L-Tyrosine Limits Mycobacterial Survival in Tuberculous Granuloma
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Cells/Strains
2.2. Zebrafish Strains and Maintenance
2.3. AAs and Reagents
2.4. Larvae Infections, Treatments, and Image Analyses
2.5. Acute Toxicity Assays
2.6. Adult Zebrafish Infections and Treatments
2.7. Cell Infections and Image Analyses
2.8. Quantitative RT-PCR (qRT-PCR)
2.9. Statistical Analyses
3. Results
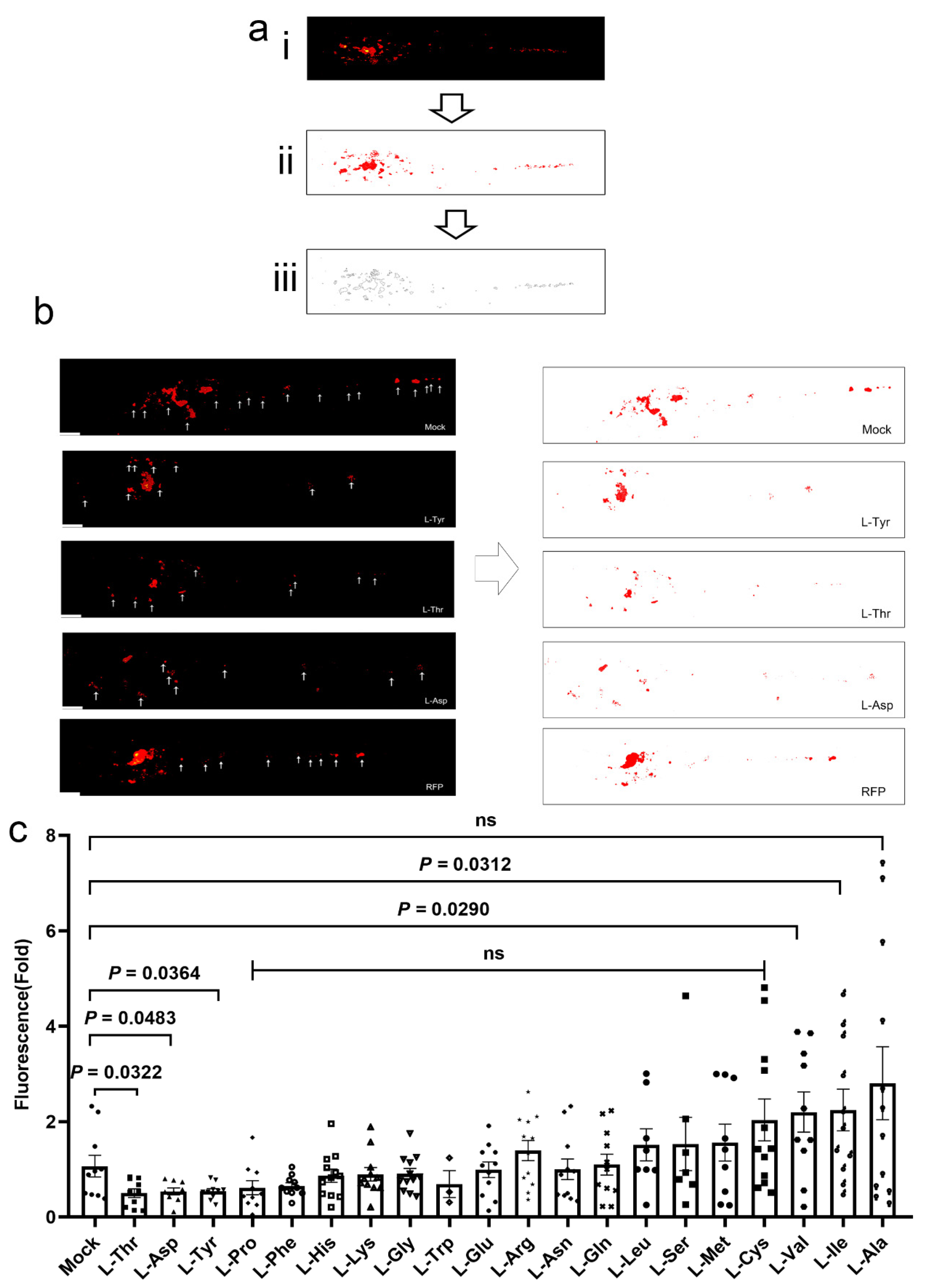
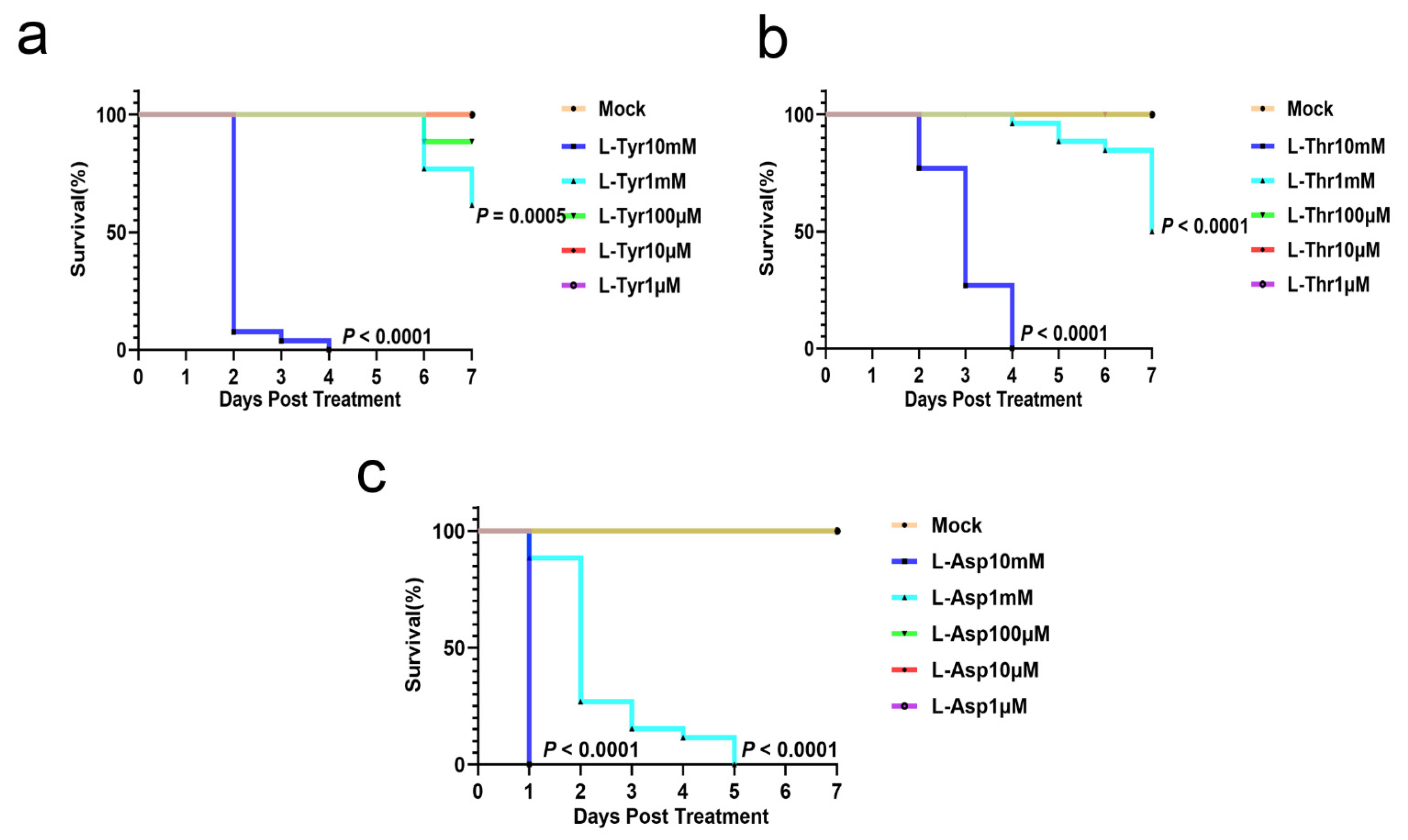
3.1. L-Asp, L-Thr, and L-Tyr Inhibit M. marinum Proliferation in Larva Granulomas
3.2. L-Asp, L-Thr, and L-Tyr Do Not Affect Zebrafish Larvae Development
3.3. L-Tyr Reduces M. marinum Levels in Adult Zebrafish Granulomas
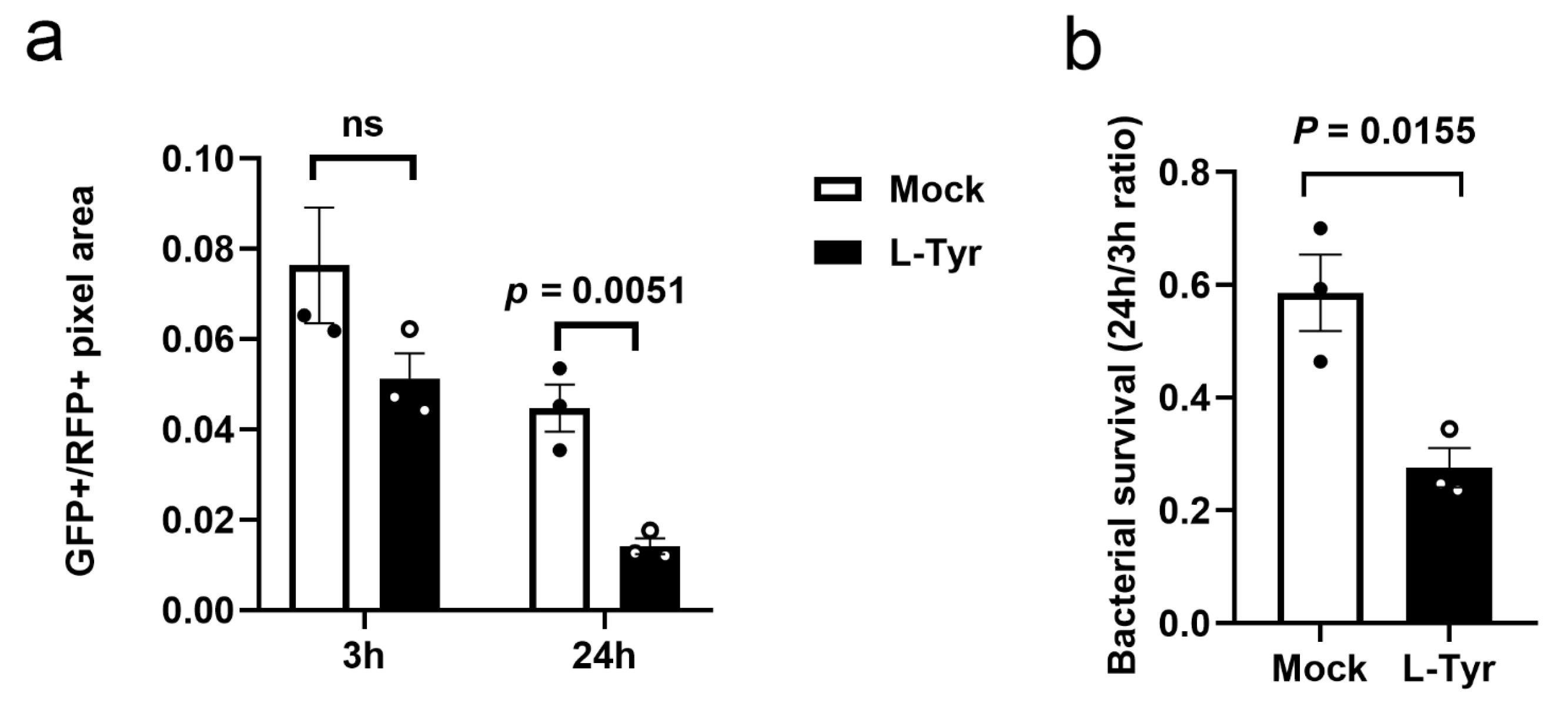
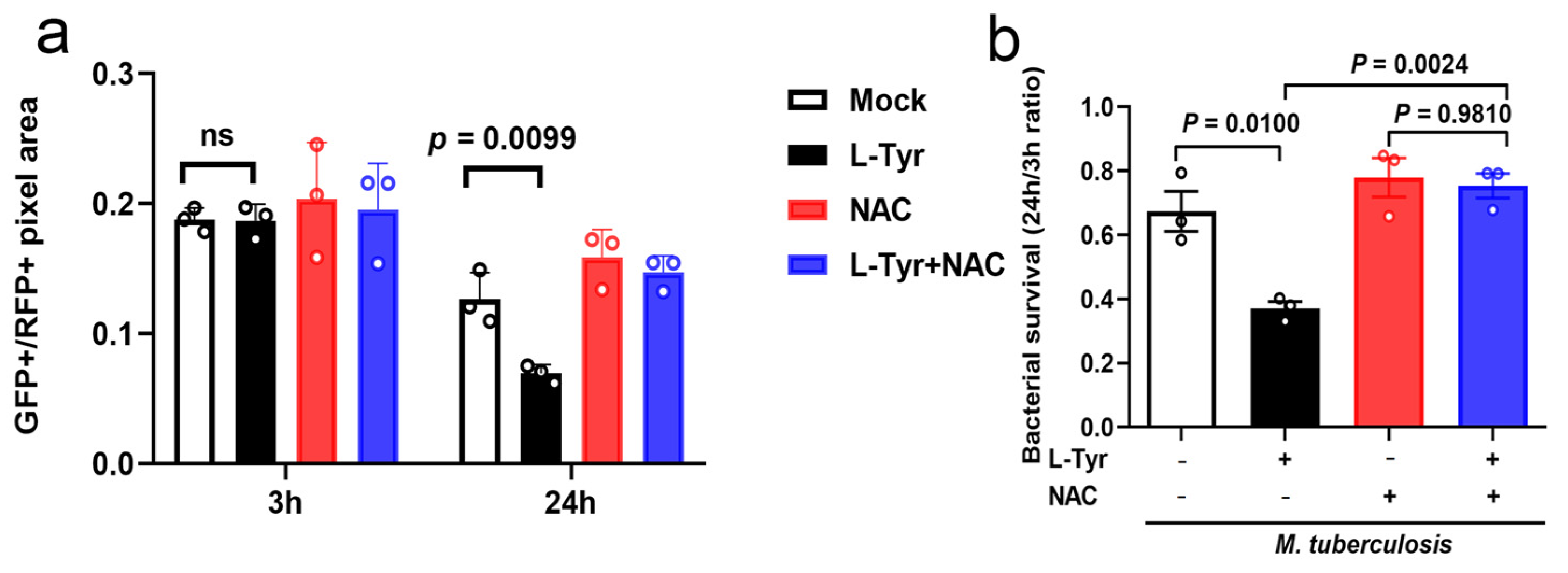
3.4. L-Tyr Reduces Mtb Intracellular Survival
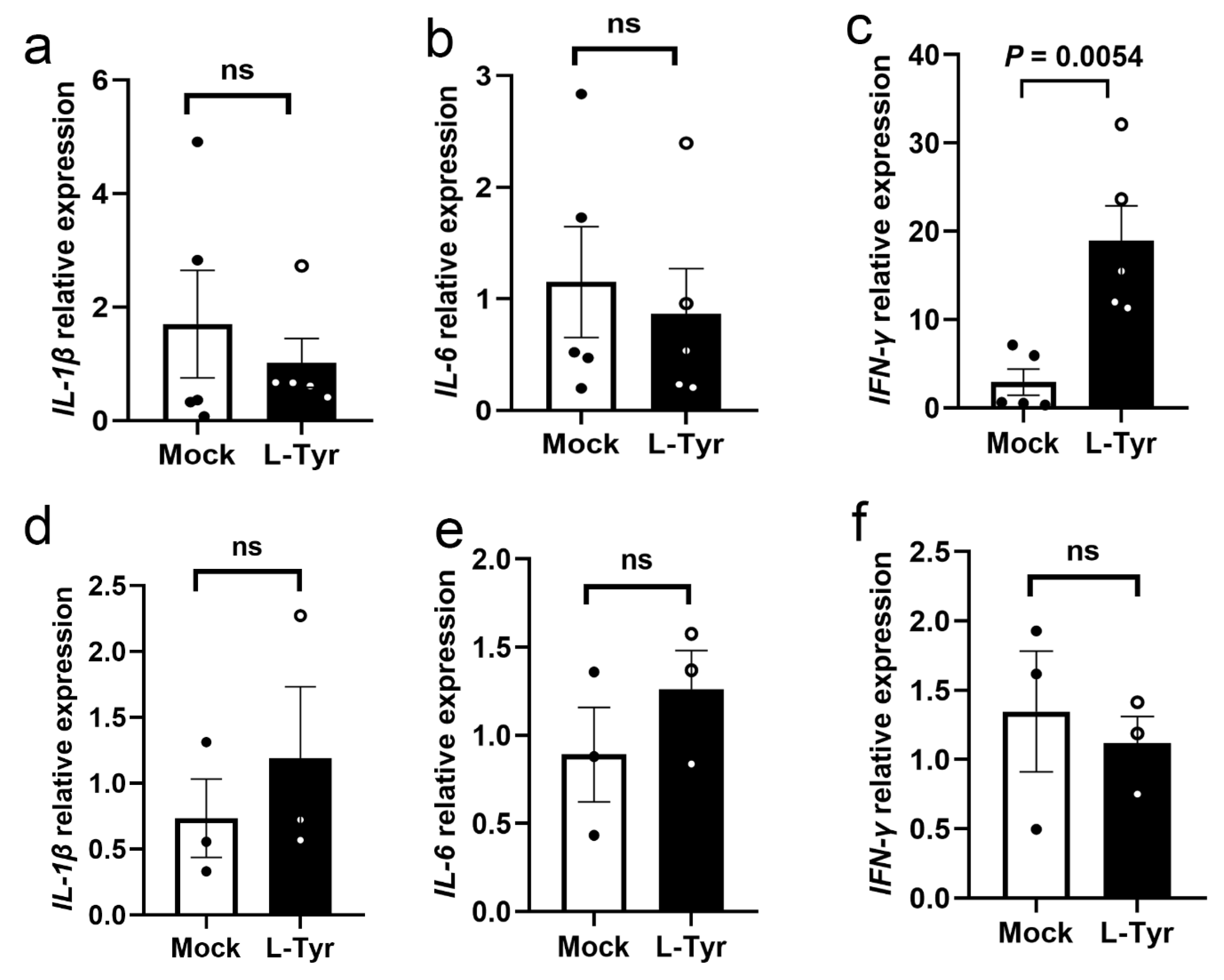
3.5. L-Tyr Upregulates IFN-γ Transcription in M. marinum-Infected Adult Zebrafish
3.6. L-Tyr Inhibits Mtb Intracellular Survival via ROS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2022. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 4 February 2023).
- Zimmer, A.J.; Klinton, J.S.; Oga-Omenka, C.; Heitkamp, P.; Nawina, N.C.; Furin, J.; Pai, M. Tuberculosis in times of COVID-19. J. Epidemiol. Commun. Health 2022, 76, 310–316. [Google Scholar] [CrossRef]
- Yoshiyama, T.; Takaki, A.; Aono, A.; Mitarai, S.; Okumura, M.; Ohta, K.; Kato, S. Multidrug Resistant Tuberculosis with Simultaneously Acquired Drug Resistance to Bedaquiline and Delamanid. Clin. Infect. Dis. 2021, 73, 2329–2331. [Google Scholar] [CrossRef]
- Singh, R.; Dwivedi, S.P.; Gaharwar, U.S.; Meena, R.; Rajamani, P.; Prasad, T. Recent updates on drug resistance in Mycobacterium tuberculosis. J. Appl. Microbiol. 2020, 128, 1547–1567. [Google Scholar] [CrossRef]
- Chao, M.C.; Rubin, E.J. Letting sleeping dos lie: Does dormancy play a role in tuberculosis? Annu. Rev. Microbiol. 2010, 64, 293–311. [Google Scholar] [CrossRef]
- Pagan, A.J.; Ramakrishnan, L. The Formation and Function of Granulomas. Annu. Rev. Immunol. 2018, 36, 639–665. [Google Scholar] [CrossRef]
- Davis, J.M.; Ramakrishnan, L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 2009, 136, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Prideaux, B.; Via, L.E.; Zimmerman, M.D.; Eum, S.; Sarathy, J.; O’Brien, P.; Chen, C.; Kaya, F.; Weiner, D.M.; Chen, P.Y.; et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat. Med. 2015, 21, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Crowther, R.R.; Qualls, J.E. Metabolic Regulation of Immune Responses to Mycobacterium tuberculosis: A Spotlight on L-Arginine and L-Tryptophan Metabolism. Front. Immunol. 2020, 11, 628432. [Google Scholar] [CrossRef] [PubMed]
- Amalia, F.; Syamsunarno, M.; Triatin, R.D.; Fatimah, S.N.; Chaidir, L.; Achmad, T.H. The Role of Amino Acids in Tuberculosis Infection: A Literature Review. Metabolites 2022, 12, 933. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Ren, W.; Rajendran, R.; Zhao, Y.; Tan, B.; Wu, G.; Bazer, F.W.; Zhu, G.; Peng, Y.; Huang, X.; Deng, J.; et al. Amino Acids As Mediators of Metabolic Cross Talk between Host and Pathogen. Front. Immunol. 2018, 9, 319. [Google Scholar] [CrossRef]
- Qualls, J.E.; Murray, P.J. Immunometabolism within the tuberculosis granuloma: Amino acids, hypoxia, and cellular respiration. Semin. Immunopathol. 2016, 38, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Tayeh, M.A.; Marletta, M.A. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J. Biol. Chem. 1989, 264, 19654–19658. [Google Scholar] [CrossRef] [PubMed]
- Grimble, G.K. Adverse gastrointestinal effects of arginine and related amino acids. J. Nutr. 2007, 137, 1693S–1701S. [Google Scholar] [CrossRef] [PubMed]
- Farazi, A.; Shafaat, O.; Sofian, M.; Kahbazi, M. Arginine adjunctive therapy in active tuberculosis. Tuberc. Res. Treat. 2015, 2015, 205016. [Google Scholar] [CrossRef]
- Schon, T.; Elias, D.; Moges, F.; Melese, E.; Tessema, T.; Stendahl, O.; Britton, S.; Sundqvist, T. Arginine as an adjuvant to chemotherapy improves clinical outcome in active tuberculosis. Eur. Respir. J. 2003, 21, 483–488. [Google Scholar] [CrossRef]
- Schon, T.; Idh, J.; Westman, A.; Elias, D.; Abate, E.; Diro, E.; Moges, F.; Kassu, A.; Ayele, B.; Forslund, T.; et al. Effects of a food supplement rich in arginine in patients with smear positive pulmonary tuberculosis—A randomised trial. Tuberculosis 2011, 91, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Parish, T.; Stoker, N.G.; Bancroft, G.J. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect. Immun. 2001, 69, 1142–1150. [Google Scholar] [CrossRef]
- Desvignes, L.; Ernst, J.D. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity 2009, 31, 974–985. [Google Scholar] [CrossRef]
- Luukinen, H.; Hammaren, M.M.; Vanha-Aho, L.M.; Parikka, M. Modeling Tuberculosis in Mycobacterium marinum Infected Adult Zebrafish. J. Vis. Exp. 2018, 140, 58299. [Google Scholar] [CrossRef]
- Stinear, T.P.; Seemann, T.; Harrison, P.F.; Jenkin, G.A.; Davies, J.K.; Johnson, P.D.; Abdellah, Z.; Arrowsmith, C.; Chillingworth, T.; Churcher, C.; et al. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 2008, 18, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Cronan, M.R.; Tobin, D.M. Fit for consumption: Zebrafish as a model for tuberculosis. Dis. Model Mech. 2014, 7, 777–784. [Google Scholar] [CrossRef]
- Varela, M.; Meijer, A.H. A fresh look at mycobacterial pathogenicity with the zebrafish host model. Mol. Microbiol. 2022, 117, 661–669. [Google Scholar] [CrossRef]
- van Leeuwen, L.M.; van der Sar, A.M.; Bitter, W. Animal models of tuberculosis: Zebrafish. Cold Spring Harb. Perspect. Med. 2014, 5, a18580. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Bryson, B.D.; Rosebrock, T.R.; Tafesse, F.G.; Itoh, C.Y.; Nibasumba, A.; Babunovic, G.H.; Corleis, B.; Martin, C.; Keegan, C.; Andrade, P.; et al. Heterogeneous GM-CSF signaling in macrophages is associated with control of Mycobacterium tuberculosis. Nat. Commun. 2019, 10, 2329. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, F.; Guo, X.; Liu, F.; Liu, Z.; Wu, X.; Zhao, M.; Ma, M.; Liu, H.; Qin, L.; et al. Interception of host fatty acid metabolism by mycobacteria under hypoxia to suppress anti-TB immunity. Cell Discov. 2021, 7, 90. [Google Scholar] [CrossRef]
- Swaim, L.E.; Connolly, L.E.; Volkman, H.E.; Humbert, O.; Born, D.E.; Ramakrishnan, L. Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect. Immun. 2006, 74, 6108–6117. [Google Scholar] [CrossRef]
- Dalton, J.P.; Uy, B.; Okuda, K.S.; Hall, C.J.; Denny, W.A.; Crosier, P.S.; Swift, S.; Wiles, S. Screening of anti-mycobacterial compounds in a naturally infected zebrafish larvae model. J. Antimicrob. Chemoth. 2017, 72, 421–427. [Google Scholar] [CrossRef]
- Messineo, A.M.; Gineste, C.; Sztal, T.E.; McNamara, E.L.; Vilmen, C.; Ogier, A.C.; Hahne, D.; Bendahan, D.; Laing, N.G.; Bryson-Richardson, R.J.; et al. L-tyrosine supplementation does not ameliorate skeletal muscle dysfunction in zebrafish and mouse models of dominant skeletal muscle alpha-actin nemaline myopathy. Sci. Rep. 2018, 8, 11490. [Google Scholar] [CrossRef]
- Ramakrishnan, L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 2012, 12, 352–366. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, A.; Ortega-Muro, F.; Alameda-Martin, L.; Mitchison, D.; Coates, A. High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo. Front. Microbiol. 2015, 6, 641. [Google Scholar] [CrossRef]
- Goyal, N.; Kashyap, B.; Singh, N.P.; Kaur, I.R. Neopterin and oxidative stress markers in the diagnosis of extrapulmonary tuberculosis. Biomarkers 2017, 22, 648–653. [Google Scholar] [CrossRef]
- Gouzy, A.; Poquet, Y.; Neyrolles, O. Amino acid capture and utilization within the Mycobacterium tuberculosis phagosome. Future Microbiol. 2014, 9, 631–637. [Google Scholar] [CrossRef]
- Ding, Y.; Raterink, R.J.; Marin-Juez, R.; Veneman, W.J.; Egbers, K.; van den Eeden, S.; Haks, M.C.; Joosten, S.A.; Ottenhoff, T.; Harms, A.C.; et al. Tuberculosis causes highly conserved metabolic changes in human patients, mycobacteria-infected mice and zebrafish larvae. Sci. Rep. 2020, 10, 11635. [Google Scholar] [CrossRef] [PubMed]
- Conde, R.; Laires, R.; Goncalves, L.G.; Rizvi, A.; Barroso, C.; Villar, M.; Macedo, R.; Simoes, M.J.; Gaddam, S.; Lamosa, P.; et al. Discovery of serum biomarkers for diagnosis of tuberculosis by NMR metabolomics including cross-validation with a second cohort. Biomed. J. 2022, 45, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Gouzy, A.; Poquet, Y.; Neyrolles, O. Aspartate: An essential amino acid for the physiology and virulence of the tuberculosis bacillus. Med. Sci. 2014, 30, 242–244. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J.; McNeal, C.J.; Bazer, F.W.; Rhoads, J.M. Role of L-Arginine in Nitric Oxide Synthesis and Health in Humans. Adv. Exp. Med. Biol. 2021, 1332, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Oehlers, S.H.; Cronan, M.R.; Scott, N.R.; Thomas, M.I.; Okuda, K.S.; Walton, E.M.; Beerman, R.W.; Crosier, P.S.; Tobin, D.M. Interception of host angiogenic signalling limits mycobacterial growth. Nature 2015, 517, 612–615. [Google Scholar] [CrossRef]
- Ralph, A.P.; Waramori, G.; Pontororing, G.J.; Kenangalem, E.; Wiguna, A.; Tjitra, E.; Sandjaja; Lolong, D.B.; Yeo, T.W.; Chatfield, M.D.; et al. L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: A randomised, double-blind, placebo-controlled trial. PLoS ONE 2013, 8, e70032. [Google Scholar] [CrossRef]
- Rivas-Santiago, B.; Rivas-Santiago, C.; Sada, E.; Hernandez-Pando, R. Prophylactic potential of defensins and L-isoleucine in tuberculosis household contacts: An experimental model. Immunotherapy 2015, 7, 207–213. [Google Scholar] [CrossRef]
- He, Y.C.; Wang, Y.H.; Cao, J.; Chen, J.W.; Pan, D.Y.; Zhou, Y.K. Effect of complex amino acid imbalance on growth of tumor in tumor-bearing rats. World J. Gastroenterol. 2003, 9, 2772–2775. [Google Scholar] [CrossRef] [PubMed]
- Jongkees, B.J.; Hommel, B.; Kuhn, S.; Colzato, L.S. Effect of tyrosine supplementation on clinical and healthy populations under stress or cognitive demands—A review. J. Psychiatr. Res. 2015, 70, 50–57. [Google Scholar] [CrossRef] [PubMed]
- van de Rest, O.; Bloemendaal, M.; de Heus, R.; Aarts, E. Dose-Dependent Effects of Oral Tyrosine Administration on Plasma Tyrosine Levels and Cognition in Aging. Nutrients 2017, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Hase, A.; Jung, S.E.; Aan, H.R.M. Behavioral and cognitive effects of tyrosine intake in healthy human adults. Pharmacol. Biochem. Behav. 2015, 133, 1–6. [Google Scholar] [CrossRef]
- Shurtleff, D.; Thomas, J.R.; Schrot, J.; Kowalski, K.; Harford, R. Tyrosine reverses a cold-induced working memory deficit in humans. Pharmacol. Biochem. Behav. 1994, 47, 935–941. [Google Scholar] [CrossRef]
- van Spronsen, F.J.; van Rijn, M.; Bekhof, J.; Koch, R.; Smit, P.G. Phenylketonuria: Tyrosine supplementation in phenylalanine-restricted diets. Am. J. Clin. Nutr. 2001, 73, 153–157. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-gamma): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Green, A.M.; Difazio, R.; Flynn, J.L. IFN-gamma from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J. Immunol. 2013, 190, 270–277. [Google Scholar] [CrossRef]
- Singh, B.; Wasita, B.; Reviono, R. Kinetics of Granuloma, IFN-gamma and IP-10 in a Wistar Rat Model Infected with Mycobacterium Tuberculosis. Med. Arch. 2022, 76, 248–251. [Google Scholar] [CrossRef]
- Ehlers, S.; Benini, J.; Held, H.D.; Roeck, C.; Alber, G.; Uhlig, S. Alphabeta T cell receptor-positive cells and interferon-gamma, but not inducible nitric oxide synthase, are critical for granuloma necrosis in a mouse model of mycobacteria-induced pulmonary immunopathology. J. Exp. Med. 2001, 194, 1847–1859. [Google Scholar] [CrossRef] [PubMed]
- Dandare, S.U.; Ezeonwumelu, I.J.; Shinkafi, T.S.; Magaji, U.F.; Adio, A.A.; Ahmad, K. L-alanine supplementation improves blood glucose level and biochemical indices in alloxan-induced diabetic rats. J. Food Biochem. 2021, 45, e13590. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xin, D.; Wang, L.; Zhang, T.; Bai, X.; Li, T.; Xie, Y.; Xue, H.; Bo, S.; Liu, D.; et al. Therapeutic effects of L-Cysteine in newborn mice subjected to hypoxia-ischemia brain injury via the CBS/H(2)S system: Role of oxidative stress and endoplasmic reticulum stress. Redox Biol. 2017, 13, 528–540. [Google Scholar] [CrossRef]
- Jiang, M.; Gong, Q.Y.; Lai, S.S.; Cheng, Z.X.; Chen, Z.G.; Zheng, J.; Peng, B. Phenylalanine enhances innate immune response to clear ceftazidime-resistant Vibrio alginolyticus in Danio rerio. Fish Shellfish Immunol. 2019, 84, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Hu, L.; Zhu, J.; Chen, J.; Wang, Z.; Yue, Z.; Qiu, M.; Shan, A. Valine Supplementation Does Not Reduce Lipid Accumulation and Improve Insulin Sensitivity in Mice Fed High-Fat Diet. ACS Omega 2020, 5, 30937–30945. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, Y.; Zhang, Y.; Zhu, X.; Jin, F. L-Arginine supplementation inhibits the growth of breast cancer by enhancing innate and adaptive immune responses mediated by suppression of MDSCs in vivo. BMC Cancer 2016, 16, 343. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.A.; Patel, P.D.; Gordillo, R.; Wert, K.J. Replenishment of TCA cycle intermediates provides photoreceptor resilience against neurodegeneration during progression of retinitis pigmentosa. JCI Insight 2021, 6, 17. [Google Scholar] [CrossRef]
- Mantilla, B.S.; Marchese, L.; Casas-Sanchez, A.; Dyer, N.A.; Ejeh, N.; Biran, M.; Bringaud, F.; Lehane, M.J.; Acosta-Serrano, A.; Silber, A.M. Proline Metabolism is Essential for Trypanosoma brucei brucei Survival in the Tsetse Vector. PLoS Pathog. 2017, 13, e1006158. [Google Scholar] [CrossRef]
- Deng, L.; Yao, P.; Li, L.; Ji, F.; Zhao, S.; Xu, C.; Lan, X.; Jiang, P. p53-mediated control of aspartate-asparagine homeostasis dictates LKB1 activity and modulates cell survival. Nat. Commun. 2020, 11, 1755. [Google Scholar] [CrossRef]
- Schobel, F.; Jacobsen, I.D.; Brock, M. Evaluation of lysine biosynthesis as an antifungal drug target: Biochemical characterization of Aspergillus fumigatus homocitrate synthase and virulence studies. Eukaryot. Cell 2010, 9, 878–893. [Google Scholar] [CrossRef]
- Gaifem, J.; Goncalves, L.G.; Dinis-Oliveira, R.J.; Cunha, C.; Carvalho, A.; Torrado, E.; Rodrigues, F.; Saraiva, M.; Castro, A.G.; Silvestre, R. L-Threonine Supplementation During Colitis Onset Delays Disease Recovery. Front. Physiol. 2018, 9, 1247. [Google Scholar] [CrossRef] [PubMed]
- Dever, J.T.; Elfarra, A.A. L-methionine toxicity in freshly isolated mouse hepatocytes is gender-dependent and mediated in part by transamination. J. Pharmacol. Exp. Ther. 2008, 326, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Wessler, L.B.; Farias, H.R.; Ronsani, J.F.; Candiotto, G.; Dos, S.P.; de Oliveira, J.; Rico, E.P.; Streck, E.L. Acute exposure to leucine modifies behavioral parameters and cholinergic activity in zebrafish. Int. J. Dev. Neurosci. 2019, 78, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Tsuji-Tamura, K.; Sato, M.; Fujita, M.; Tamura, M. Glycine exerts dose-dependent biphasic effects on vascular development of zebrafish embryos. Biochem. Biophys. Res. Commun. 2020, 527, 539–544. [Google Scholar] [CrossRef]
- Giacomini, A.; Piassetta, A.S.; Genario, R.; Bonan, C.D.; Piato, A.; Barcellos, L.; de Abreu, M.S. Tryptophan alleviates neuroendocrine and behavioral responses to stress in zebrafish. Behav. Brain Res. 2020, 378, 112264. [Google Scholar] [CrossRef]

| Primer | Sequence (5′-3′) |
|---|---|
| β-actin-F | ATGGATGAGGAAATCGCTGCC |
| β-actin-R | CTCCCTGATGTCTGGGTCGTC |
| IL-1β-F | TGGACTTCGCAGCACAAAATG |
| IL-1β-R | GTTCACTTCACGCTCTTGGATG |
| IL6-F | AGACCGCTGCCTGTCTAAAA |
| IL6-R | TTTGATGTCGTTCACCAGGA |
| IFN-γ-F | AAATGGTGCTACTCTGTGGAC |
| IFN-γ-R | TTCCAACCCAATCCTTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Li, J.; Guo, X.; Guan, L.; Wang, J.; Huang, X.; Wang, W.; Yang, H. L-Tyrosine Limits Mycobacterial Survival in Tuberculous Granuloma. Pathogens 2023, 12, 654. https://doi.org/10.3390/pathogens12050654
Gao Y, Li J, Guo X, Guan L, Wang J, Huang X, Wang W, Yang H. L-Tyrosine Limits Mycobacterial Survival in Tuberculous Granuloma. Pathogens. 2023; 12(5):654. https://doi.org/10.3390/pathogens12050654
Chicago/Turabian StyleGao, Yaxian, Jiaqing Li, Xinya Guo, Liru Guan, Jie Wang, Xiaochen Huang, Wenjuan Wang, and Hua Yang. 2023. "L-Tyrosine Limits Mycobacterial Survival in Tuberculous Granuloma" Pathogens 12, no. 5: 654. https://doi.org/10.3390/pathogens12050654
APA StyleGao, Y., Li, J., Guo, X., Guan, L., Wang, J., Huang, X., Wang, W., & Yang, H. (2023). L-Tyrosine Limits Mycobacterial Survival in Tuberculous Granuloma. Pathogens, 12(5), 654. https://doi.org/10.3390/pathogens12050654





