed]
- Roca-Feltrer, A.; Khim, N.; Kim, S.; Chy, S.; Canier, L.; Kerleguer, A.; Tor, P.; Chuor, C.M.; Kheng, S.; Siv, S.; et al. Field Trial Evaluation of the Performances of Point-of-Care Tests for Screening G6PD Deficiency in Cambodia. PLoS ONE 2014, 9, e116143. [Google Scholar] [CrossRef]
- Bancone, G.; Chu, C.S.; Chowwiwat, N.; Somsakchaicharoen, R.; Wilaisrisak, P.; Charunwatthana, P.; Bansil, P.; McGray, S.; Domingo, G.J.; Nosten, F.H. Suitability of Capillary Blood for Quantitative Assessment of G6PD Activity and Performances of G6PD Point-of-Care Tests. Am. J. Trop. Med. Hyg. 2015, 92, 818–824. [Google Scholar] [CrossRef]
- Espino, F.E.; Bibit, J.-A.; Sornillo, J.B.; Tan, A.; Seidlein, L.v.; Ley, B. Comparison of Three Screening Test Kits for G6PD Enzyme Deficiency: Implications for Its Use in the Radical Cure of Vivax Malaria in Remote and Resource-Poor Areas in the Philippines. PLoS ONE 2016, 11, e0148172. [Google Scholar] [CrossRef]
- Oo, N.N.; Bancone, G.; Maw, L.Z.; Chowwiwat, N.; Bansil, P.; Domingo, G.J.; Htun, M.M.; Thant, K.Z.; Htut, Y.; Nosten, F. Validation of G6PD Point-of-Care Tests among Healthy Volunteers in Yangon, Myanmar. PLoS ONE 2016, 11, e0152304. [Google Scholar] [CrossRef]
- Tantular, I.S.; Kawamoto, F. An improved, simple screening method for detection of glucose-6-phosphate dehydrogenase deficiency. Trop. Med. Int. Health 2003, 8, 569–574. [Google Scholar] [CrossRef]
- Ley, B.; Alam, M.S.; O’Donnell, J.J.; Hossain, M.S.; Kibria, M.G.; Jahan, N.; Khan, W.A.; Thriemer, K.; Chatfield, M.D.; Price, R.N.; et al. A Comparison of Three Quantitative Methods to Estimate G6PD Activity in the Chittagong Hill Tracts, Bangladesh. PLoS ONE 2017, 12, e0169930. [Google Scholar] [CrossRef] [PubMed]
- Van Noorden, C.J.; Dolbeare, F.; Aten, J. Flow cytofluorometric analysis of enzyme reactions based on quenching of fluorescence by the final reaction product: Detection of glucose-6-phosphate dehydrogenase deficiency in human erythrocytes. J. Histochem. Cytochem. 1989, 37, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Thedsawad, A.; Wanachiwanawin, W.; Taka, O.; Hantaweepant, C. Cut-off values for diagnosis of G6PD deficiency by flow cytometry in Thai population. Ann. Hematol. 2022, 101, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nguon, C.; Guillard, B.; Duong, S.; Chy, S.; Sum, S.; Nhem, S.; Bouchier, C.; Tichit, M.; Christophel, E.; et al. Performance of the CareStart™ G6PD Deficiency Screening Test, a Point-of-Care Diagnostic for Primaquine Therapy Screening. PLoS ONE 2011, 6, e28357. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.K.; Dewi, M.; Subekti, D.; Elyazar, I.; Satyagraha, A.W. Noninferiority of glucose-6-phosphate dehydrogenase deficiency diagnosis by a point-of-care rapid test vs the laboratory fluorescent spot test demonstrated by copper inhibition in normal human red blood cells. Transl. Res. 2015, 165, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Satyagraha, A.W.; Rahmat, H.; Fricken, M.E.v.; Douglas, N.M.; Pfeffer, D.A.; Espino, F.; Seidlein, L.v.; Henriques, G.; Oo, N.N.; et al. Performance of the Access Bio/CareStart rapid diagnostic test for the detection of glucose-6-phosphate dehydrogenase deficiency: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002992. [Google Scholar] [CrossRef] [PubMed]
- Osorio, L.; Carter, N.; Arthur, P.; Bancone, G.; Gopalan, S.; Gupta, S.K.; Noedl, H.; Kochar, S.K.; Kochar, D.K.; Krudsood, S.; et al. Performance of BinaxNOW G6PD Deficiency Point-of-Care Diagnostic in P. vivax-Infected Subjects. Am. J. Trop. Med. Hyg. 2015, 92, 22–27. [Google Scholar] [CrossRef]
- Tinley, K.E.; Loughlin, A.M.; Jepson, A.; Barnett, E.D. Evaluation of a Rapid Qualitative Enzyme Chromatographic Test for Glucose-6-Phosphate Dehydrogenase Deficiency. Am. J. Trop. Med. Hyg. 2010, 82, 210–214. [Google Scholar] [CrossRef]
- Baird, J.K. Point-of-care G6PD diagnostics for Plasmodium vivax malaria is a clinical and public health urgency. BMC Med. 2015, 13, 296. [Google Scholar] [CrossRef]
- LaRue, N.; Kahn, M.; Murray, M.; Leader, B.T.; Bansil, P.; McGray, S.; Kalnoky, M.; Zhang, H.; Huang, H.; Jiang, H.; et al. Comparison of Quantitative and Qualitative Tests for Glucose-6-Phosphate Dehydrogenase Deficiency. Am. J. Trop. Med. Hyg. 2014, 91, 854–861. [Google Scholar] [CrossRef]
- Alam, M.S.; Kibria, M.G.; Jahan, N.; Thriemer, K.; Hossain, M.S.; Douglas, N.M.; Phru, C.S.; Khan, W.A.; Price, R.N.; Ley, B. Field evaluation of quantitative point of care diagnostics to measure glucose-6-phosphate dehydrogenase activity. PLoS ONE 2018, 13, e0206331. [Google Scholar] [CrossRef] [PubMed]
- Weppelmann, T.A.; von Fricken, M.E.; Wilfong, T.D.; Aguenza, E.; Philippe, T.T.; Okech, B.A. Field Trial of the CareStart Biosensor Analyzer for the Determination of Glucose-6-Phosphate Dehydrogenase Activity in Haiti. Am. J. Trop. Med. Hyg. 2017, 97, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Bancone, G.; Gornsawun, G.; Chu, C.S.; Porn, P.; Pal, S.; Bansil, P.; Domingo, G.J.; Nosten, F. Validation of the quantitative point-of-care CareStart biosensor for assessment of G6PD activity in venous blood. PLoS ONE 2018, 13, e0196716. [Google Scholar] [CrossRef] [PubMed]
- Pengboon, P.; Thamwarokun, A.; Changsri, K.; Kaset, C.; Chomean, S. Evaluation of quantitative biosensor for glucose-6-phosphate dehydrogenase activity detection. PLoS ONE 2019, 14, e0226927. [Google Scholar] [CrossRef]
- Zobrist, S.; Brito, M.; Garbin, E.; Monteiro, W.M.; Clementino Freitas, S.; Macedo, M.; Soares Moura, A.; Advani, N.; Kahn, M.; Pal, S.; et al. Evaluation of a point-of-care diagnostic to identify glucose-6-phosphate dehydrogenase deficiency in Brazil. PLoS Negl. Trop. Dis. 2021, 15, e0009649. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Myburgh, J.; Bansil, P.; Hann, A.; Robertson, L.; Gerth-Guyette, E.; Ambler, G.; Bizilj, G.; Kahn, M.; Zobrist, S.; et al. Reference and point-of-care testing for G6PD deficiency: Blood disorder interference, contrived specimens, and fingerstick equivalence and precision. PLoS ONE 2021, 16, e0257560. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tests for Glucose-6-Phosphate Dehydrogenase Activity: Target Product Profiles; World Health Organization: Geneva, Switzerland, 2022.
- World Health Organization. Guidelines for the Treatment of Malaria, 3rd ed.; World Health Organization: Geneva, Switzerland, 2015.
- Recht, J.; Ashley, E.A.; White, N.J. Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: Divergent policies and practices in malaria endemic countries. PLoS Negl. Trop. Dis. 2018, 12, e0006230. [Google Scholar] [CrossRef]
- Ley, B.; Thriemer, K.; Jaswal, J.; Poirot, E.; Alam, M.S.; Phru, C.S.; Khan, W.A.; Dysoley, L.; Qi, G.; Kheong, C.C.; et al. Barriers to routine G6PD testing prior to treatment with primaquine. Malar. J. 2017, 16, 329. [Google Scholar] [CrossRef]
- Adhikari, B.; Awab, G.R.; von Seidlein, L. Rolling out the radical cure for vivax malaria in Asia: A qualitative study among policy makers and stakeholders. Malar. J. 2021, 20, 164. [Google Scholar] [CrossRef]
- Berte, C.J.; Lorenzo, P.J.; Espino, F.E. Perceived challenges in implementing point-of-care testing for glucose-6-phosphate-dehydrogenase for malaria patients before primaquine treatment in the Philippines. Res. Sq. Platf. LLC 2022, preprint. [Google Scholar] [CrossRef]
- National Malaria and Leishmaniasis Control Program. National Malaria Treatment Guideline; Ministry of Public Health Islamic Republic of Afghanistan: Kabul, Afghanistan, 2017.
- National Malaria and Leishmaniasis Control Program. National Strategic Plan “From Malaria Control to Elimination in Afghanistan” 2018–2022; Ministry of Public Health Islamic Republic of Afghanistan: Kabul, Afghanistan, 2017.
- National Malaria Elimination and Aedes Transmitted Diseases Control Program. Revised Malaria Treatment Regimen, 6th ed.; Ministry of Health and Family Welfare People’s Republic of Bangladesh: Dhaka, Bangladesh, 2019.
- National Malaria Elimination Programme. National Strategic Plan for Malaria Elimination in Bangladesh: 2021–2025; Ministry of Health and Family Welfare Bangladesh: Dhaka, Bangladesh, 2020.
- Vector-borne Disease Control Programme. National Guidelines on Diagnosis and Treatment of Malaria in Bhutan, 5th ed.; Department of Public Health Bhutan: Gelephu, Bhutan, 2019.
- Vector-borne Disease Control Programme. Strategic Plan for Elimination of Malaria and Prevention of Re-Introduction in Bhutan 2020–2025; Ministry of Health Kingdom of Bhutan: Thimphu, Bhutan, 2020.
- Ministry of Health. National Treatment Guidelines for Malaria in Cambodia; Ministry of Health Kingdom of Cambodia: Phnom Penh, Cambodia, 2014.
- National Vector Borne Disease Control Programme. Guidelines for Diagnosis and Treatment of Malaria in India 2011; National Institute of Malaria Research: New Delhi, India, 2011.
- National Vector Borne Disease Control Programme. Operational Manual for Malaria Elimination in India 2016 (Version 1); Ministry of Health and Family Welfare Government of India: New Delhi, India, 2016.
- National Vector Borne Disease Control Programme. National Framework for Malaria Elimination in India 2016–2030; Ministry of Health and Family Welfare Government of India: New Delhi, India, 2016.
- Directorate General of Disease Prevention and Control. Buku Saku Tatalaksana Kasus Malaria 2020 (Malaria Case Management Pocket Book); Ministry of Health Republic of Indonesia: Jakarta, Indonesia, 2019.
- Center of Malariology Parasitology and Entomology. Malaria Treatment Guideline (Updated Nov 2017); Ministry of Health Lao People’s Democratic Republic: Vientiane, Laos, 2017.
- Center of Malariology Parasitology and Entomology. Malaria NSP 2021–2025; Ministry of Health Lao People’s Democratic Republic: Vientiane, Laos, 2020.
- Center of Malariology Parasitology and Entomology. National Guidelines for the Treatment of Malaria; Ministry of Health Lao People’s Democratic Republic: Vientiane, Laos, 2022.
- National Malaria Control Programme. Guidelines for Malaria Diagnosis and Treatment in Myanmar; Ministry of Health Republic of the Union of Myanmar: Naypyidaw, Myanmar, 2015.
- National Malaria Control Programme. Addendum—Guidelines for Malaria Diagnosis and Treatment in Myanmar; Ministry of Health Republic of the Union of Myanmar: Naypyidaw, Myanmar, 2018.
- Epidemiology and Disease Control Division. National Malaria Treatment Protocol; Ministry of Health and Population Government of Nepal: Kathmandu, Nepal, 2019.
- Epidemiology and Disease Control Division. National Malaria Strategic Plan 2014–2025; Ministry of Health and Population Government of Nepal: Kathmandu, Nepal, 2018.
- Directorate of Malaria Control. National Malaria Case Management Guidelines; Ministry of National Health Services Regulations and Coordination Islamic Republic of Pakistan: Islamabad, Pakistan, 2018.
- Directorate of Malaria Control. National Strategic Plan for Malaria Elimination in Pakistan 2021–2035; Directorate of Malaria Control Islamic Republic of Pakistan: Islamabad, Pakistan, 2020.
- National Department of Health. National Malaria Treatment Protocol; National Department of Health Independent State of Papua New Guinea: Port Moresby, Papua New Guinea, 2009.
- National Malaria Control Program. National Malaria Strategic Plan 2014–2018; National Department of Health Independent State of Papua New Guinea: Port Moresby, Papua New Guinea, 2014.
- National Malaria Control and Elimination Program. Philippines Clinical Practice Guidelines for the Diagnosis, Treatment, Prevention, and Control of Malaria in Adults and Special Risk Groups; Department of Health Republic of the Philippines: Manila, Philippines, 2018.
- National Vectorborne Disease Control Programme. Solomon Islands Strategic Plan for Malaria Control and Elimination 2021–2025; Ministry of Health and Medical Services Solomon Islands: Honiara, Solomon Islands, 2020.
- Ministry of Health and Medical Services. Solomon Islands 2018 Malaria Case Management Guideline; Ministry of Health and Medical Services Solomon Islands: Honiara, Solomon Islands, 2018.
- Korea Centers for Disease Control and Prevention. 2019년 말라리아 진료 가이드 (Malaria Practice Guide 2019); Ministry of Health and Welfare Government of South Korea: South Chungcheong, Republic of Korea, 2019.
- Division of Vector-Borne Diseases. Medical Practice Guidelines for Malaria Disease Treatment 2021; Ministry of Public Health Kingdom of Thailand: Nonthaburi, Thailand, 2021.
- National Malaria and Vector Borne Diseases Control Program. Malaria Diagnosis and Treatment Guidelines 2021; Republic of Vanuatu Ministry of Health: Port Villa, Vanuatu, 2021.
- National Malaria and Vector Borne Diseases Control Program. National Strategic Plan for Malaria Elimination 2021–2026; Republic of Vanuatu Ministry of Health: Port Vila, Vanuatu.
- National Malaria and Vector Borne Diseases Control Program. Guidelines for Malaria Diagnosis, Treatment and Prevention: MOH Decision No. 4845/QD-BYT; Ministry of Health Socialist Republic of Viet Nam: Hanoi, Viet Nam, 2016.
- National Malaria and Vector Borne Diseases Control Program. National Strategic Plan on Malaria Control and Elimination 2021–2025; Ministry of Health Socialist Republic of Viet Nam: Hanoi, Viet Nam, 2020.
- Ministry of Health. National Malaria Guidelines, 5th ed.; Ministry of Health Federal Democratic Republic of Ethiopia: Addis Ababa, Ethiopia, 2022.
- National Malaria Control Program. Guidelines for the Diagnosis and Treatment of Malaria in Somalia 2016; Ministry of Health Federal Government of Somalia Puntland and Somaliland: Mogadishu, Somaliland, 2016.
- Directorate of Communicable and Non-communicable Diseases Control. Sudan Malaria Diagnosis and Treatment Protocol 2017; Federal Ministry of Health Republic of Sudan: Khartoum, Sudan, 2017.
- Directorate for the Fight against Malaria. Prise en charge des cas de paludism—Manuel de Référence (Malaria Case Management Reference Manual); Ministry of Public Health Republic of Madagascar: Antananarivo, Madagascar, 2015.
- Pharmacotherapeutic Committee of the Malaria Program. Manual de Tratamiento de la Malaria (Malaria Treatment Manual); Ministry of Health and Sports Bolivian Plurinational State: La Paz, Bolivia, 2013.
- Department of Immunization and Communicable Diseases. Guia de Tratamento da Malária no Brasil (Malaria Treatment guidelines for Brazil); Ministry of Health Federative Republic of Brazil: Brasilia, Brazil, 2020.
- Ministry of Health and Social Protection. Guía de Práctica Clínica Diagnóstico y Tratamiento de la Malaria (Clinical Practice Guidelines Diagnosis and Treatment of Malaria); Ministry of Health and Social Protection Republic of Colombia: Bogotá, Colombia, 2022.
- Costa Rican Social Security Fund. Protocolo Para La Atención de la Persona Con Malaria Según Nivel de Atención (Protocol for the Care of the Person with Malaria by Level of Attention); Directorate of Development of Health Services Republic of Costa Rica: San José, Costa Rica, 2020.
- Ministry of Public Health. Diagnóstico y Tratamiento de Malaria (Diagnosis and Treatment of Malaria); Ministry of Public Health Republic of Ecuador: Quito, Ecuador, 2019.
- Regional Health Agency. Plan de Lutte Contre le Paludism en Guyane 2015–2018 (Plan against Malaria in Guiana 2015–2018); Regional Health Agency Territorial Collectivity of French Guiana: Cayenne, French Guiana, 2015. [Google Scholar]
- National Malaria Control Program. Malaria Treatment Guideline for Health Facilities in Guyana 2015; Ministry of Public Health Co-Operative Republic of Guyana: Georgetown, Guyana, 2015.
- Ministry of Health. Protocolo De Atención Integral a la Malaria (Comprehensive Malaria Care Protocol); Ministry of Health Republic of Honduras: Tegucigalpa, Honduras, 2018.
- National Center for Disease Control and Preventive Programs. Manual de Tratamientos Medicos para la Atención de Casos Confirmados de Paludismo en México (Manual of Medical Treatments for the Care of Confirmed Cases of Malaria in Mexico); Secretariat of Health Mexico: Mexico City, Mexico, 2022.
- Ministry of Health. Guía para el Manejo Clínico de la Malaria (Guide to the Clinical Management of Malaria); Ministry of Health Republic of Nicaragua: Managua, Nicaragua, 2022.
- Directorate General of Public Health. Guía de Abordaje Integral para la Eliminación de la Malaria en la República de Panamá (Integral Approach Guide for the Elimination of Malaria in the Republic of Panama); Ministry of Health Republic of Panama: Panama City, Panama, 2022.
- Directorate General of People’s Health. Norma Tecnica de Salud Para la Atencion de la Malaria y Malaria Grave en el Peru (Technical Health Standard for Malaria Care and Severe Malaria in Peru); Ministry of Health Republic of Peru: Lima, Peru, 2015.
- National Malaria Board. Malaria Preventie en Therapie Protocol 2018 (Malaria Prevention and Therapy Protocol 2018); Ministry of Health Republic of Suriname: Paramaribo, Suriname, 2018.
- Ministry of Popular Power for Health. Pautas de Tratamiento en Casos de Malaria (Treatment Guidelines in Malaria Cases); Ministry of Popular Power for Health Bolivarian Republic of Venezuela: Caracas, Venezuela, 2017.
- Yoshida, A.; Beutler, E.; Motulsky, A.G. Human glucose-6-phosphate dehydrogenase variants. Bull. World Health Organ. 1971, 45, 243–253. [Google Scholar] [PubMed]
- Vanisaveth, V.C.K.; Vongviengxay, S.; Cassidy-Seyoum, S.; Nguyen, H.; Tsai, Y. GORCoP: Early Experiences with Point-of-Care G6PD Testing in Malaria Case Management. GORCoP Zoom 2021. Available online: https://www.youtube.com/watch?v=Up9oVdYvG60 (accessed on 18 October 2022).
- Engel, N.; Ghergu, C.; Matin, M.A.; Kibria, M.G.; Thriemer, K.; Price, R.N.; Ding, X.C.; Howes, R.E.; Ley, B.; Incardona, S.; et al. Implementing radical cure diagnostics for malaria: User perspectives on G6PD testing in Bangladesh. Malar. J. 2021, 20, 217. [Google Scholar] [CrossRef]
- Adhikari, B.; Tripura, R.; Dysoley, L.; Callery, J.J.; Peto, T.J.; Heng, C.; Vanda, T.; Simvieng, O.; Cassidy-Seyoum, S.; Ley, B.; et al. Glucose 6 Phosphate Dehydrogenase (G6PD) quantitation using biosensors at the point of first contact: A mixed method study in Cambodia. Malar. J. 2022, 21, 282. [Google Scholar] [CrossRef]
- Alam, M.S. GORCoP: A Training for Health Service Providers on the SD Biosensor STANDARD G6PD Test in Bangladesh. GORCoP Zoom 2020. Available online: https://www.youtube.com/watch?v=Cze6OeHYQXk (accessed on 24 October 2022).
- Gerth-Guyette, E.; Cassidy-Seyoum, S.; Nguyen, H. Integrating G6PD Point-of-Care Testing into Malaria Case Management to Support Radical Cure: An Assessment of Health Worker Skills and Knowledge in Laos and Vietnam. In Proceedings of the 70th ASTMH Annual Meeting 2021 Poster Presentation, Virtual, 17–21 November 2021. [Google Scholar]
- Brito-Sousa, J.D. GORCoP: Training Healthcare Providers for Point of Care G6PD Testing in Brazil. GORCoP Zoom 2020. Available online: https://www.youtube.com/watch?v=TmJI67YNb_U (accessed on 14 October 2022).
- CHAI Cambodia and Laos. G6PD Testing in Cambodia and Laos—Piloting and Implementation; P. vivax Information Hub: Phnom Penh, Cambodia, 2021. [Google Scholar]
- Finn, T. Expanding access to safe radical cure: A study to assess the operational feasibility of reactive case detection and integrating quantitative G6PD testing into P. vivax malaria case management in Lao PDR. In Proceedings of the International Congress for Tropical Medicine and Malaria 2022, Bangkok, Thailand, 24–28 October 2022. [Google Scholar]
- Kitchakarn, S.; Lek, D.; Thol, S.; Hok, C.; Saejeng, A.; Huy, R.; Chinanonwait, N.; Thimasarn, K.; Wongsrichanalai, C. Implementation of G6PD testing and primaquine for P. vivax radical cure: Operational perspectives from Thailand and Cambodia. WHO South-East Asia J. Public Health 2017, 6, 60. [Google Scholar] [CrossRef]
- Gerth-Guyette, E.; Adissu, W.; Brito, M.; Garbin, E.; Macedo, M.; Sharma, A.; Das, S.; Lacerda, M.V.G.; Pereira, D.; Talukdar, A.; et al. Usability of a point-of-care diagnostic to identify glucose-6-phosphate dehydrogenase deficiency: A multi-country assessment of test label comprehension and results interpretation. Malar. J. 2021, 20, 307. [Google Scholar] [CrossRef] [PubMed]
- PATH. G6PD Operational Research Community of Practice (GORCOP) G6PD Training Materials. Available online: https://www.path.org/programs/diagnostics/gorcop-g6pd-test-training-materials/ (accessed on 7 December 2022).
- Chindavongsa, K. Paving the way to elimination by optimizing P. vivax radical cure: Roll-out of Point of Care G6PD Testing in Lao PDR. In Proceedings of the 71st ASTMH Annual Meeting, Seattle, WA, USA, 30 October–3 November 2022. [Google Scholar]
- Bancone, G. G6PD Tests Training. In Proceedings of the APMEN Vivax Working Group Annual Meeting 2022, Hilton, Bangkok, 12–14 December 2022. [Google Scholar]
- Lacerda, M.V.G. TRuST: Assess operational feasibility of providing appropriate radical cure after G6PD testing. In Proceedings of the APMEN Vivax Working Group Annual Meeting 2022, Hilton, Bangkok, 12–14 December 2022. [Google Scholar]
- VivAccess. Compliance and Adherence to Primaquine Treatment in Myanmar: A Brief of Available Evidence. 2021. Available online: https://www.vivaxmalaria.org/resources/compliance-and-adherence-to-primaquine-treatment-in-myanmar-%E2%80%94-a-brief-of-available (accessed on 24 October 2022).
- Dysoley, L. From evidence to nationwide policy: The rollout of P. vivax radical cure with G6PD testing in Cambodia, November 2019 to June 2021. In Proceedings of the APMEN Vivax Working Group 2021 Poster Presentation, Online, 9–11 August 2021; Available online: https://www.vivaxmalaria.org/resources/poster-from-evidence-to-nationwide-policy-the-rollout-of-p-vivax-radical-cure-with-g6pd (accessed on 18 October 2022).
- Brito-Sousa, J.D.; Murta, F.; Vitor-Silva, S.; Sampaio, V.; Mendes, M.; Souza, B.; Batista, T.; Santos, A.; Marques, L.; Barbosa, L.; et al. Quantitative G6PD Deficiency Screening in Routine Malaria Diagnostic Units in the Brazilian Amazon (SAFEPRIM): An Operational Mixed-Methods Study. Pathogens 2022, 11, 1328. [Google Scholar] [CrossRef] [PubMed]
- Chhim, S.; Piola, P.; Housen, T.; Herbreteau, V.; Tol, B. Malaria in Cambodia: A Retrospective Analysis of a Changing Epidemiology 2006–2019. Int. J. Environ. Res. Public Health 2021, 18, 1960. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. How Village Malaria Workers Lead the Fight against Malaria in Purworejo; World Health Organization: Geneva, Switzerland, 2021.
- Dysoley, L.; Iqubal, S.; Chhun, B.; Samphor, N.T.; Nguon, S.; Peng, S.; Dunn, J.C.; Cheung, H.C.; Tsai, Y.; Sovannaroth, S.; et al. Applying an effective coverage cascade to measure gaps in case management of P. vivax malaria, improve coverage, and maximize impact in Cambodia. In Proceedings of the 71st ASTMH Annual Meeting 2022, Seattle, WA, USA, 30 October–3 November 2022. [Google Scholar]
- Kheang, S.T.; Ridley, R.; Ngeth, E.; Ir, P.; Ngor, P.; Sovannaroth, S.; Lek, D.; Phon, S.; Kak, N.; Yeung, S. G6PD testing and radical cure for Plasmodium vivax in Cambodia: A mixed methods implementation study. PLoS ONE 2022, 17, e0275822. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Tripura, R.; Dysoley, L.; Peto, T.J.; Callery, J.J.; Heng, C.; Vanda, T.; Simvieng, O.; Cassidy-Seyoum, S.; Thriemer, K.; et al. Glucose-6-Phosphate Dehydrogenase (G6PD) Measurement Using Biosensors by Community-Based Village Malaria Workers and Hospital Laboratory Staff in Cambodia: A Quantitative Study. Pathogens 2023, 12, 400. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Sikes, H.D. Addressing Barriers to the Development and Adoption of Rapid Diagnostic Tests in Global Health. Nanobiomedicine 2015, 2, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Starr, C. Social benefit versus technological risk. Science 1969, 165, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Engel, N.; Ochodo, E.A.; Karanja, P.W.; Schmidt, B.M.; Janssen, R.; Steingart, K.R.; Oliver, S. Rapid molecular tests for tuberculosis and tuberculosis drug resistance: A qualitative evidence synthesis of recipient and provider views. Cochrane Database Syst. Rev. 2022, 4, CD014877. [Google Scholar] [CrossRef] [PubMed]
- Averilla, L. Supply chain issues—Why did stock data come up as such a high priority across so many countries? In Proceedings of the APMEN Vivax Working Group Annual Meeting 2022, Hilton, Bangkok, 12–14 December 2022. [Google Scholar]
- Mwaura, M.; Engel, N. Constructing confidence: User perspectives on AlereLAM testing for tuberculosis. Int. J. Infect. Dis. 2021, 112, 237–242. [Google Scholar] [CrossRef]
- Engel, N.; Wolffs, P.F.G. Aligning diagnostics to the point-of-care: Lessons for innovators, evaluators and decision-makers from tuberculosis and HIV. BMJ Glob. Health 2020, 5, e003457. [Google Scholar] [CrossRef]
- World Health Organization. WHO Information Notice for Users 2020/1. Available online: https://www.who.int/news/item/13-02-2020-who-information-notice-for-users-2020-1 (accessed on 15 December 2022).
- World Health Organization. WHO List of Prequalified In Vitro Diagnostic Products; World Health Organization: Geneva, Switzerland, 2023.
- The Global Fund. List of Rapid Diagnostic Test (Rdt) Kits for Malaria Classified According to the Global Fund Quality Assurance Policy; The Global Fund: Geneva, Switzerland, 2022.
- Devine, A.; Pasaribu, A.P.; Teferi, T.; Pham, H.-T.; Awab, G.R.; Contantia, F.; Nguyen, T.-N.; Ngo, V.-T.; Tran, T.-H.; Hailu, A.; et al. Provider and household costs of Plasmodium vivax malaria episodes: A multicountry comparative analysis of primary trial data. Bull. World Health Organ. 2019, 97, 828–836. [Google Scholar] [CrossRef]
- Devine, A.; Howes, R.E.; Price, D.J.; Moore, K.A.; Ley, B.; Simpson, J.A.; Dittrich, S.; Price, R.N. Cost-Effectiveness Analysis of Sex-Stratified Plasmodium vivax Treatment Strategies Using Available G6PD Diagnostics to Accelerate Access to Radical Cure. Am. J. Trop. Med. Hyg. 2020, 103, 394–403. [Google Scholar] [CrossRef]
- Brito-Sousa, J.D.; Peixoto, H.M.; Devine, A.; Silva-Neto, A.V.; Balieiro, P.C.S.; Sampaio, V.S.; Vitor-Silva, S.; Mendes, M.O.; Souza, B.K.A.; Lacerda, M.V.G.; et al. Real-life quantitative G6PD screening in Plasmodium vivax patients in the Brazilian Amazon: A cost-effectiveness analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010325. [Google Scholar] [CrossRef]
- Ruwanpura, V.S.H.; Nowak, S.; Gerth-Guyette, E.; Theodora, M.; Dysoley, L.; Haile, M.; Peeters Grietens, K.; Price, R.N.; Lynch, C.A.; Thriemer, K. Further evidence needed to change policy for the safe and effective radical cure of vivax malaria: Insights from the 2019 annual APMEN Vivax Working Group meeting. Asia Pac. Policy Stud. 2021, 8, 208–242. [Google Scholar] [CrossRef]
- Paulden, M.; O’Mahony, J.; McCabe, C. Determinants of Change in the Cost-effectiveness Threshold. Med Decis. Mak. 2017, 37, 264–276. [Google Scholar] [CrossRef] [PubMed]


| Diagnostic to Detect G6PD Deficiency (Year First Reported) | Output | Blood Volume Required | Time to Result | Pipetting Steps in Sample Preparation | Cost * | Performance ** |
|---|---|---|---|---|---|---|
| Laboratory Assays | ||||||
| Spectrophotometry (1967) [48] | Quantitative | 10 µL | 15 min + calculation time | 4–5 + sample or buffer preparation steps | Trinity Biotech (Ireland) USD 3.6 Pointe Scientific (USA) USD 2.0 [49] | Used as diagnostic reference; substantial inter-lab variability [34] |
| Fluorescent Spot Test (1966) [50,51] | Qualitative | 10 µL | 15 min + drying time | 5 | USD 0.1–3.0 [52] |
|
| WST-8/1-methoxy PMS (2003) [57,58] | Quantitative or qualitative | 5 µL | 15–60 min | 4 | USD 0.1–3.2 [55,58] |
|
| Flow Cytometry (1989) [40,41,59] | Cytochemical | 1 mL [59] | 3 h | At least 14 + buffer preparation steps | USD 20 [60] |
|
| Point-of-care Assays | ||||||
| CareStart G6PD RDT (2011; AccessBio, Somerset, NJ, USA) [61] | Qualitative (2.7 U/g Hb threshold) | 2 µL | 10 min | 2 | USD 1.5 [62] |
|
| BinaxNOW G6PD Test (2010; Alere, Waltham, MA, USA) [64,65] | Qualitative (4.0 U/g Hb threshold) | 10 µL | 7 min | 3 | USD 15 [66] |
|
| CareStart G6PD Biosensor (2017; AccessBio, Somerset, NJ, USA) | Quantitative | 5 µL | 4 min | 0 | USD 670 (device) + USD 3.4 (test strip) [68] USD 500 (device) + 2.5 (test strip) [58] |
|
| STANDARD G6PD Test (2018; SD Biosensor, Suwon, Republic of Korea) | Quantitative | 10 µL | 2 min | 2 | USD 380 (device) + USD 3 (test device) [68] |
|
| Characteristics | Acceptable | Desirable |
|---|---|---|
| TPP #1: PoC Screening Test for G6PD | ||
| Performance in percent positive agreement (PPA) or percent negative agreement (PNA) |
|
|
| Kit storage |
|
|
| Pricing |
|
|
| TPP #2: One-time Quantitative Test for G6PD | ||
| Agreement | Systematic difference (bias):
| Systematic difference (bias):
|
| Kit storage |
|
|
| Pricing |
|
|
| Themes to be addressed |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadhewa, A.; Cassidy-Seyoum, S.; Acharya, S.; Devine, A.; Price, R.N.; Mwaura, M.; Thriemer, K.; Ley, B. A Review of the Current Status of G6PD Deficiency Testing to Guide Radical Cure Treatment for Vivax Malaria. Pathogens 2023, 12, 650. https://doi.org/10.3390/pathogens12050650
Sadhewa A, Cassidy-Seyoum S, Acharya S, Devine A, Price RN, Mwaura M, Thriemer K, Ley B. A Review of the Current Status of G6PD Deficiency Testing to Guide Radical Cure Treatment for Vivax Malaria. Pathogens. 2023; 12(5):650. https://doi.org/10.3390/pathogens12050650
Chicago/Turabian StyleSadhewa, Arkasha, Sarah Cassidy-Seyoum, Sanjaya Acharya, Angela Devine, Ric N. Price, Muthoni Mwaura, Kamala Thriemer, and Benedikt Ley. 2023. "A Review of the Current Status of G6PD Deficiency Testing to Guide Radical Cure Treatment for Vivax Malaria" Pathogens 12, no. 5: 650. https://doi.org/10.3390/pathogens12050650
APA StyleSadhewa, A., Cassidy-Seyoum, S., Acharya, S., Devine, A., Price, R. N., Mwaura, M., Thriemer, K., & Ley, B. (2023). A Review of the Current Status of G6PD Deficiency Testing to Guide Radical Cure Treatment for Vivax Malaria. Pathogens, 12(5), 650. https://doi.org/10.3390/pathogens12050650







