New Insights on the Neglected Avian Nematode Hystrichis tricolor: Hystrichiosis-Induced Proventriculitis in Synanthropic Egyptian Geese (Alopochen aegyptiaca Linnaeus, 1766) in Germany
Abstract
1. Introduction
2. Methods
2.1. Macroscopic and Microscopic Analysis
2.1.1. Coprological Analysis
2.1.2. Histopathological Analyses
2.1.3. Scanning Electron Microscopy (SEM) Analysis
2.2. Molecular Analyses
2.2.1. Sequencing
2.2.2. Phylogenetic Analyses
3. Results
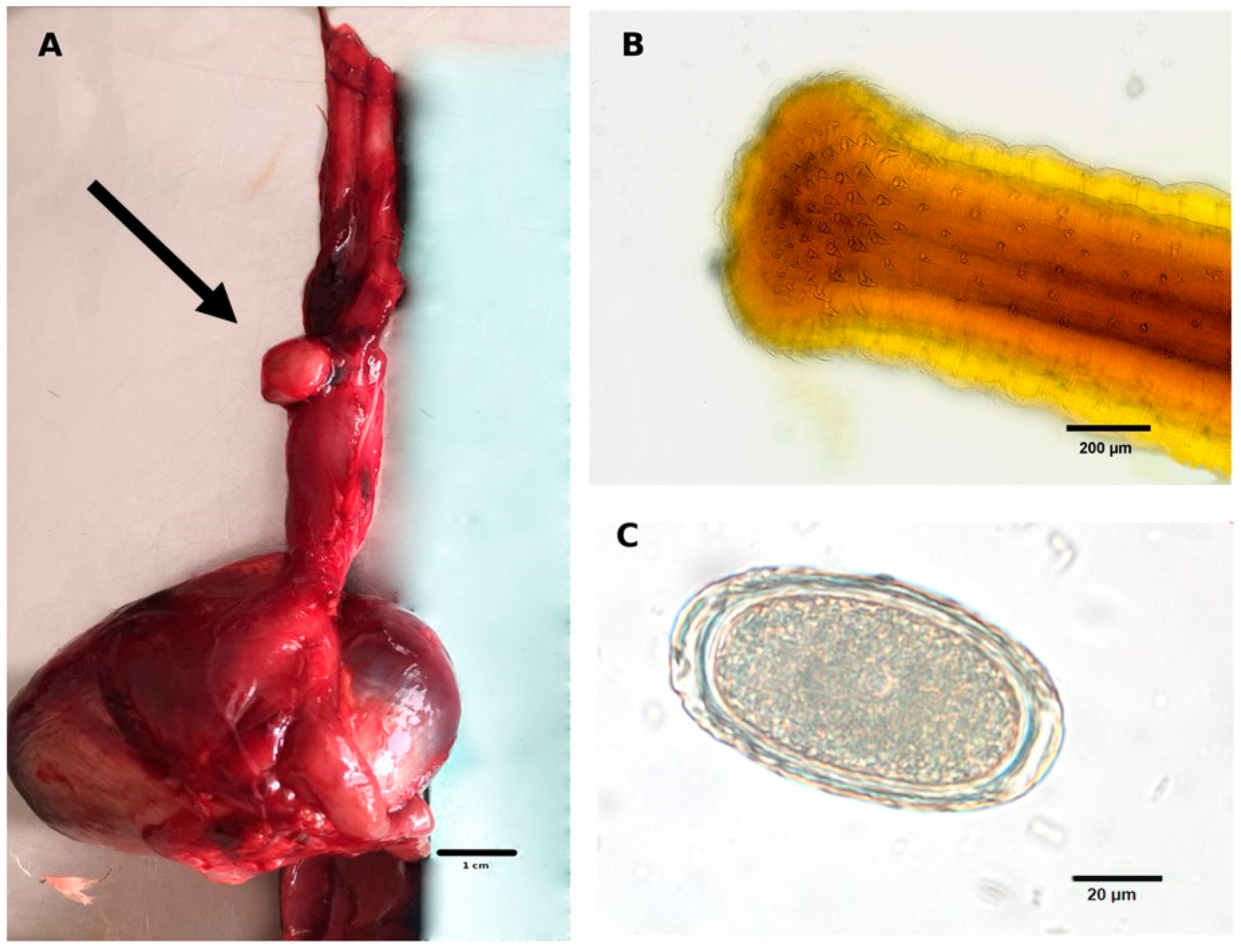
3.1. Macroscopic Findings
3.2. Microscopic Findings
3.2.1. Coprological Findings
3.2.2. Adult Nematodes
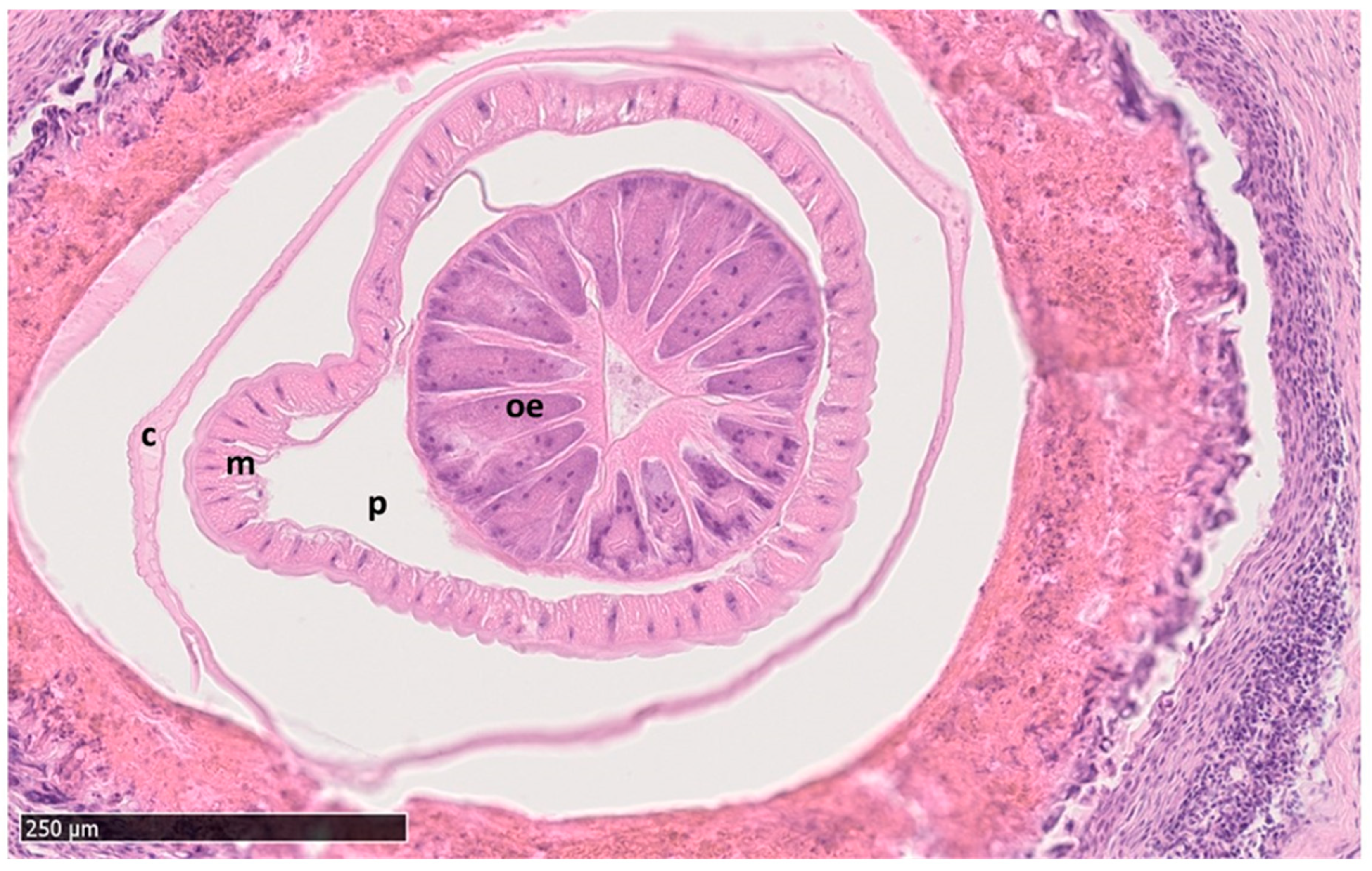
3.2.3. Histopathological Findings
3.2.4. Scanning Electron Microscopy (SEM)
3.3. Molecular Findings
Sequence and Phylogenetic Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt-Rhaesa, A. (Ed.) Gastrotricha, Cycloneuralia and Gnathifera: Nematoda; De Gruyter: Berlin, Germany, 2014; ISBN 9783110274257. [Google Scholar]
- McDonald, M.E. Key to Nematodes Reported in Waterfowl; Department of the interior, Bureau of Sport Fisheries and Wildlife: Washington, DC, USA, 1974. [Google Scholar]
- Avery, R.A. Helminth parasites of wildfowl from Slimbridge, Gloucestershire. I. Parasites of captive Anatidae. J. Helminthol. 1966, 40, 269–280. [Google Scholar] [CrossRef]
- Gharagozlou, M.J.; Mobedi, I.; Aghaebrahimi Samani, R.; Taghizadeh, F.; Mowlavi, G. Esophageal-Crop Capillariasis and Proventriculus Ventriculus Hystrichisiasis in a Migratory Duck (Anas crecca). Iran. J. Parasitol. 2019, 14, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kavetska, K.M.; Pilarczyk, B.; Królaczyk, K. Stomach Nematodes of Wild Ducks (Subfamily Anatinae) Wintering in the North-Western Poland. Bull. Vet. Inst. Pulawy 2012, 56, 27–31. [Google Scholar] [CrossRef]
- Farias, J.D.; Canaris, A.G. Gastrointestinal helminths of the Mexican duck, Anas platyrhynchos diazi Ridgway, from north central Mexico and southwestern United States. J. Wildl. Dis. 1986, 22, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Rommel, M.; Eckert, J.; Kutzer, E.; Körting, W.; Schnieder, T.; Boch, J. Veterinärmeogie: 100 Tabellen, 5; vollst. neubearb. Aufl.; Parey: Berlin, Germany, 2000; ISBN 3-8263-3178-8. [Google Scholar]
- McDonald, M.E. Catalogue of Helminths of Waterfowl (Anatidae); Special scientific Report Wildlife No 126; Bureau of Sport Fisheries and Wildlife: Washington, DC, USA, 1969. [Google Scholar]
- Hendricks, L.D.; Harkema, R.; Miller, G.C. Hystrichis corvi sp. n. (Nematoda: Dioctophymatidae) from the Crow, and a Revised Key to the Species of Hystrichis. J. Parasitol. 1969, 55, 1201. [Google Scholar] [CrossRef]
- Kavetska, K.M.; Rzad, I.; Kornyushin, V.V.; Korol, E.N.; Sitko, J.; Szałańska, K. Helmintofauna przewodu pokarmowego krzyzówki Anas platyrhynchos L., 1758 północno-zachodniej Polski. Wiad. Parazytol. 2008, 54, 23–29. [Google Scholar] [PubMed]
- Kinsella, J.M.; Hon, L.T.; Reed, P.B. A Comparison of the Helminth Parasites of the Common Gallinule (Gallinula chloropus cachinnans) and the Purple Gallinule (Porphyrula martinica) in Florida. Am. Midl. Nat. 1973, 89, 467. [Google Scholar] [CrossRef]
- Canaris, A.G.; Ortiz, R.; Canaris, G.J. A predictable suite of helminth parasites in the long-billed dowitcher, Limnodromus scolopaceus, from the Chihuahua desert in Texas and Mexico. J. Parasitol. 2010, 96, 1060–1065. [Google Scholar] [CrossRef]
- Zukovic, M.; Mikacic, D. Vorkommen und Bestimmung von Parasiten in den Organen von Huhn, Pute, Gans, und Ente bei Sektion und Schlachtung. Tierärztliche Praxis 1976, 4, 317–338. [Google Scholar]
- Bernauer, D.; Jansen, W. Recent invasions of alien macroinvertebrates and loss of native species in the upper Rhine River, Germany. Aquat. Invasions 2006, 1, 55–71. [Google Scholar] [CrossRef]
- Hart, A.L.; Downs, C.T. Introduction. In Invasive Birds: Global Trends and Impacts; Downs, C.T., Hart, L.A., Eds.; CABI: Wallingford, UK; Boston, MA, USA, 2020; ISBN 9781789242065. [Google Scholar]
- Svensson, L. Der Kosmos Vogelführer: Alle Arten Europas, Nordafrikas und Vorderasiens; Neue Einbandgestaltung 2017, 2. Auflage; Kosmos: Stuttgart, Germany, 2017; ISBN 978-3-440-15635-3. [Google Scholar]
- Brown, L.H.; Urban, E.K.; Newman, K. The Birds of Africa: Volume 1; Academic Press Inc. (London) Ltd.: London, UK, 1982; ISBN 0-12-137301-0. [Google Scholar]
- Huysentruyt, F.; Callaghan, C.T.; Strubbe, D.; Winston, K.; Adriaens, T.; Brooks, D.M. Egyptian Goose (Alopochen aegyptiaca Linnaeus, 1766). In Invasive Birds: Global Trends and Impacts; Downs, C.T., Hart, L.A., Eds.; CABI: Wallingford, UK; Boston, MA, USA, 2020; ISBN 9781789242065. [Google Scholar]
- Sraml, M.; Christidis, L.; Easteal, S.; Horn, P.; Collet, C. Molecular Relationships Within Australasian Waterfowl (Anseriformes). Aust. J. Zool. 1996, 44, 47. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Allport, G. The distribution and ecology of naturalized Egyptian Geese Alopochen aegyptiacus in Britain. Bird Study 1991, 38, 128–134. [Google Scholar] [CrossRef]
- Gyimesi, A.; Lensink, R. Risk Analysis of the Egyptian Goose in the Netherlands; Bureau Waardenburg bv: Culemborg, The Netherlands, 2010. [Google Scholar]
- Koehler, A.V.A.; Hoberg, E.P.; Torres-Pérez, F.; Cook, J.A. A Molecular View of the Superfamily Dioctophymatoidea (Nematoda). Comp. Parasitol. 2009, 76, 100–104. [Google Scholar] [CrossRef]
- DNASTAR. Lasergene Genomics|NGS Analysis Software|DNASTAR. Available online: https://www.dnastar.com/software/lasergene/genomics/ (accessed on 31 January 2022).
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- Holmes, S. Bootstrapping Phylogenetic Trees: Theory and Methods. Statist. Sci. 2003, 18, 241–255. [Google Scholar] [CrossRef]
- Romeo, C.; Cafiso, A.; Fesce, E.; Martinez-Rondan, F.J.; Panzeri, M.; Ferrari, N. Hystrichis Tricolor Isolate racIT03 Small Subunit Ribosomal RNA Gene, Partial Sequence: GenBank: MT141133.1. Available online: https://www.ncbi.nlm.nih.gov/nuccore/1818403297 (accessed on 20 April 2023).
- Romeo, C.; Cafiso, A.; Fesce, E.; Martínez-Rondán, F.J.; Panzeri, M.; Martinoli, A.; Cappai, N.; Defilippis, G.; Ferrari, N. Lost and found: Helminths infecting invasive raccoons introduced to Italy. Parasitol. Int. 2021, 83, 102354. [Google Scholar] [CrossRef]
- Wobeser, G.A. Parasitism: Costs und Effects. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, D.B., Eds.; Wiley-Blackwell: Oxford, UK, 2008; ISBN 0813804574. [Google Scholar]
- Poulin, R. Evolutionary Ecology of Parasites, 2nd ed.; Princeton University Press: Princeton, NJ, USA; Woodstock, GA, USA, 2007; ISBN 978-0-691-12085-0. [Google Scholar]
- Lachish, S.; Knowles, S.C.L.; Alves, R.; Wood, M.J.; Sheldon, B.C. Fitness effects of endemic malaria infections in a wild bird population: The importance of ecological structure. J. Anim. Ecol. 2011, 80, 1196–1206. [Google Scholar] [CrossRef]
- Toft, C.A. Current theory of host-parasite interactions. In Bird-Parasite Interactions: Ecology, Evolution and Behaviour; Loye, J.E., Zuk, M., Eds.; Oxford University Press: New York, NY, USA, 1991; ISBN 0-19-857738-9. [Google Scholar]
- Scheer, S.; Macedo, M.R.P.; Soares, M.P.; Schramm, C.C.; Muller, G. Pathology and morphometry of Hystrichis acanthocephalicus (Nematoda) from Phimosus infuscatus (Pelecaniformes) in southern Brazil. Rev. Bras. Parasitol. Vet. 2017, 26, 34–38. [Google Scholar] [CrossRef]
- Ferreira, V.L.; Medeiros, F.P.; July, J.R.; Raso, T.F. Dioctophyma renale in a dog: Clinical diagnosis and surgical treatment. Vet. Parasitol. 2010, 168, 151–155. [Google Scholar] [CrossRef]
- Spalding, M.G.; Forrester, D.J. Pathogenesis of Eustrongylides ignotus (Nematoda: Dioctophymatoidea) in Ciconiiformes. J. Wildl. Dis. 1993, 29, 250–260. [Google Scholar] [CrossRef]
- Xiong, F.; Wang, G.T.; Wu, S.G.; Nie, P. Development of Eustrongylides ignotus (Nematoda: Dioctophmida) in domestic ducks (Anas platyrhynchos domestica (L.)). J. Parasitol. 2009, 95, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Sorci, G.; Skarstein, F.; Morand, S.; Hugot, J.-P. Correlated evolution between host immunity and parasite life histories in primates and oxyurid parasites. Proc. Biol. Sci. 2003, 270, 2481–2484. [Google Scholar] [CrossRef] [PubMed]
- Rossin, M.A.; Poulin, R.; Timi, J.T.; Malizia, A.-I. Causes of inter-individual variation in reproductive strategies of the parasitic nematode Graphidioides subterraneus. Parasitol. Res. 2005, 96, 335–339. [Google Scholar] [CrossRef]
- Stear, M.J.; Bairden, K.; Duncan, J.L.; Holmes, P.H.; McKellar, Q.A.; Park, M.; Strain, S.; Murray, M.; Bishop, S.C.; Gettinby, G. How hosts control worms. Nature 1997, 389, 27. [Google Scholar] [CrossRef]
- Skorping, A.; Read, A.F.; Keymer, A.E. Life History Covariation in Intestinal Nematodes of Mammals. Oikos 1991, 60, 365. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, E.F.; Schlohsarczyk, E.K.; Gröf, M.; Gärtner, U.; Taubert, A.; Hermosilla, C. New Insights on the Neglected Avian Nematode Hystrichis tricolor: Hystrichiosis-Induced Proventriculitis in Synanthropic Egyptian Geese (Alopochen aegyptiaca Linnaeus, 1766) in Germany. Pathogens 2023, 12, 663. https://doi.org/10.3390/pathogens12050663
Fischer EF, Schlohsarczyk EK, Gröf M, Gärtner U, Taubert A, Hermosilla C. New Insights on the Neglected Avian Nematode Hystrichis tricolor: Hystrichiosis-Induced Proventriculitis in Synanthropic Egyptian Geese (Alopochen aegyptiaca Linnaeus, 1766) in Germany. Pathogens. 2023; 12(5):663. https://doi.org/10.3390/pathogens12050663
Chicago/Turabian StyleFischer, Ella F., Elfi K. Schlohsarczyk, Manuela Gröf, Ulrich Gärtner, Anja Taubert, and Carlos Hermosilla. 2023. "New Insights on the Neglected Avian Nematode Hystrichis tricolor: Hystrichiosis-Induced Proventriculitis in Synanthropic Egyptian Geese (Alopochen aegyptiaca Linnaeus, 1766) in Germany" Pathogens 12, no. 5: 663. https://doi.org/10.3390/pathogens12050663
APA StyleFischer, E. F., Schlohsarczyk, E. K., Gröf, M., Gärtner, U., Taubert, A., & Hermosilla, C. (2023). New Insights on the Neglected Avian Nematode Hystrichis tricolor: Hystrichiosis-Induced Proventriculitis in Synanthropic Egyptian Geese (Alopochen aegyptiaca Linnaeus, 1766) in Germany. Pathogens, 12(5), 663. https://doi.org/10.3390/pathogens12050663







