Sequential Administration of SARS-CoV-2 Strains-Based Vaccines Effectively Induces Potent Immune Responses against Previously Unexposed Omicron Strain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, Vaccines and Animals
2.2. Immunizations
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. IFN-γ Enzyme-Linked Immunospot Assay (IFN-γ ELISPOT)
2.5. Flow Cytometry
2.6. Authentic SARS-CoV-2 Neutralization Assay
2.7. Statistical Analysis
3. Results
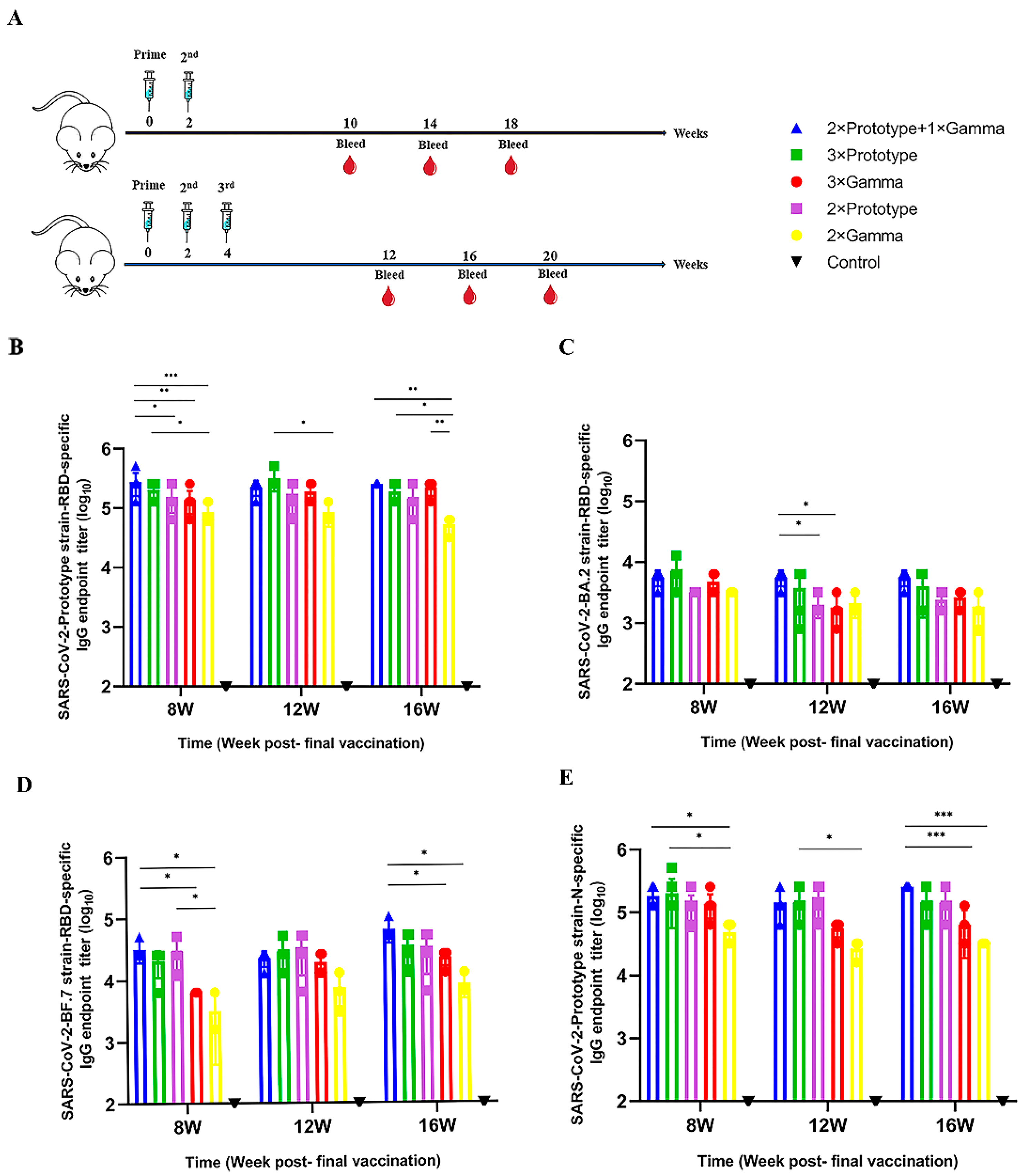
3.1. The Third Vaccination Elicits Higher Levels of Binding Antibodies
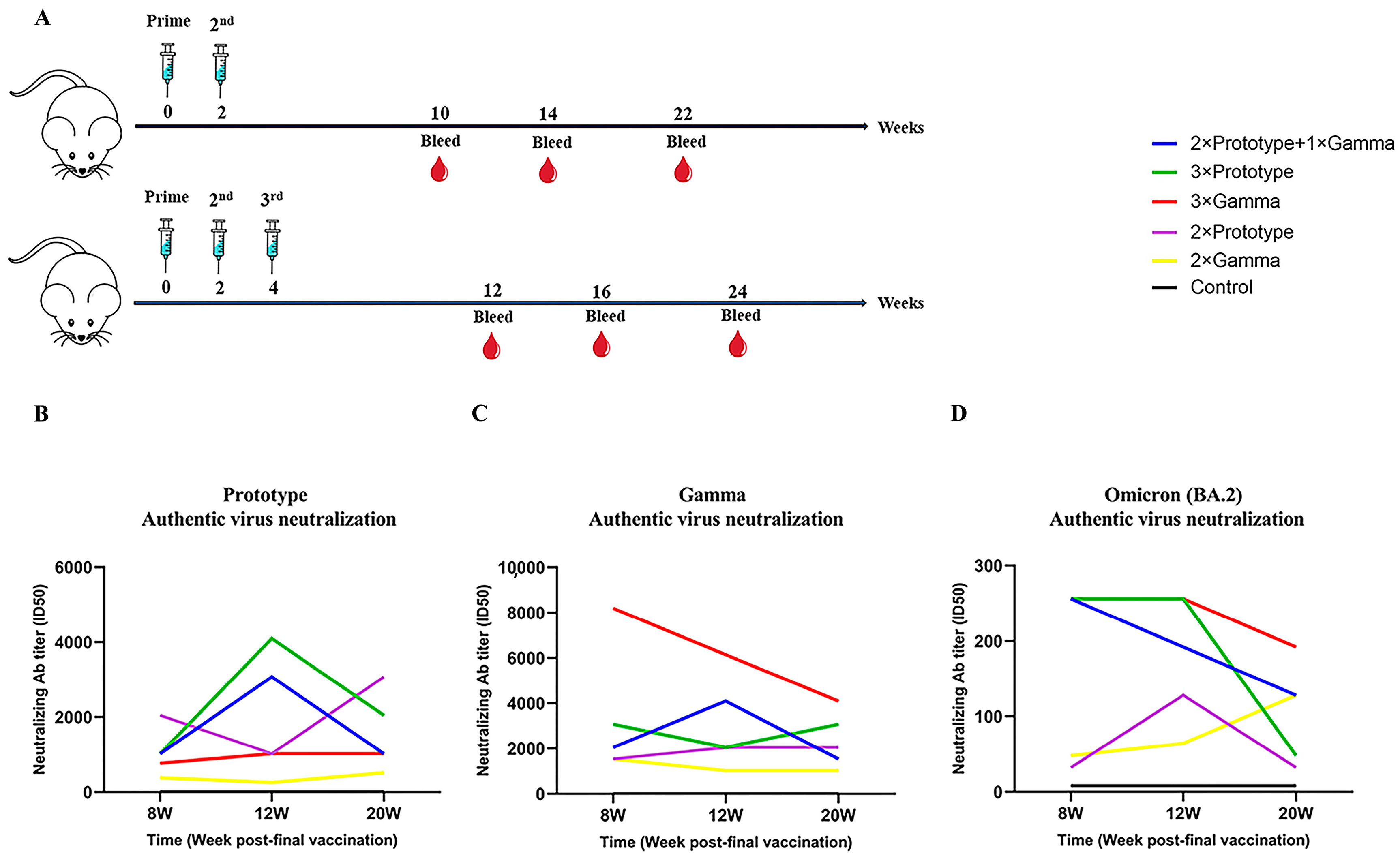
3.2. Durable and Cross-Reactive Neutralizing Antibody Responses Induced by the Third Vaccination
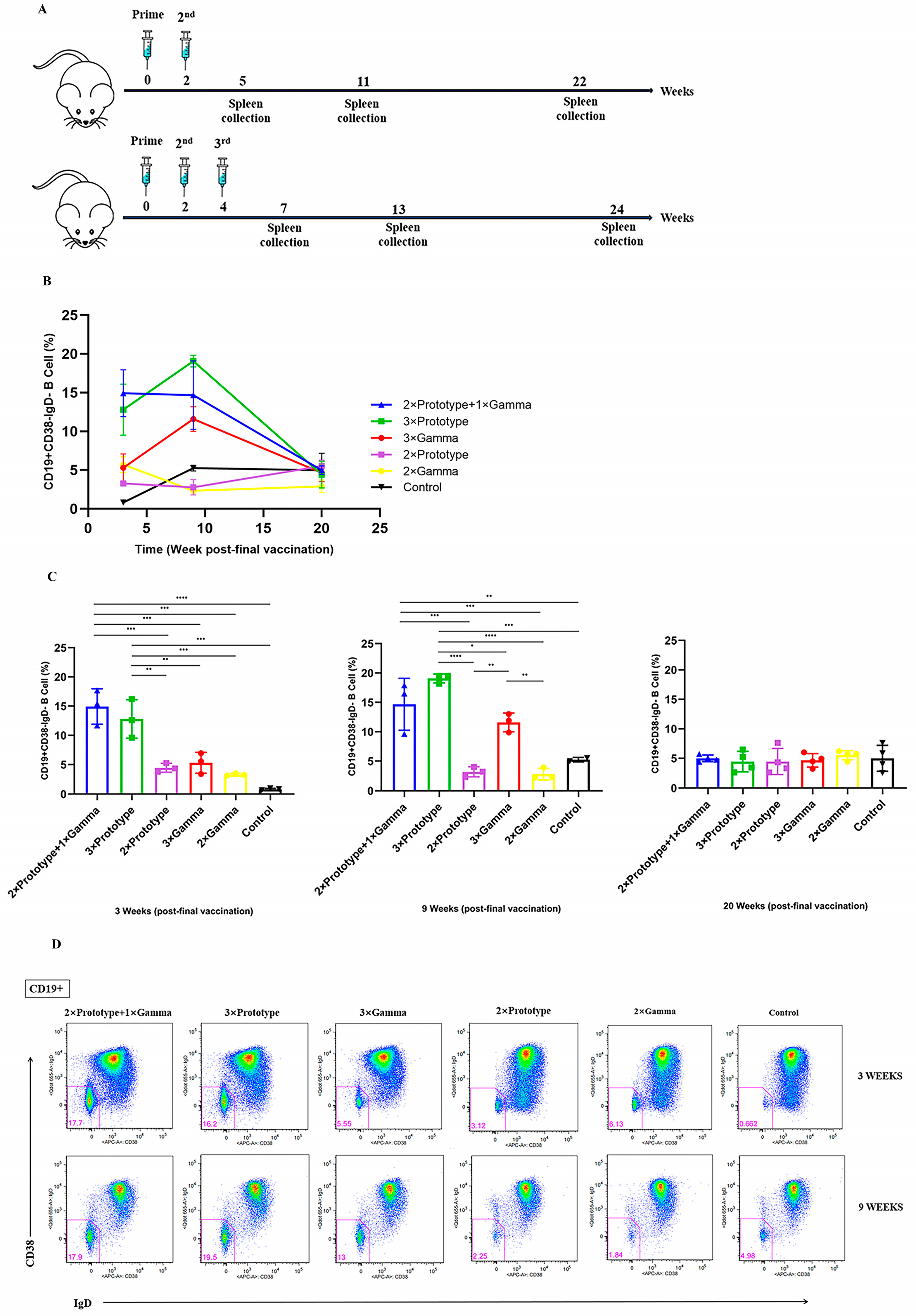
3.3. Activation of Memory B Cells by the Third Vaccination
3.4. Higher SARS-CoV-2-Specific T Cell Responses Elicited by Sequential Immunization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Vaccine Tracker and Landscape. 2023. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 30 March 2023).
- Ren, W.; Sun, H.; Gao, G.F.; Chen, J.; Sun, S.; Zhao, R.; Gao, G.; Hu, Y.; Zhao, G.; Chen, Y.; et al. Recombinant SARS-CoV-2 spike S1-Fc fusion protein induced high levels of neutralizing responses in nonhuman primates-ScienceDirect. Vaccine 2020, 38, 5653–5658. [Google Scholar] [CrossRef]
- Cohen, J. Vaccine designers take first shots at COVID-19. Science 2020, 368, 14–16. [Google Scholar] [CrossRef] [Green Version]
- Burki, T.K. Omicron variant and booster COVID-19 vaccines. Lancet Respir. Med. 2022, 10, e17. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Vaccine brands can be mixed in “extremely rare occasions”, says Public Health England. BMJ 2021, 372, n12. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Repulic of China. Available online: http://www.nhc.gov.cn/xcs/yqfkdt/202212/acd8ba68d934488983909e81642dc337.shtml (accessed on 1 December 2022).
- International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health. VIEW-hub. Available online: http://www.view-hub.org (accessed on 18 April 2023).
- Qin, C.; Du, M.; Wang, Y.; Liu, Q.; Yan, W.; Tao, L.; Liu, M.; Liu, J. Assessing acceptability of the fourth dose against COVID-19 among Chinese adults: A population-based survey. Hum. Vaccin Immunother. 2023, 19, 2186108. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.J.; McKay, P.F.; Belij-Rammerstorfer, S.; Ulaszewska, M.; Bissett, C.D.; Hu, K.; Samnuan, K.; Blakney, A.K.; Wright, D.; Sharpe, H.R.; et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat. Commun. 2021, 12, 2893. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- World Health Organization. Interim Recommendations for Heterologous COVID-19 Vaccine Schedules. 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-heterologous-schedules (accessed on 18 April 2023).
- Sapkota, B.; Saud, B.; Shrestha, R.; Al-Fahad, D.; Sah, R.; Shrestha, S.; Rodriguez-Morales, A.J. Heterologous prime-boost strategies for COVID-19 vaccines. J. Travel. Med. 2022, 29, taab191. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, L.; Guo, X.; Jin, P.; Wu, S.; Zhu, J.; Pan, H.; Wang, X.; Song, Z.; Wan, J.; et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: A randomized phase 4 trial. Nat. Med. 2022, 28, 401–409. [Google Scholar] [CrossRef]
- He, Q.; Mao, Q.; An, C.; Zhang, J.; Gao, F.; Bian, L.; Li, C.; Liang, Z.; Xu, M.; Wang, J. Heterologous prime–boost: Breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg. Microbes Infect. 2021, 10, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Then, E.; Lucas, C.; Monteiro, V.S.; Miric, M.; Brache, V.; Cochon, L.; Vogels, C.B.F.; Malik, A.A.; De la Cruz, E.; Jorge, A. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat. Med. 2022, 28, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Otter, A.D.; Lin, K.M.; Muñoz Sandoval, D.; Pieper, F.P.; Butler, D.K.; Liu, S.; Joy, G.; et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science 2022, 377, eabq1841. [Google Scholar] [CrossRef] [PubMed]
- Fierz, W.; Walz, B. Antibody Dependent Enhancement Due to Original Antigenic Sin and the Development of SARS. Front. Immunol. 2020, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.L.; Essigmann, H.T. Original Antigenic Sin: The Downside of Immunological Memory and Implications for COVID-19. mSphere 2021, 6, e00056-21. [Google Scholar] [CrossRef]
- Noori, M.; Nejadghaderi, S.A.; Rezaei, N. “Original antigenic sin”: A potential threat beyond the development of booster vaccination against novel SARS-CoV-2 variants. Infect. Control. Hosp. Epidemiol. 2022, 43, 1091–1092. [Google Scholar] [CrossRef]
- Gao, B.; He, L.; Bao, Y.; Chen, Y.; Lu, G.; Zhang, Y.; Xu, Y.; Su, B.; Xu, J.; Wang, Y.; et al. Repeated vaccination of inactivated SARS-CoV-2 vaccine dampens neutralizing antibodies against Omicron variants in breakthrough infection. Cell Res. 2023, 25, 258–261. [Google Scholar] [CrossRef]
- Güzel, E.Ç.; Çelikkol, A.; Erdal, B.; Sedef, N. Immunogenicity after CoronaVac vaccination. Rev. Da Assoc. Med. Bras. 2021, 67, 1403–1408. [Google Scholar] [CrossRef]
- Kremsner, P.G.; Mann, P.; Kroidl, A.; Leroux-Roels, I.; Schindler, C.; Gabor, J.J.; Schunk, M.; Leroux-Roels, G.; Bosch, J.J.; Fendel, R.; et al. Safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2: A phase 1 randomized clinical trial. Wien. Klin. Wochenschr. 2021, 133, 931–941. [Google Scholar] [CrossRef]
- China Food and Drug Administration. Conditional Use Approval for CoronaVac. 2021. Available online: https://wwwnmpagovcn/yaopin/ypjgdt/20210206154636109html (accessed on 2 February 2021).
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2022, 22, 483–495. [Google Scholar] [CrossRef]
- Li, M.; Yang, J.; Wang, L.; Wu, Q.; Wu, Z.; Zheng, W.; Wang, L.; Lu, W.; Deng, X.; Peng, C.; et al. A booster dose is immunogenic and will be needed for older adults who have completed two doses vaccination with CoronaVac: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Zhao, X.; Li, D.; Ruan, W.; Chen, Z.; Zhang, R.; Zheng, A.; Qiao, S.; Zheng, X.; Zhao, Y.; Dai, L.; et al. Effects of a Prolonged Booster Interval on Neutralization of Omicron Variant. N. Engl. J. Med. 2022, 386, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, L.; Guo, X.; Jin, P.; Wu, S.; Zhu, J.; Pan, H.; Wang, X.; Song, Z.; Wan, J.; et al. Heterologous prime-boost immunization with CoronaVac and Convidecia. medRxiv 2021. [Google Scholar] [CrossRef]
- Wanlapakorn, N.; Suntronwong, N.; Phowatthanasathian, H.; Yorsaeng, R.; Vichaiwattana, P.; Thongmee, T.; Auphimai, C.; Srimuan, D.; Thatsanatorn, T.; Assawakosri, S.; et al. Safety and immunogenicity of heterologous and homologous inactivated and adenoviral-vectored COVID-19 vaccine regimens in healthy adults: A prospective cohort study. Hum. Vaccin. Immunother. 2022, 18, 2029111. [Google Scholar] [CrossRef]
- Andrews, S.F.; Chambers, M.J.; Schramm, C.A.; Plyler, J.; Raab, J.E.; Kanekiyo, M.; Gillespie, R.A.; Ransier, A.; Darko, S.; Hu, J.; et al. Activation Dynamics and immunoglobulin evolution of pre-existing and newly generated human memory B cell responses to influenza hemagglutinin. Immunity 2019, 51, 398–410.e5. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, W.; Lou, Z.; Zhao, Y.; Zhang, J.; Liang, H.; Li, N.; Zhu, X.; Ding, L.; Huang, B.; et al. Vaccination with Omicron Inactivated Vaccine in Pre-vaccinated Mice Protects against SARS-CoV-2 Prototype and Omicron Variants. Vaccines 2022, 10, 1149. [Google Scholar] [CrossRef]
- Jin, L.; Li, Z.; Zhang, X.; Li, J.; Zhu, F. CoronaVac: A review of efficacy, safety, and immunogenicity of the inactivated vaccine against SARS-CoV-2. Hum. Vaccin. Immunother. 2022, 18, 2096970. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Hitchings, M.D.T.; Dorion, M.; D’Agostini, T.L.; de Paula, R.C.; de Paula, O.F.P.; Villela, E.F.M.; Torres, M.S.S.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of COVID-19 in Brazil: Test negative case-control study. BMJ 2021, 374, n2015. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128, Erratum in Lancet 2022, 399, 142. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yisimayi, A.; Bai, Y.; Huang, W.; Li, X.; Zhang, Z.; Yuan, T.; An, R.; Wang, J.; Xiao, T.; et al. Humoral immune response to circulating SARS-CoV-2 variants elicited by inactivated and RBD-subunit vaccines. Cell Res. 2021, 31, 732–741. [Google Scholar]
- Harvey, R.A.; Rassen, J.A.; Kabelac, C.A.; Turenne, W.; Leonard, S.; Klesh, R.; Meyer, W.A., 3rd; Kaufman, H.W.; Anderson, S.; Cohen, O.; et al. Association of SARS-CoV-2 Seropositive Antibody Test with Risk of Future Infection. JAMA Intern. Med. 2021, 181, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, Q.; An, C.; Mao, Q.; Gao, F.; Bian, L.; Wu, X.; Wang, Q.; Liu, P.; Song, L.; et al. Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS-CoV-2 vaccine. Emerg. Microbes Infect. 2021, 10, 1598–1608. [Google Scholar] [CrossRef]
- Yue, L.; Zhou, J.; Zhou, Y.; Yang, X.; Xie, T.; Yang, M.; Zhao, H.; Zhao, Y.; Yang, T.; Li, H.; et al. Antibody response elicited by a third boost dose of inactivated SARS-CoV-2 vaccine can neutralize SARS-CoV-2 variants of concern. Emerg. Microbes Infect. 2021, 10, 2125–2127. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Peng, Y.; Du, N.; Lei, Y.; Dorje, S.; Qi, J.; Luo, T.; Gao, G.F.; Song, H. Structures of the SARS-CoV-2 nucleocapsid and their perspectives for drug design. EMBO J. 2020, 39, e105938. [Google Scholar] [CrossRef]
- Ai, J.; Wang, X.; He, X.; Zhao, X.; Zhang, Y.; Jiang, Y.; Li, M.; Cui, Y.; Chen, Y.; Qiao, R.; et al. Antibody evasion of SARS-CoV-2 Omicron BA.1, BA.1.1, BA.2, and BA.3 sub-lineages. Cell Host Microbe 2022, 30, 1077–1083.e4. [Google Scholar] [CrossRef]
- Juno, J.A.; Tan, H.X.; Lee, W.S.; Reynaldi, A.; Kelly, H.G.; Wragg, K.; Esterbauer, R.; Kent, H.E.; Batten, C.J.; Mordant, F.L.; et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020, 26, 1428–1434. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef]
- He, X.; Ding, L.; Cao, K.; Peng, H.; Gu, C.; Li, Y.; Li, D.; Dong, L.; Hong, X.; Wang, X.; et al. A human cell-based SARS-CoV-2 vaccine elicits potent neutralizing antibody responses and protects mice from SARS-CoV-2 challenge. Emerg. Microbes Infect. 2021, 10, 1555–1573. [Google Scholar] [CrossRef]
- Vaisman-Mentesh, A.; Dror, Y.; Tur-Kaspa, R.; Markovitch, D.; Kournos, T.; Dicker, D.; Wine, Y. SARS-CoV-2 specific memory B cells frequency in recovered patient remains stable while antibodies decay over time. medRxiv 2020. [Google Scholar] [CrossRef]
- Kreer, C.; Zehner, M.; Weber, T.; Ercanoglu, M.S.; Gieselmann, L.; Rohde, C.; Halwe, S.; Korenkov, M.; Schommers, P.; Vanshylla, K.; et al. Longitudinal Isolation of Potent Near-Germline SARS-CoV-2-Neutralizing Antibodies from COVID-19 Patients. Cell 2020, 182, 843–854.e12. [Google Scholar] [CrossRef] [PubMed]
- Kissler, S.M.; Tedijanto, C.; Goldstein, E.; Grad, Y.H.; Lipsitch, M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 2020, 368, 860–868. [Google Scholar] [CrossRef]
- Wilson, P.; Stamper, C.; Dugan, H.; Li, L.; Asby, N.; Halfmann, P. Distinct B cell subsets give rise to antigen-specific antibody responses against SARS-CoV-2. Res. Sq. 2020, 25, rs.3.rs-80476. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Lundgreen, K.A.; Apostolidis, S.A.; Baxter, A.E.; Giles, J.R.; Mathew, D.; Pattekar, A.; Reynaldi, A.; Khoury, D.S.; et al. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell 2022, 185, 1875–1887.e8. [Google Scholar] [CrossRef]
- Wang, K.; Cao, Y.R.; Zhou, Y.; Wu, J.; Jia, Z.; Hu, Y.; Yisimayi, A.; Fu, W.; Wang, L.; Liu, P.; et al. A third dose of inactivated vaccine augments the potency, breadth, and duration of anamnestic responses against SARS-CoV-2. medRxiv 2021. [Google Scholar] [CrossRef]
- Muecksch, F.; Wang, Z.; Cho, A.; Gaebler, C.; Ben Tanfous, T.; DaSilva, J.; Bednarski, E.; Ramos, V.; Zong, S.; Johnson, B.; et al. Increased Memory B Cell Potency and Breadth After a SARS-CoV-2 mRNA Boost. Nature 2022, 607, 128–134. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, S.; Tong, X.; Tao, Y.; Ni, J.; Pan, J.; Li, M.; Wan, Y.; Mao, M.; Xiong, Y.; et al. Dynamic SARS-CoV-2-specific B-cell and T-cell responses following immunization with an inactivated COVID-19 vaccine. Clin. Microbiol. Infect. 2022, 28, 410–418. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Karlsson, A.C.; Humbert, M.; Buggert, M. The known unknowns of T cell immunity to COVID-19. Sci. Immunol. 2020, 5, eabe8063. [Google Scholar] [CrossRef]
- Kent, S.J.; Khoury, D.S.; Reynaldi, A.; Juno, J.A.; Wheatley, A.K.; Stadler, E.; John, W.E.; Triccas, J.; Sasson, S.C.; Cromer, D.; et al. Disentangling the relative importance of T cell responses in COVID-19: Leading actors or supporting cast? Nat. Rev. Immunol. 2022, 22, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Kim, C.U.; Seo, S.H.; Kim, D.J. Current Status of COVID-19 Vaccine Development: Focusing on Antigen Design and Clinical Trials on Later Stages. Immune. Netw. 2021, 21, e4. [Google Scholar] [CrossRef] [PubMed]
- Dagotto, G.; Yu, J.; Barouch, D.H. Approaches and Challenges in SARS-CoV-2 Vaccine Development. Cell Host Microbe 2020, 28, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Gagne, M.; Moliva, J.I.; Foulds, K.E.; Andrew, S.F.; Flynn, B.J.; Werner, A.P.; Wagner, D.A.; Teng, I.T.; Lin, B.C.; Moore, C.; et al. mRNA-1273 or mRNA-Omicron boost in vaccinated macaques elicits similar B cell expansion, neutralizing responses, and protection from Omicron. Cell 2022, 185, 1556–1571.e18. [Google Scholar] [CrossRef]

| Abbreviation | Type of Vaccine | Vaccine Platform Description | Dose (SU/mL) |
|---|---|---|---|
| Prototype strain vaccine | COVID-19 Vaccine (Vero cell), Inactivated CoronaVac [32] | Inactivated virus | 1200 |
| Gamma strain vaccine | COVID-19 Vaccine (Vero cell), Inactivated, Gamma strain | Inactivated virus | 1200 |
| Group | 1st | 2nd | 3rd |
|---|---|---|---|
| Negative control | PBS | PBS | PBS |
| 2 × Prototype + 1 × Gamma | Prototype strain vaccine | Prototype strain vaccine | Gamma strain vaccine |
| 3 × Prototype | Prototype strain vaccine | Prototype strain vaccine | Prototype strain vaccine |
| 3 × Gamma | Gamma strain vaccine | Gamma strain vaccine | Gamma strain vaccine |
| 2 × Prototype | Prototype strain vaccine | Prototype strain vaccine | |
| 2 × Gamma | Gamma strain vaccine | Gamma strain vaccine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, S.; Liu, Y.; Wang, S.; Peng, H.; Hao, Y.; Hong, K.; Li, D.; Shao, Y. Sequential Administration of SARS-CoV-2 Strains-Based Vaccines Effectively Induces Potent Immune Responses against Previously Unexposed Omicron Strain. Pathogens 2023, 12, 655. https://doi.org/10.3390/pathogens12050655
Wang Q, Wang S, Liu Y, Wang S, Peng H, Hao Y, Hong K, Li D, Shao Y. Sequential Administration of SARS-CoV-2 Strains-Based Vaccines Effectively Induces Potent Immune Responses against Previously Unexposed Omicron Strain. Pathogens. 2023; 12(5):655. https://doi.org/10.3390/pathogens12050655
Chicago/Turabian StyleWang, Qianying, Shuhui Wang, Ying Liu, Shuo Wang, Hong Peng, Yanling Hao, Kunxue Hong, Dan Li, and Yiming Shao. 2023. "Sequential Administration of SARS-CoV-2 Strains-Based Vaccines Effectively Induces Potent Immune Responses against Previously Unexposed Omicron Strain" Pathogens 12, no. 5: 655. https://doi.org/10.3390/pathogens12050655
APA StyleWang, Q., Wang, S., Liu, Y., Wang, S., Peng, H., Hao, Y., Hong, K., Li, D., & Shao, Y. (2023). Sequential Administration of SARS-CoV-2 Strains-Based Vaccines Effectively Induces Potent Immune Responses against Previously Unexposed Omicron Strain. Pathogens, 12(5), 655. https://doi.org/10.3390/pathogens12050655






