Comparative Analysis of Human Hepatic Lesions in Dengue, Yellow Fever, and Chikungunya: Revisiting Histopathological Changes in the Light of Modern Knowledge of Cell Pathology
Abstract
1. Introduction
2. Materials and Methods
2.1. Histological Analysis
2.2. Immunohistochemistry
2.3. Molecular Biology
2.4. Statistical Analysis
3. Results
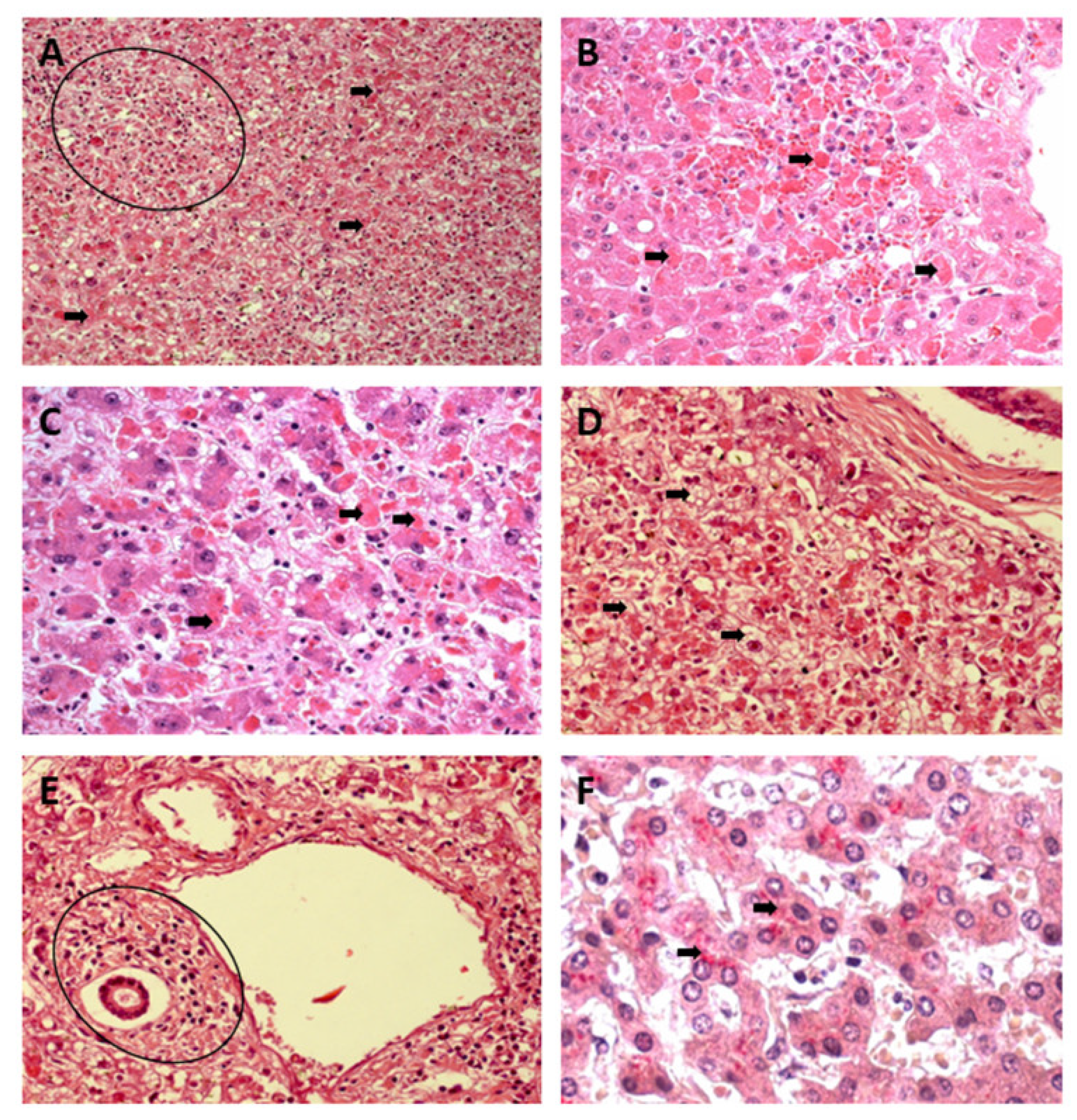
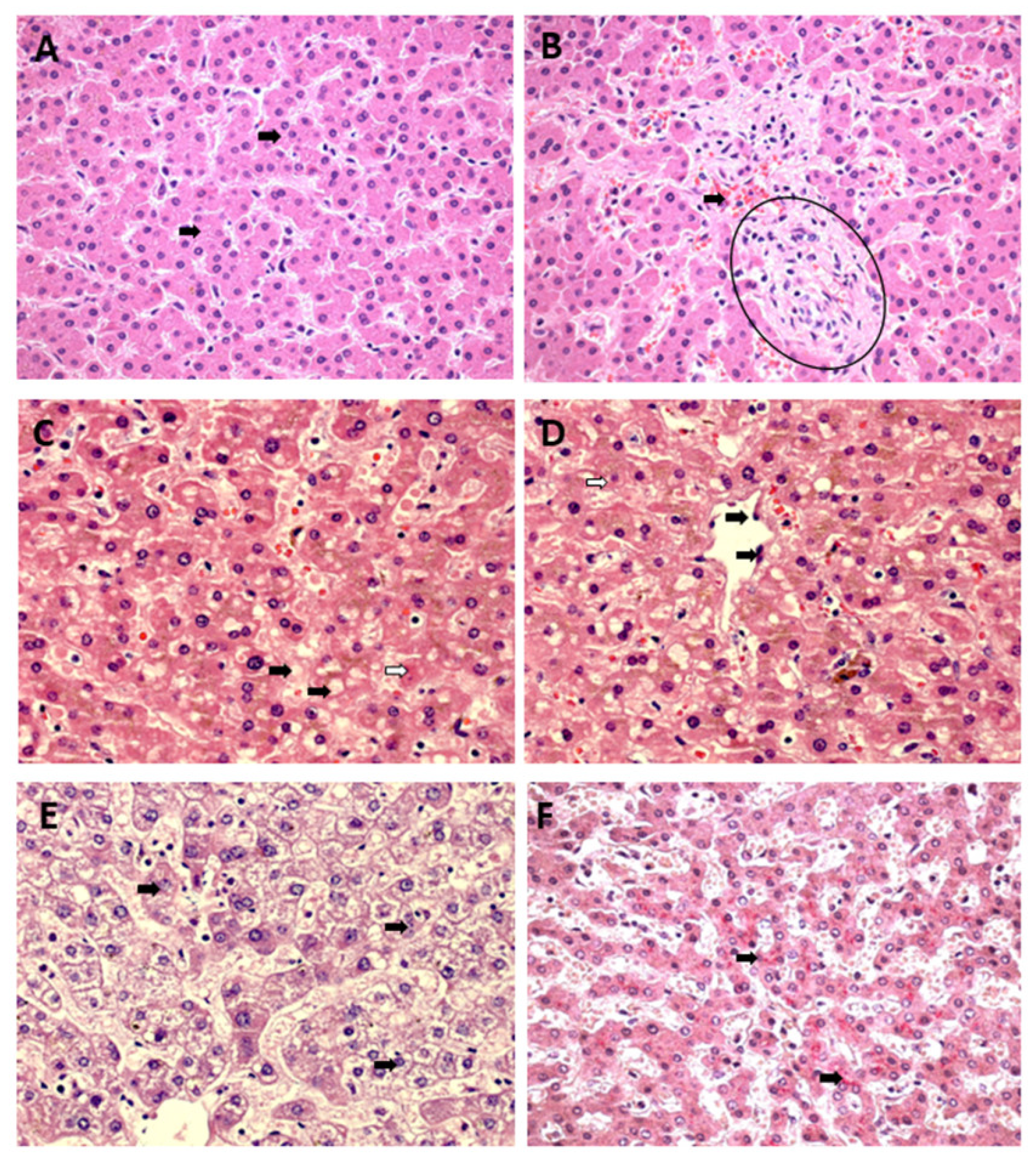
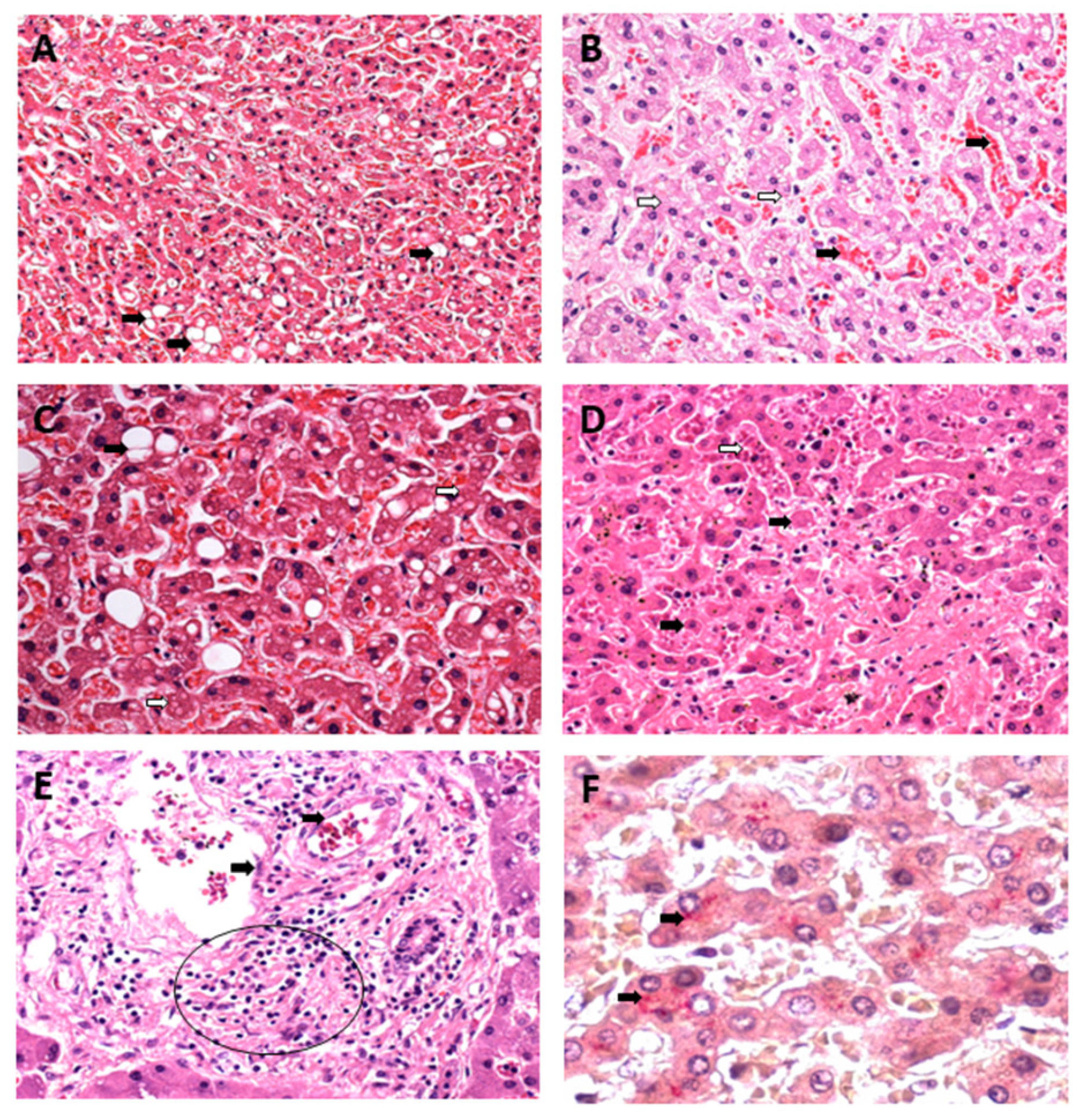
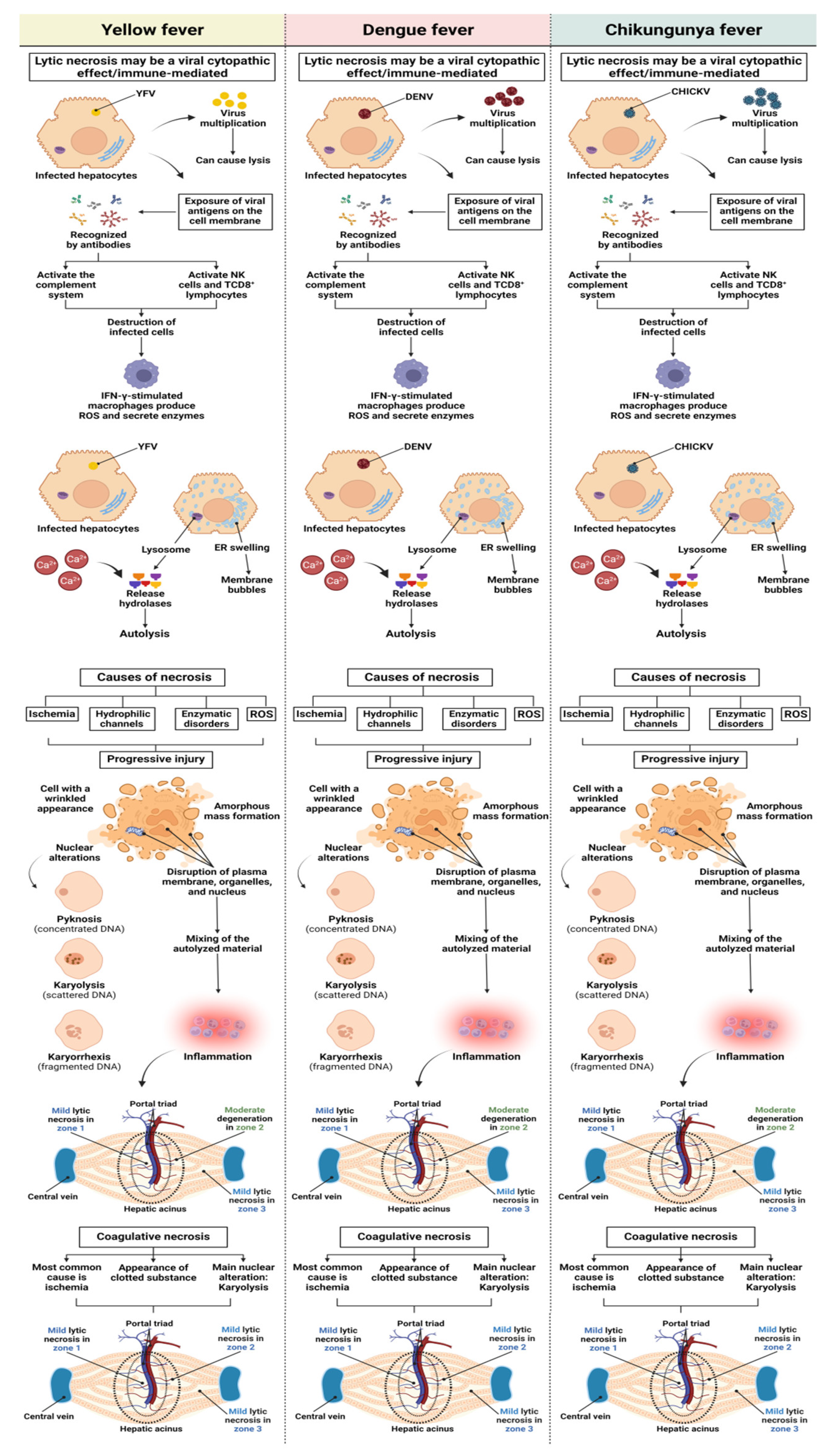
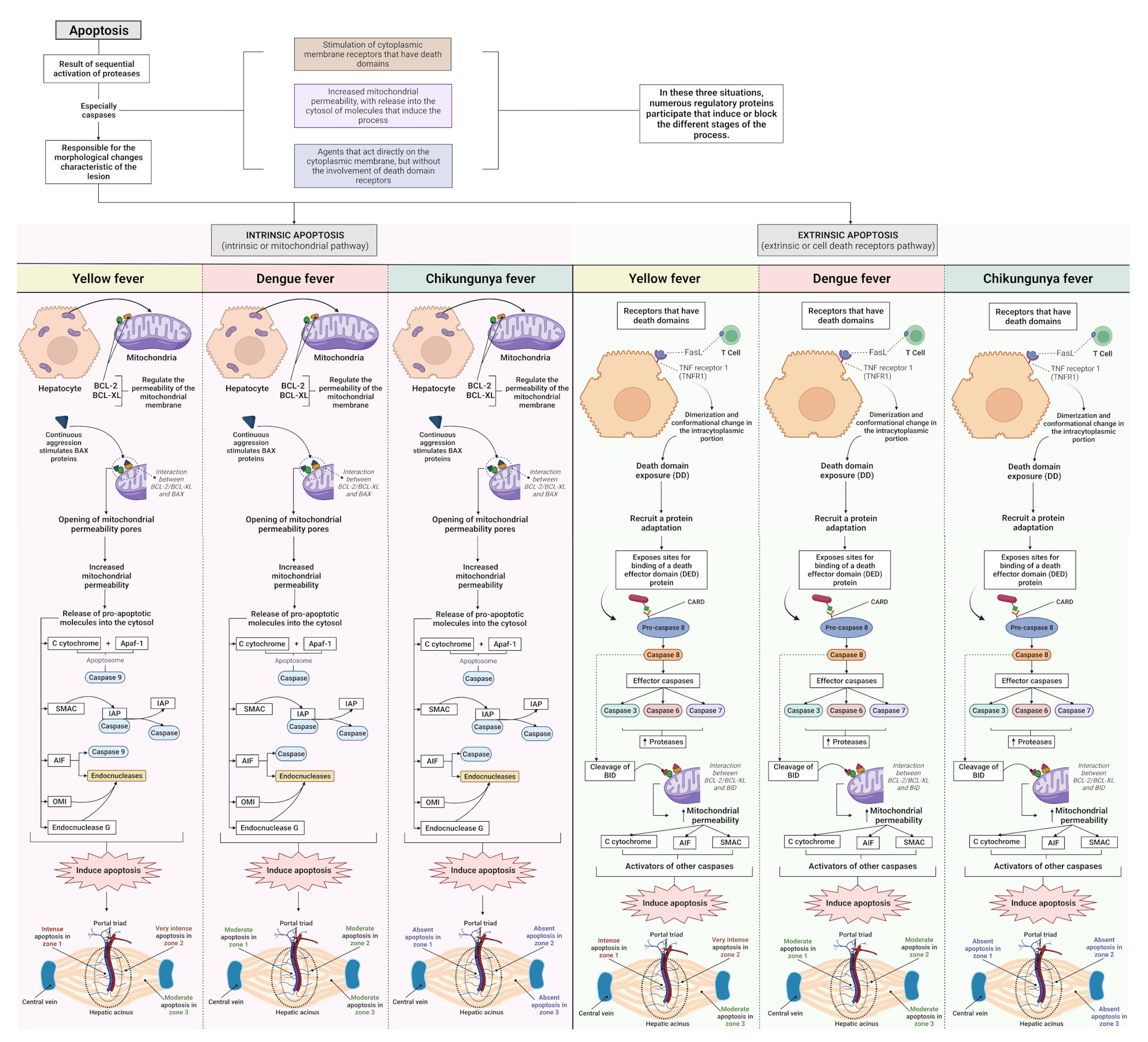
3.1. Qualitative Histology
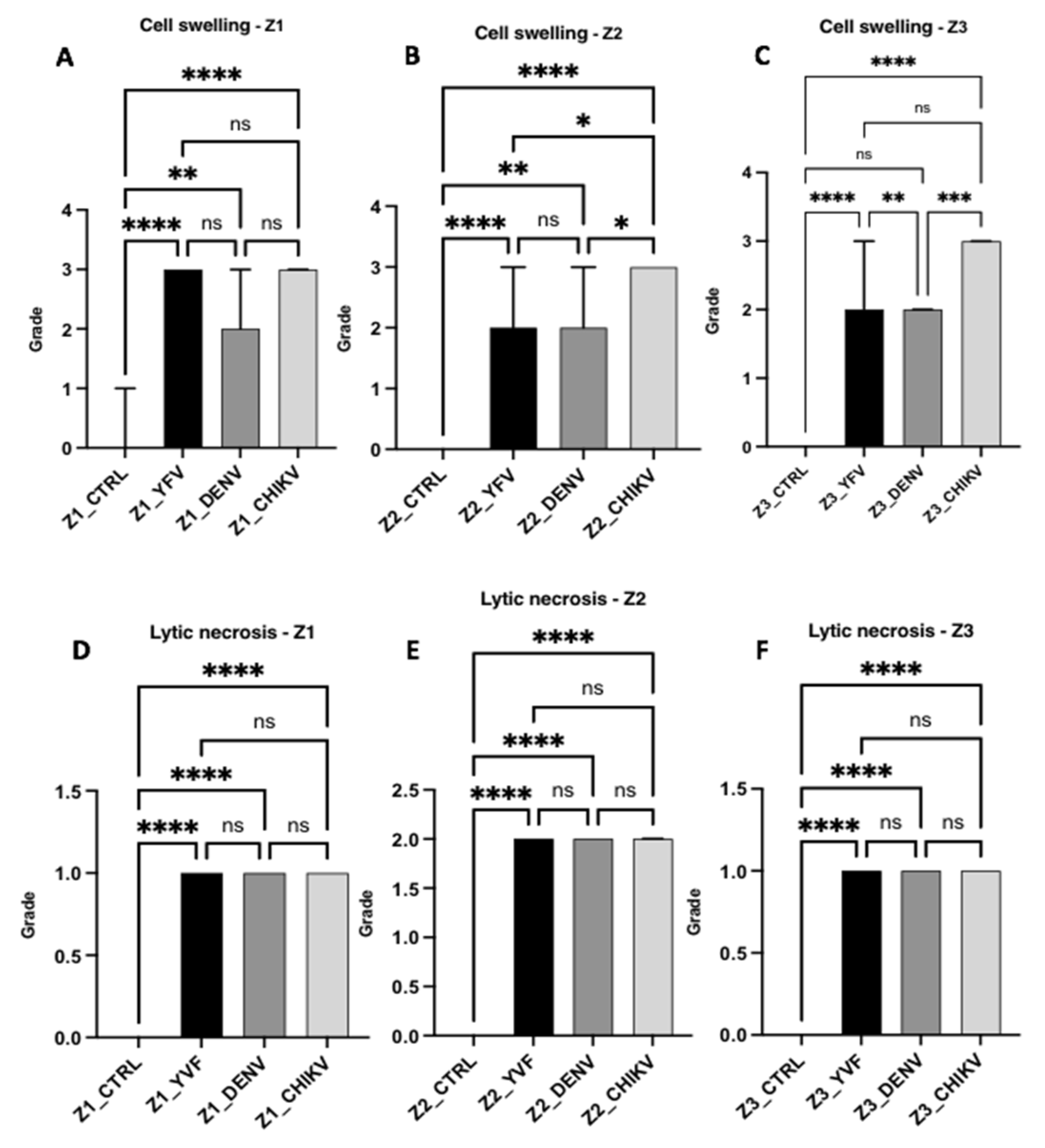
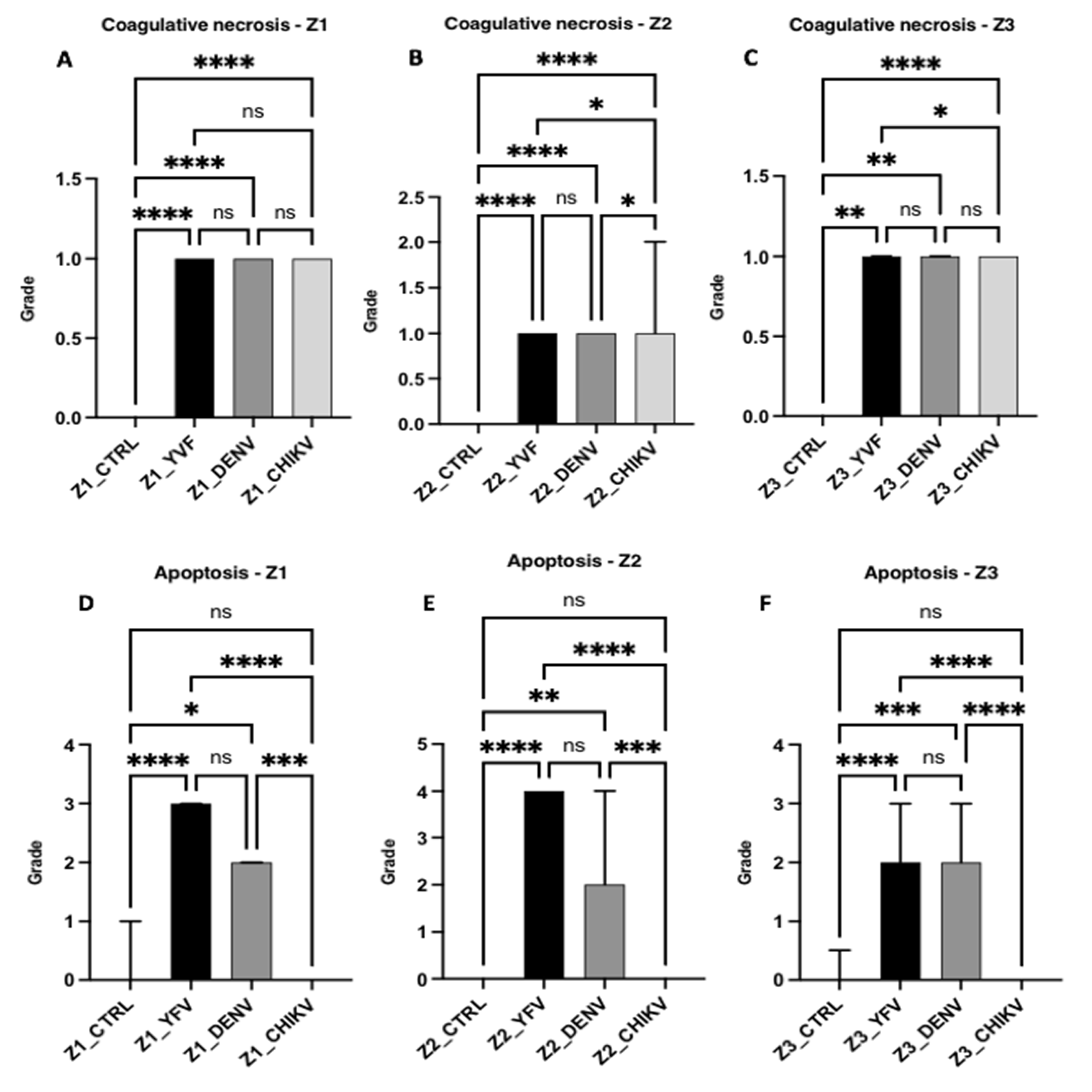
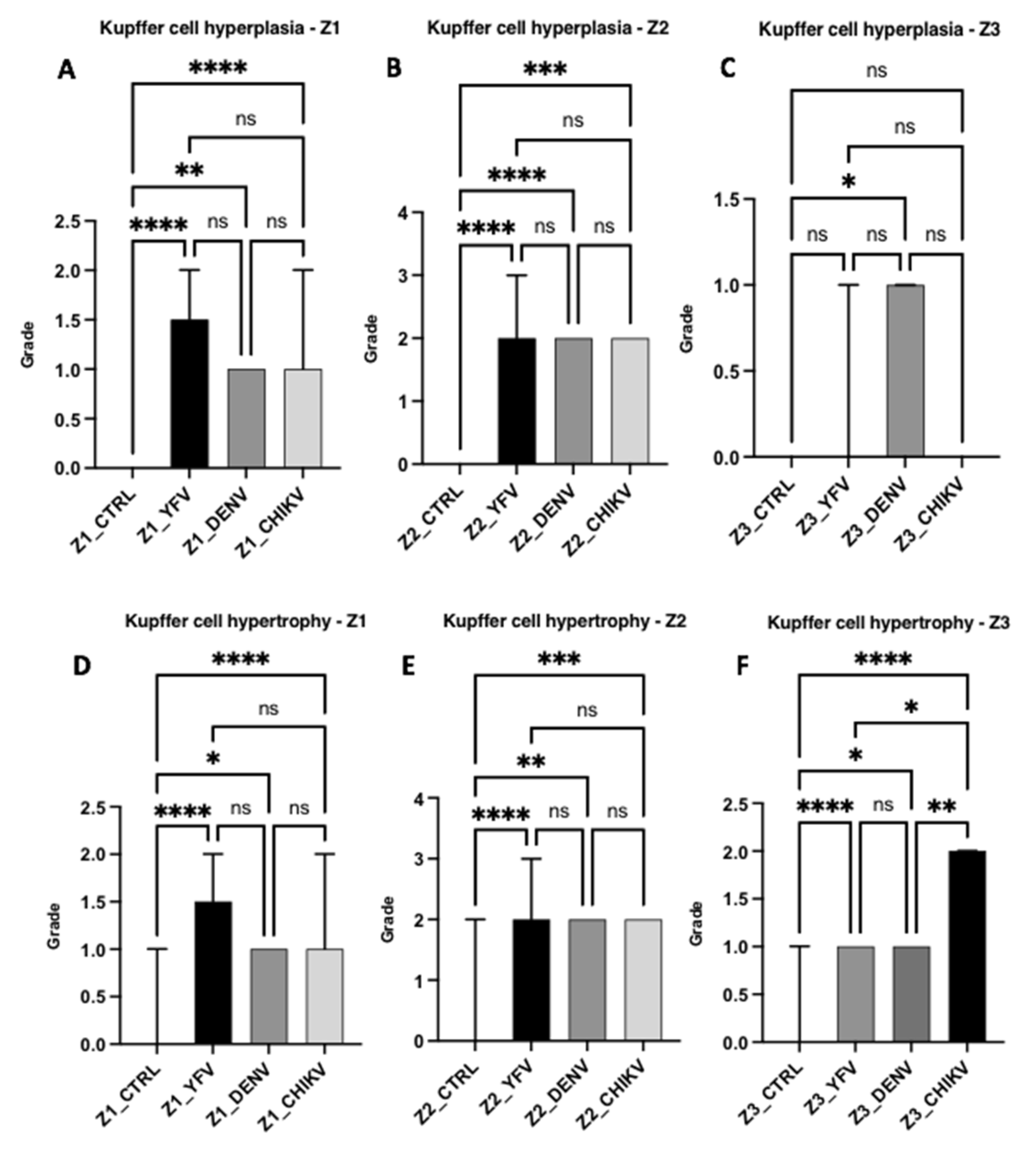
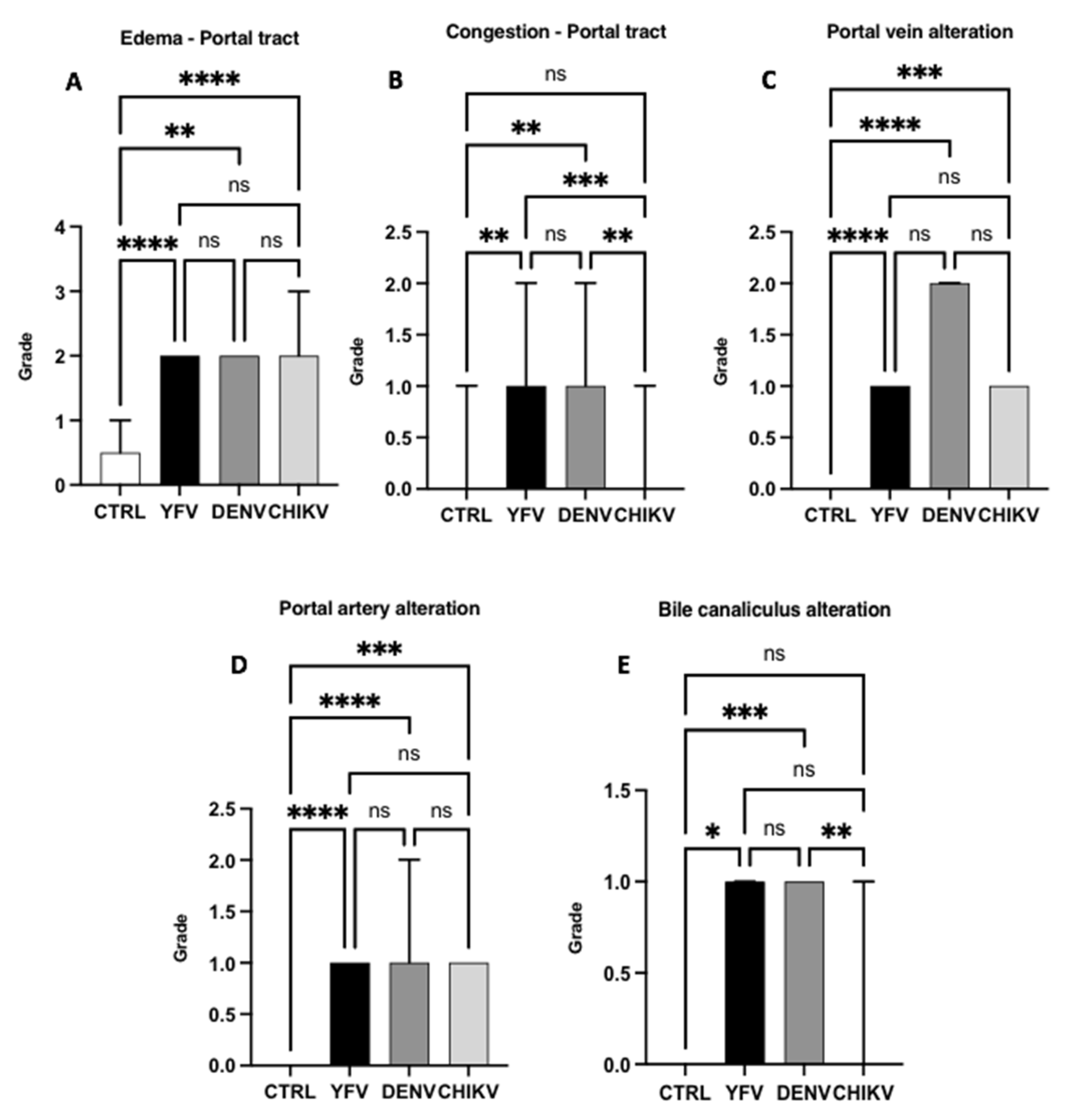
3.2. Semiquantitative Histology
Histopathological Changes in Hepatic Acinus
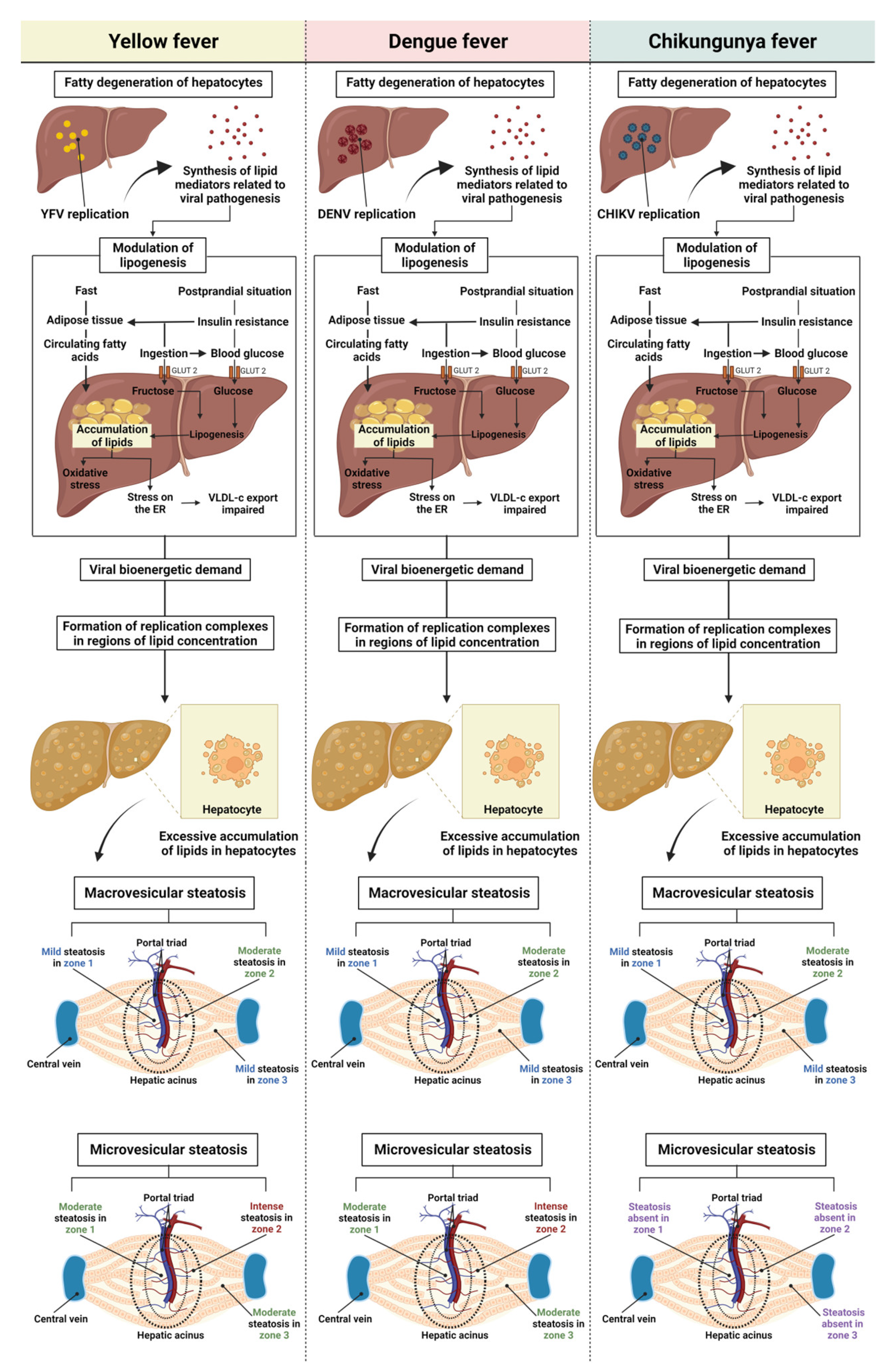
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, P.R. Arboviruses: A Family on the Move. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1062, pp. 1–10. [Google Scholar] [CrossRef]
- Halstead, S.B. Travelling arboviruses: A historical perspective. Travel Med. Infect. Dis. 2019, 31, 101471. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Higgs, S.; Vanlandingham, D.L. Arbovirus-Mosquito Vector-Host Interactions and the Impact on Transmission and Disease Pathogenesis of Arboviruses. Front. Microbiol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.L.; Cheng, Q.; Marshall, J.M.; Hu, W.B.; Yang, Z.C.; Lu, J.H. Imported cases and minimum temperature drive dengue transmission in Guangzhou, China: Evidence from ARIMAX model. Epidemiol. Infect. 2018, 146, 1226–1235. [Google Scholar] [CrossRef]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Mukhopadhyay, S.; Merits, A.; Bolling, B.; Nasar, F.; Coffey, L.L.; Powers, A.; Weaver, S.C. International Committee on Taxonomy of Viruses (ICTV) Virus Taxonomy Profile: Togaviridae. J. Gen. Virol. 2018, 99, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Hamlet, A.; Jean, K.; Perea, W.; Yactayo, S.; Biey, J.; Van Kerkhove, M.; Ferguson, N.; Garske, T. The seasonal influence of climate and environment on yellow fever transmission across Africa. PLoS Negl. Trop. Dis. 2018, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti vector competence studies: A review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef]
- Goes de Jesus, J.; Gräf, T.; Giovanetti, M.; Mares-Guia, M.A.; Xavier, J.; Lima Maia, M.; Fonseca, V.; Fabri, A.; Dos Santos, R.F.; Mota Pereira, F.; et al. Yellow fever transmission in non-human primates, Bahia, Northeastern Brazil. PLoS Negl. Trop. Dis. 2020, 14, 8. [Google Scholar] [CrossRef]
- Auguste, A.J.; Lemey, P.; Bergren, N.A.; Giambalvo, D.; Moncada, M.; Morón, D.; Hernandez, R.; Navarro, J.C.; Weaver, S.C. Enzootic Transmission of Yellow Fever Virus, Venezuela. Emerg. Infect. Dis. 2015, 21, 99–102. [Google Scholar] [CrossRef]
- Lovera, D.; Martínez-Cuellar, C.; Galeano, F.; Amarilla, S.; Vazquez, C.; Arbo, A. Clinical manifestations of primary and secondary dengue in Paraguay and its relation to virus serotype. J. Infect. Dev. Ctries. 2019, 13, 1127–1134. [Google Scholar] [CrossRef]
- Oliveira, L.G.; Peron, J.P.S. Viral receptors for flaviviruses: Not only gatekeepers. J. Leukoc. Biol. 2019, 106, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Kril, V.; Aïqui-Reboul-Paviet, O.; Briant, L.; Amara, A. New Insights into. Chikungunya Virus Infection and Pathogenesis. Annu. Rev. Virol. 2021, 8, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.S.; Pagliari, C.; Medeiros, D.B.A.; Duarte, M.I.S.; Vasconcelos, P.F.C. Immunity and immune response, pathology and pathologic changes: Progress and challenges in the immunopathology of yellow fever. Rev. Med. Virol. 2013, 23, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, L.; Lv, Y.; Zhang, W.; Li, J.; Zhang, Y.; Di, T.; Zhang, S.; Liu, J.; Li, J.; et al. A fatal yellow fever virus infection in China: Description and lessons. Emerg. Microbes Infect. 2016, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Yung, C.F.; Lee, K.S.; Thein, T.L.; Tan, L.K.; Gan, V.C.; Wong, J.G.; Lye, D.C.; Ng, L.C.; Leo, Y.S. Dengue serotype-specific differences in clinical manifestation, laboratory parameters and risk of severe disease in adults, Singapore. Am. J. Trop. Med. Hyg. 2015, 92, 999–1005. [Google Scholar] [CrossRef]
- Honório, N.A.; Câmara, D.C.P.; Calvet, G.A.; Brasil, P. Chikungunya: An arbovirus infection in the process of establishment and expansion in Brazil. Rep. Public Health 2015, 31, 906–908. [Google Scholar] [CrossRef]
- Gasque, P.; Bandjee, M.C.J.; Reyes, M.M.; Viasus, D. Chikungunya Pathogenesis: From the Clinics to the Bench. J. Infect. Dis. 2016, 214, S446–S448. [Google Scholar] [CrossRef]
- Póvoa, T.F.; Alves, A.M.B.; Oliveira, C.A.B.; Nuovo, G.J.; Chagas, V.L.A.; Paes, M.V. The Pathology of Severe Dengue in Multiple Organs of Human Fatal Cases: Histopathology, Ultrastructure and Virus Replication. PLoS ONE 2014, 9, e83386. [Google Scholar] [CrossRef]
- Kularatne, S.A.M.; Rajapakse, M.M.; Ralapanawa, U.; Waduge, R.; Pathirage, L.P.M.M.K.; Rajapakse, R.P.V.J. Heart and liver are infected in fatal cases of dengue: Three PCR based case studies. BMC Infect. Dis. 2018, 18, 681. [Google Scholar] [CrossRef]
- Mercado-Reyes, M.; Acosta-Reyes, J.; Navarro-Lechuga, E.; Corchuelo, S.; Rico, A.; Parra, E.; Tolosa, N.; Pardo, L.; González, M.; Martìn-Rodriguez-Hernández, J.; et al. Dengue, chikungunya and zika virus coinfection: Results of the national surveillance during the zika epidemic in Colombia. Epidemiol. Infect. 2019, 147, e77. [Google Scholar] [CrossRef]
- Sharp, T.M.; Keating, M.K.; Shieh, W.J.; Bhatnagar, J.; Bollweg, B.C.; Levine, R.; Blau, D.M.; Torres, J.V.; Rivera, A.; Perez-Padilla, J.; et al. Clinical Characteristics, Histopathology, and Tissue Immunolocalization of Chikungunya Virus Antigen in Fatal Cases. Clin. Infect. Dis. 2021, 73, e345–e354. [Google Scholar] [CrossRef] [PubMed]
- Ustafa, M.; Illzam, E.M.; Jeffree, M.S. Dengue Fever: Clinical Spectrum, And Management. IOSR JDMS 2017, 16, 53–59. [Google Scholar] [CrossRef]
- Chia, P.Y.; Thein, T.-L.; Ong, S.W.X.; Lye, D.C.; Leo, Y.S. Severe dengue and liver involvement: An overview and review of the literature. Expert Rev. Anti Infect. Ther. 2020, 18, 181–189. [Google Scholar] [CrossRef]
- Dissanayake, H.A.; Seneviratne, S.L. Liver involvement in dengue viral infections. Rev. Med. Virol. 2018, 28, e1971. [Google Scholar] [CrossRef]
- Valentine, M.J.; Murdock, C.C.; Kelly, P.J. Sylvatic cycles of arboviruses in non-human primates. Parasit. Vectors 2019, 12, 463. [Google Scholar] [CrossRef]
- Kerkhof, K.; Falconi-Agapito, F.; Van Esbroeck, M.; Talledo, M.; Ariën, K.K. Reliable Serological Diagnostic Tests for Arboviruses: Feasible or Utopia? Trends Microbiol. 2020, 28, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Araújo, L.J.T.D.; Gonzalez, L.L.; Buss, L.F.; Guerra, J.M.; Gomez, D.S.; Ferreira, C.S.D.S.; Cirqueira, C.S.; Ghillardi, F.; Witkin, S.S.; Sabino, E.C. Surveillance of hemorrhagic fever and/or neuroinvasive disease: Challenges of diagnosis. J. Public Health. 2021, 55, 41. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Jo, W.K.; Haage, V.; Moreira-Soto, A.; Filho, E.F.O.; Drexler, J.F. Challenges towards serologic diagnostics of emerging arboviroses. Clin. Microbiol. Infect. 2021, 27, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Pomari, E.; Deiana, M.; Perandin, F.; Caldrer, S.; Formenti, F.; Mistretta, M.; Orza, P.; Ragusa, A.; Piubelli, C. Molecular techniques for the genomic viral RNA detection of West Nile, Dengue, Zika and Chikungunya arboviruses: A narrative review. Expert Rev. Mol. Diagn. 2021, 21, 591–612. [Google Scholar] [CrossRef]
- Barrantes Murillo, D.F.; Piche-Ovares, M.; Gamboa-Solano, J.C.; Romero, L.M.; Soto-Garita, C.; Alfaro-Alarcón, A.; Corrales-Aguilar, E. Serological Positivity against Selected Flaviviruses and Alphaviruses in Free-Ranging Bats and Birds from Costa Rica Evidence Exposure to Arboviruses Seldom Reported Locally in Humans. Viruses 2022, 14, 93. [Google Scholar] [CrossRef]
- Campos, S.; Figueredo Thiel, S.; Bellassai, J.; Rodríguez, I. Immunohistochemistry in the diagnosis of yellow fever. Experience in the Department of Pathology, Health Sciences Research Institute and the Department of Pathology, Faculty of Medical Sciences, National University of Asuncion. Mem. Inst. Investig. Cienc. Salud 2009, 7, 33–39. [Google Scholar]
- Melo-Lima, B.L.; Espósito, D.L.A.; Lopes da Fonseca, B.A.; Figueiredo, L.T.M.; Moreau, P.; Donadi, E.A. The Attenuated Live Yellow Fever Virus 17D Infects the Thymus and Induces Thymic Transcriptional Modifications of Immunomodulatory Genes in C57BL/6 and BALB/C Mice. Autoimmune Dis. 2015, 2015, 503087. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Guzman, H.; Da Rosa, A.P.T.; Vasconcelos, P.F.; Dias, L.B.; Bunnell, J.E.; Zhang, H.; Xiao, S.Y. Experimental yellow fever virus infection in the Golden Hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J. Infect. Dis. 2001, 183, 1431–1436. [Google Scholar] [CrossRef]
- Quaresma, J.A.; Barros, V.L.; Pagliari, C.; Fernandes, E.R.; Guedes, F.; Takakura, C.F.; Andrade, H.F., Jr.; Vasconcelos, P.F.; Duarte, M.I. Revisiting the liver in human yellow fever: Virus-induced apoptosis in hepatocytes associated with TGF-beta, TNF-alpha and NK cells activity. Virology 2006, 345, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.S.; Barros, V.L.R.S.; Fernandes, E.R.; Pagliari, C.; Guedes, F.; da Costa Vasconcelos, P.F.; de Andrade Junior, H.F.; Duarte, M.I.S. Immunohistochemical examination of the role of Fas ligand and lymphocytes in the pathogenesis of human liver yellow fever. Virus Res. 2006, 116, 91–97. [Google Scholar] [CrossRef]
- Idirisinghe, K.A.P. Histopathological study of Dengue Haemorrhagic Fever. J. Diagn. Pathol. 2014, 8, 50–58. [Google Scholar] [CrossRef]
- Rathi, K.R.; Arora, M.M.; Sahai, K.; Tripathi, S.; Singh, S.P.; Raman, D.K.; Anand, K.B. Autopsy findings in fatal dengue haemorrhagic fever-06 Cases. Med. J. Armed Forces India 2013, 69, 254–259. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Ganga, D.; Menon, M.; Kothari, K.; Singh, R. Dengue hepatitis with acute liver failure: Clinical, biochemical, histopathological characteristics and predictors of outcome. J. Gastroenterol. Hepat. 2020, 35, 1223–1228. [Google Scholar] [CrossRef]
- Dhanoa, A.; Hassan, S.S.; Ngim, C.F.; Lau, C.F.; Chan, T.S.; Adnan, N.A.A.; Eng, W.W.H.; Gan, H.M.; Rajasekaram, G. Impact of dengue virus (DENV) co-infection on clinical manifestations, disease severity and laboratory parameters. BMC Infect. Dis. 2016, 16, 406. [Google Scholar] [CrossRef]
- Londono-Renteria, B.; Marinez-Angarita, J.C.; Troupin, A.; Colpitts, T.M. Role of Mast Cells in Dengue Virus Pathogenesis. DNA Cell. Biol. 2017, 36, 423–427. [Google Scholar] [CrossRef]
- Gaythorpe, K.A.; Hamlet, A.; Jean, K.; Garkauskas Ramos, D.; Cibrelus, L.; Garske, T.; Ferguson, N. The global burden of yellow fever. eLife 2021, 10, e64670. [Google Scholar] [CrossRef]
- Quaresma, J.A.S.; Barros, V.L.R.S.; Fernandes, E.R.; Pagliari, C.; Takakura, C.; da Costa Vasconcelos, P.F.; de Andrade, H.F., Jr.; Duarte, M.I.S. Reconsideration of histopathology and ultrastructural aspects of the human liver in yellow fever. Acta Trop. 2005, 94, 116–127. [Google Scholar] [CrossRef]
- Azevedo, R.S.; Araujo, M.T.; Oliveira, C.S.; Martins Filho, A.J.; Nunes, B.T.; Henriques, D.F.; Silva, E.V.; Carvalho, V.L.; Chiang, J.O.; Martins, L.C.; et al. Zika Virus Epidemic in Brazil. II. Post-Mortem Analyses of Neonates with Microcephaly, Stillbirths, and Miscarriage. J. Clin. Med. 2018, 7, 496. [Google Scholar] [CrossRef]
- Goodman, A.G.; Rasmussen, A.L. Editorial: Host-Pathogen Interactions during Arboviral Infections. Front. Cell. Infect. Microbiol. 2019, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Akhrymuk, I.; Kulemzin, S.V.; Frolova, E.I. Evasion of the Innate Immune Response: The Old World Alphavirus nsP2 Protein Induces Rapid Degradation of Rpb1, a Catalytic Subunit of RNA Polymerase II. J. Virol. 2012, 86, 7180–7191. [Google Scholar] [CrossRef]
- Randall, G. Lipid Droplet Metabolism during Dengue Virus Infection. Trends Microbiol. 2018, 26, 640–642. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, Y.; Li, M.Y.; Lamers, M.M.; Fusade-Boyer, M.; Klemm, E.; Thiele, C.; Ashour, J.; Sanyal, S. Flaviviruses Exploit the Lipid Droplet Protein AUP1 to Trigger Lipophagy and Drive Virus Production. Cell. Host Microbe. 2018, 23, 819–831.e5. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Gyeltshen, S.; Chaijaroenkul, W.; Na-Bangchang, K. Significance of Autophagy in Dengue Virus Infection: A Brief Review. Am. J. Trop. Med. Hyg. 2019, 100, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Akhrymuk, I.; Lukash, T.; Frolov, I.; Frolova, E.I. Novel Mutations in nsP2 Abolish Chikungunya Virus-Induced Transcriptional Shutoff and Make the Virus Less Cytopathic without Affecting Its Replication Rates. J. Virol. 2019, 93, e02062-18. [Google Scholar] [CrossRef]
- Bovay, A.; Marraco, S.A.F.; Speiser, D.E. Yellow fever virus vaccination: An emblematic model to elucidate robust human immune responses. Hum. Vaccines Immunother. 2021, 17, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Mercado, M.; Acosta-Reyes, J.; Parra, E.; Pardo, L.; Rico, A.; Campo, A.; Navarro, E.; Viasus, D. Clinical and histopathological features of fatal cases with dengue and chikungunya virus co-infection in Colombia, 2014 to 2015. Eurosurveillance 2016, 21, 22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, C.F.; Wan, S.W.; Chen, M.C.; Lin, S.C.; Cheng, C.C.; Chiu, S.C.; Hsiao, Y.L.; Lei, H.Y.; Liu, H.S.; Yeh, T.M.; et al. Liver injury caused by antibodies against dengue virus nonstructural protein 1 in a murine model. Lab. Invest. 2008, 88, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Paes, M.V.; Lenzi, H.L.; Nogueira, A.C.M.; Nuovo, G.J.; Pinhao, A.T.; Mota, E.M.; Basilio-de-Oliveira, C.A.; Schatzmayr, H.; Barth, O.M.; de Barcelos Alves, A.M. Hepatic damage associated with dengue-2 virus replication in liver cells of BALB/c mice. Lab. Investig. 2009, 89, 1140–1151. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Gao, N.; Wang, Y.; Tian, Y.; Wu, J.; Zhang, J.; Zhu, J.; Fan, D.; An, J. Inhibitory effect of glutathione on oxidative liver injury induced by dengue virus serotype 2 infections in mice. PLoS ONE 2013, 8, e55407. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, F.; Josset, L.; Girke, T.; Park, B.; Barron, A.; Dewane, J.; Hammarlund, E.; Lewis, A.; Axthelm, M.K.; Slifka, M.K.; et al. Pathophysiologic and Transcriptomic Analyses of Viscerotropic Yellow Fever in a Rhesus Macaque Model. PLoS Negl. Trop. Dis. 2014, 8, e3295. [Google Scholar] [CrossRef] [PubMed]
- Drumond, B.P.; Fagundes, L.G.D.S.; Rocha, R.P.; Fumagalli, M.J.; Araki, C.S.; Colombo, T.E.; Nogueira, M.L.; Castilho, T.E.; Silveira, N.J.F.D.; Malaquias, L.C.C.; et al. Phylogenetic analysis of Dengue virus 1 isolated from South Minas Gerais, Brazil. Braz. J. Microbiol. 2016, 47, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Sakinah, S.; Priya, S.P.; Kumari, S.; Amira, F.; Poorani, K.; Alsaeedy, H.; Ling, M.P.; Chee, H.Y.; Higuchi, A.; Alarfaj, A.A.; et al. Impact of dengue virus (serotype DENV-2) infection on liver of BALB/c mice: A histopathological analysis. Tissue Cell 2017, 49, 86–94. [Google Scholar] [CrossRef]
- Nogueira, R.M.R.; Schatzmayr, H.G.; De Filippis, A.M.B.; Dos Santos, F.B.; Da Cunha, R.V.; Coelho, J.O.; De Souza, L.J.; Guimarães, F.R.; De Araújo, E.S.M.; De Simone, T.S.; et al. Dengue Virus Type 3, Brazil, 2002. Emerg. Infect. Dis. 2005, 11, 1376–1381. [Google Scholar] [CrossRef]
- Dias, L.B., Jr.; Alves, V.A.F.; Kanamura, C.; Oikawa, R.T.C.; Wakamatsu, A. Fulminant hepatic failure in northern Brazil: Morphological, immunohistochemical and pathogenic aspects of Lábrea hepatitis and yellow fever. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 831–839. [Google Scholar] [CrossRef]
- Pierrotti, L.C.; Duarte-Neto, A.N.; Song, A.T.W.; Ventura, C.G.; David-Neto, E.; Azevedo, L.S. Fatal Yellow Fever in a Kidney Transplant Patient. Clin. Infect. Dis. 2020, 70, 144–148. [Google Scholar] [CrossRef]
- Olímpio, F.A.; Falcão, L.F.M.; Carvalho, M.L.G.; da Costa Lopes, J.; Mendes, C.C.H.; Filho, A.J.M.; da Silva, C.A.M.; Miranda, V.D.S.C.; Santos, L.C.D.; da Silva Vilacoert, F.S.; et al. Endothelium Activation during Severe Yellow Fever Triggers an Intense Cytokine-Mediated Inflammatory Response in the Liver Parenchyma. Pathogens 2022, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, C.; Quaresma, J.A.S.; Fernandes, E.R.; Stegun, F.W.; Brasil, R.A.; de Andrade, H.F., Jr.; Barros, V.; Vasconcelos, P.F.C.; Duarte, M.I.S. Immunopathogenesis of dengue hemorrhagic fever: Contribution to the study of human liver lesions. J. Med. Virol. 2014, 86, 1193–1197. [Google Scholar] [CrossRef]
- Rivera, J.A.; Rengifo, A.C.; Parra, É.A.; Castellanos, J.E.; Caldas, M.L. Illustrated histopathological features of fatal dengue cases in Colombia. Biomedica 2020, 40, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Win, M.M.; Charngkaew, K.; Punyadee, N.; Aye, K.S.; Win, N.; Chaisri, U.; Chomanee, N.; Avirutnan, P.; Yoksan, S.; Malasit, P. Ultrastructural Features of Human Liver Specimens from Patients Who Died of Dengue Hemorrhagic Fever. Trop. Med. Infect. Dis. 2019, 4, 63. [Google Scholar] [CrossRef]
- Cui, L.; Lee, Y.H.; Kumar, Y.; Xu, F.; Lu, K.; Ooi, E.E.; Tannenbaum, S.R.; Ong, C.N. Serum Metabolome and Lipidome Changes in Adult Patients with Primary Dengue Infection. PLoS Negl. Trop. Dis. 2013, 7, e2373. [Google Scholar] [CrossRef]
- Murakami, M. Bioactive Lipid Mediators: Current Reviews and Protocols; Springer: Tokyo, Japan, 2015. [Google Scholar]
- Melo, C.F.O.R.; De Oliveira, D.N.; Lima, E.D.O.; Guerreiro, T.M.; Esteves, C.Z.; Beck, R.M.; Padilla, M.A.; Milanez, G.P.; Arns, C.W.; Proenca-Modena, J.L.; et al. A Lipidomics Approach in the Characterization of Zika-Infected Mosquito Cells: Potential Targets for Breaking the Transmission Cycle. PLoS ONE 2016, 11, e0164377. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Jia, J.; Zhang, B.; Mi, S.; Zhang, L.; Xie, X.; Guo, H.; Shi, J.; Tu, C. Serum Metabolomic Profiling of Piglets Infected with Virulent Classical Swine Fever Virus. Front. Microbiol. 2017, 8, 731. [Google Scholar] [CrossRef]
- Melo, C.F.O.R.; Delafiori, J.; Dabaja, M.Z.; de Oliveira, D.N.; Guerreiro, T.M.; Colombo, T.E.; Nogueira, M.L.; Proenca-Modena, J.L.; Catharino, R.R. The role of lipids in the inception, maintenance and complications of dengue virus infection. Sci. Rep. 2018, 8, 11826. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Heaton, N.S.; Randall, G. Dengue virus-induced autophagy regulates lipid metabolism. Cell. Host Microbe 2010, 8, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Sagan, S.M.; Rouleau, Y.; Leggiadro, C.; Supekova, L.; Schultz, P.G.; Su, A.I.; Pezacki, J.P. The influence of cholesterol and lipid metabolism on host cell structure and hepatitis C virus replication. Biochem. Cell. Biol. 2006, 4, 67–79. [Google Scholar] [CrossRef]
- Albulescu, L.; Wubbolts, R.; Van Kuppeveld, F.J.M.; Strating, J.R.P.M. Cholesterol shuttling is important for RNA replication of coxsackievirus B3 and encephalomyocarditis virus. Cell. Microbiol. 2015, 17, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.; Chukkapalli, V.; Nchoutmboube, J.A.; Li, J.; Randall, G.; Belov, G.A.; Wang, X. Positive-strand RNA viruses stimulate host phosphatidylcholine synthesis at viral replication sites. Proc. Natl. Acad. Sci. USA 2016, 113, E1064–E1073. [Google Scholar] [CrossRef] [PubMed]
- Stoeck, I.K.; Lee, J.Y.; Tabata, K.; Romero-Brey, I.; Paul, D.; Schult, P.; Lohmann, V.; Kaderali, L.; Bartenschlager, R. Hepatitis C Virus Replication Depends on Endosomal Cholesterol Homeostasis. J. Virol. 2017, 92, e01196-17. [Google Scholar] [CrossRef]
- Hong, C.; Tontonoz, P. Liver X receptors in lipid metabolism: Opportunities for drug Discovery. Nat. Rev. Drug Discov. 2014, 13, 433–444. [Google Scholar] [CrossRef]
- Howe, V.; Sharpe, L.J.; Alexopoulos, S.J.; Kunze, S.V.; Chua, N.K.; Li, D.; Brown, A.J. Cholesterol homeostasis: How do cells sense sterol excess? Chem. Phys. Lipids 2016, 199, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Wang, Y.; Fikrig, E. Inhibition of Chikungunya Virus Replication in Primary Human Fibroblasts by Liver X Receptor Agonist. Antimicrob. Agents Chemother. 2019, 63, e01220-19. [Google Scholar] [CrossRef]
- França, R.F.O.; Zucoloto, S.; Lopes da Fonseca, B.A. A BALB/c mouse model shows that liver involvement in dengue disease is immune-mediated. Exp. Mol. Pathol. 2010, 89, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Mascheretti, M.; Tengan, C.H.; Sato, H.K.; Suzuki, A.; Souza, R.P.D.; Maeda, M.; Brasil, R.; Pereira, M.; Tubaki, R.M.; Wanderley, D.; et al. Yellow fever: Reemerging in the state of Sao Paulo, Brazil, 2009. Public Health J. 2013, 47, 5. [Google Scholar] [CrossRef]
- Casadio, L.; Nastri, A.C.; Malta, F.M.; Araujo, J.; Silva, J.B.; Salomao, J.; Yamashiro, J.; Salles, A.P.; Gouvea, M.G.; Kanamura, C.; et al. Late-Onset Relapsing Hepatitis Associated with Yellow Fever. N. Engl. J. Med. 2020, 382, 2059–2061. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, A.; Wang, M.; Yin, Z.; Jia, R. The Dual Regulation of Apoptosis by Flavivirus. Front. Microbiol. 2021, 12, 654494. [Google Scholar] [CrossRef] [PubMed]
- Paes, M.V.; Pinhao, A.T.; Barreto, D.F.; Costa, S.M.; Oliveira, M.P.; Nogueira, A.C.; Takiya, C.M.; Farias-Filho, J.C.; Schatzmayr, H.G.; Alves, A.M.B.; et al. Liver injury and viremia in mice infected with dengue-2 virus. Virology 2005, 338, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Volkova, E.; Adams, A.P.; Forrester, N.; Xiao, S.Y.; Frolov, I.; Weaver, S.C. Chimeric Alphavirus Vaccine Candidates for Chikungunya. Vaccine 2008, 26, 5030–5039. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.A.; Lu, L.; Travassos da Rosa, A.P.A.; Xiao, S.-Y.; Tesh, R.B. An animal model for studying the pathogenesis of chikungunya virus infection. Am. J. Trop. Med. Hyg. 2008, 79, 133–139. [Google Scholar] [CrossRef]
- Mallilankaraman, K.; Shedlock, D.J.; Bao, H.; Kawalekar, O.U.; Fagone, P.; Ramanathan, A.A.; Ferraro, B.; Stabenow, J.; Vijayachari, P.; Sundaram, S.G.; et al. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl. Trop. Dis. 2011, 5, e928. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Joshi, G.; Nagar, D.P.; Sharma, A.K.; Sukumaran, D.; Pant, S.C.; Parida, M.M.; Dash, P.K. Mosquito saliva induced cutaneous events augment Chikungunya virus replication and disease progression. Infect. Genet. Evol. 2016, 40, 126–135. [Google Scholar] [CrossRef]
- Sitia, G.; Iannacone, M.; Aiolfi, R.; Isogawa, M.; van Rooijen, N.; Scozzesi, C.; Bianchi, M.E.; von Andrian, U.H.; Chisari, F.V.; Guidotti, L.G. Kupffer Cells Hasten Resolution of Liver Immunopathology in Mouse Models of Viral Hepatitis. PLoS Pathog. 2011, 7, e1002061. [Google Scholar] [CrossRef]
- Leowattana, W.; Leowattana, T. Dengue hemorrhagic fever and the liver. World J. Hepatol. 2021, 13, 1968–1976. [Google Scholar] [CrossRef]
- Bailey, A.L.; Kang, L.I.; de Assis Barros D’Elia Zanella, L.G.F.; Silveira, C.G.; Ho, Y.L.; Foquet, L.; Bial, G.; McCune, B.T.; Duarte-Neto, A.N.; Thomas, A.; et al. Consumptive coagulopathy of severe yellow fever occurs independently of hepatocellular tropism and massive hepatic injury. Proc. Natl. Acad. Sci. USA 2020, 117, 32648–32656. [Google Scholar] [CrossRef]
- Tuboi, S.H.; Costa, Z.G.A.; Vasconcelos, P.F.C.; Hatch, D. Clinical and epidemiological characteristics of yellow fever in Brazil: Analysis of reported cases 1998-2002. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 169–175. [Google Scholar] [CrossRef]
- Holz, L.E.; Bowen, D.G.; Bertolino, P. Mechanisms of T cell death in the liver: To Bim or not to Bim? Dig. Dis. 2010, 28, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Meertens, L.; Carnec, X.; Lecoin, M.P.; Ramdasi, R.; Guivel-Benhassine, F.; Lew, E.; Lemke, G.; Schwartz, O.; Amara, A. The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry. Cell. Host Microbe 2012, 12, 544–557. [Google Scholar] [CrossRef]
- Moller-Tank, S.; Kondratowicz, A.S.; Davey, R.A.; Rennert, P.D.; Maury, W. Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J. Virol. 2013, 87, 8327–8341. [Google Scholar] [CrossRef]
- Jemielity, S.; Wang, J.J.; Chan, Y.K.; Ahmed, A.A.; Li, W.; Monahan, S.; Bu, X.; Farzan, M.; Freeman, G.J.; Umetsu, D.T.; et al. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013, 9, e1003232. [Google Scholar] [CrossRef]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus Entry Receptors: An Update. Viruses 2014, 6, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Morizono, K.; Chen, I.S.Y. Role of Phosphatidylserine Receptors in Enveloped Virus Infection. J. Virol. 2014, 88, 4275–4290. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.; Mercer, J. Viral apoptotic mimicry. Nat. Rev. Microbiol. 2015, 13, 461–469. [Google Scholar] [CrossRef]
- Duarte-Neto, A.N.; Cunha, M.D.P.; Marcilio, I.; Song, A.T.; de Martino, R.B.; Ho, Y.L.; Pour, S.Z.; Dolhnikoff, M.; Saldiva, P.H.; Duarte, M.I.; et al. Yellow fever and orthotopic liver transplantation: New insights from the autopsy room for an old but re-emerging disease. Histopathology 2019, 75, 638–648. [Google Scholar] [CrossRef]
- Smith, D.R.; Khakpoor, A. Involvement of the liver in dengue infections. Dengue Bull. 2009, 33, 75–86. [Google Scholar]
- Aye, K.S.; Charngkaew, K.; Win, N.; Wai, K.Z.; Moe, K.; Punyadee, N.; Thiemmeca, S.; Suttitheptumrong, A.; Sukpanichnant, S.; Prida, M.; et al. Pathologic highlights of dengue hemorrhagic fever in 13 autopsy cases from Myanmar. Hum. Pathol. 2014, 45, 1221–1233. [Google Scholar] [CrossRef]
- Huerre, M.R.; Trong Lan, N.; Marianneau, P.; Bac Hue, N.; Khun, H.; Thanh Hung, N.; Thi Khen, N.; Drouet, M.T.; Que Huong, V.T.; Quang Ha, D.; et al. Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Virchows Arch. 2001, 438, 107–115. [Google Scholar] [CrossRef]
- Limonta, D.; Capó, V.; Torres, G.; Pérez, A.B.; Guzmán, M.G. Apoptosis in tissues from fatal dengue shock syndrome. J. Clin. Virol. 2007, 40, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Jessie, K.; Fong, M.Y.; Devi, S.; Lam, S.K.; Wong, K.T. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 2004, 189, 1411–1418. [Google Scholar] [CrossRef]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhang, R.; Cheng, G. Progress towards understanding the pathogenesis of dengue hemorrhagic fever. Virol. Sin. 2017, 32, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Prestwood, T.R.; Shresta, S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell. Host Microbe. 2010, 7, 128–139. [Google Scholar] [CrossRef]
- Khongphatthanayothin, A.; Lertsapcharoen, P.; Supachokchaiwattana, P.; Satupan, P.; Thongchaiprasit, K.; Poovorawan, Y.; Thisyakorn, C. Hepatosplanchnic circulatory dysfunction in acute hepatic infection: The case of dengue hemorrhagic fever. Shock 2005, 24, 407–411. [Google Scholar] [CrossRef]
- Basílio-de-Oliveira, C.A.; Aguiar, G.R.; Baldanza, M.S.; Barth, O.M.; Eyer-Silva, W.A.; Paes, M.V. Pathologic study of a fatal case of dengue-3 virus infection in Rio de Janeiro, Brazil. Braz. J. Infect. Dis. 2005, 9, 341–347. [Google Scholar] [CrossRef]
- Kumaria, R. Correlation of disease spectrum among four Dengue serotypes: A five years hospital based study from India. Braz. J. Infect. Dis. 2010, 14, 141–146. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lu, J.-W.; Yeh, C.-T.; Lin, T.-Y.; Liu, F.-C.; Ho, Y.-J. Micafungin Inhibits Dengue Virus Infection through the Disruption of Virus Binding, Entry, and Stability. Pharmaceuticals 2021, 14, 338. [Google Scholar] [CrossRef]
- Suppiah, J.; Ching, S.M.; Amin-Nordin, S.; Mat-Nor, L.A.; Ahmad-Najimudin, N.A.; Low, G.K.K.; Abdul-Wahid, M.Z.; Thayan, R.; Chee, H.Y. Clinical manifestations of dengue in relation to dengue serotype and genotype in Malaysia: A retrospective observational study. PLoS Negl. Trop. Dis. 2018, 12, e0006817. [Google Scholar] [CrossRef] [PubMed]
- Halsey, E.S.; Marks, M.A.; Gotuzzo, E.; Fiestas, V.; Suarez, L.; Vargas, J.; Aguayo, N.; Madrid, C.; Vimos, C.; Kochel, T.J.; et al. Correlation of serotype-specific dengue virus infection with clinical manifestations. PLoS Negl. Trop. Dis. 2012, 6, e1638. [Google Scholar] [CrossRef] [PubMed]
- Baltina, L.A.; Tasi, Y.T.; Huang, S.H.; Lai, H.C.; Baltina, L.A.; Petrova, S.F.; Yunusov, M.S.; Lin, C.W. Glycyrrhizic acid derivatives as Dengue virus inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 126645. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Yellow fever: An update. Lancet Infect. Dis. 2001, 1, 11–20. [Google Scholar] [CrossRef]
- Xavier, E.A.; GonÇalves, D.; de Queiroz Prado, R.; Almeida Xavier, E.; Cristina de Oliveira, N.; da Matta Guedes, P.M.; da Silva, J.S.; Tadeu Moraes Figueiredo, L.; Hugo Aquino, V. Immunocompetent mice model for dengue virus infection. Sci. World J. 2012, 2012, 525947. [Google Scholar] [CrossRef]
- Fox, C.J.; Hammerman, P.S.; Thompson, C.B. Fuel feeds function: Energy metabolism and the T-cell response. Nat. Rev. Immunol. 2005, 5, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Luan, H.H.; Medzhitov, R. An evolutionary perspective on immunometabolism. Science 2019, 363, eaar3932. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.R.; Gan, E.S.; Chan, C.Y.Y.; Liang, C.; Low, J.Z.H.; Zhang, S.L.X.; Ong, E.Z.; Bhatta, A.; Wijaya, L.; Lee, Y.H.; et al. Metabolic perturbations and cellular stress underpin susceptibility to symptomatic live-attenuated yellow fever infection. Nat. Med. 2019, 25, 1218–1224. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell. Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Cappuccio, L.; Maisse, C. Infection of Mammals and Mosquitoes by Alphaviruses: Involvement of Cell Death. Cells 2020, 9, 2612. [Google Scholar] [CrossRef]
- Roy, S.G.; Sadigh, B.; Datan, E.; Lockshin, R.A.; Zakeri, Z. Regulation of cell survival and death during Flavivirus infections. World J. Biol. Chem. 2014, 5, 93–105. [Google Scholar] [CrossRef]
- Choi, M.E.; Price, D.R.; Ryter, S.W.; Choi, A.M.K. Necroptosis: A crucial pathogenic mediator of human disease. JCI Insight 2019, 4, e128834. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Karki, R.; Kanneganti, T.-D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Berghe, T.V.; Vanlangenakker, N.; Parthoens, E.; Deckers, W.; Devos, M.; Festjens, N.; Guerin, C.J.; Brunk, U.T.; Declercq, W.; Vandenabeele, P. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell. Death Differ. 2010, 17, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Kepp, O.; Galluzzi, L.; Zitvogel, L.; Kroemer, G. Pyroptosis-a cell death modality of its kind? Eur. J. Immunol. 2010, 40, 627–630. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, Y.P.; Falcão, L.F.M.; Smith, V.C.; de Sousa, J.R.; Pagliari, C.; Franco, E.C.S.; Cruz, A.C.R.; Chiang, J.O.; Martins, L.C.; Nunes, J.A.L.; et al. Comparative Analysis of Human Hepatic Lesions in Dengue, Yellow Fever, and Chikungunya: Revisiting Histopathological Changes in the Light of Modern Knowledge of Cell Pathology. Pathogens 2023, 12, 680. https://doi.org/10.3390/pathogens12050680
Ribeiro YP, Falcão LFM, Smith VC, de Sousa JR, Pagliari C, Franco ECS, Cruz ACR, Chiang JO, Martins LC, Nunes JAL, et al. Comparative Analysis of Human Hepatic Lesions in Dengue, Yellow Fever, and Chikungunya: Revisiting Histopathological Changes in the Light of Modern Knowledge of Cell Pathology. Pathogens. 2023; 12(5):680. https://doi.org/10.3390/pathogens12050680
Chicago/Turabian StyleRibeiro, Yasmin Pacheco, Luiz Fabio Magno Falcão, Vanessa Cavaleiro Smith, Jorge Rodrigues de Sousa, Carla Pagliari, Edna Cristina Santos Franco, Ana Cecília Ribeiro Cruz, Janniffer Oliveira Chiang, Livia Carício Martins, Juliana Abreu Lima Nunes, and et al. 2023. "Comparative Analysis of Human Hepatic Lesions in Dengue, Yellow Fever, and Chikungunya: Revisiting Histopathological Changes in the Light of Modern Knowledge of Cell Pathology" Pathogens 12, no. 5: 680. https://doi.org/10.3390/pathogens12050680
APA StyleRibeiro, Y. P., Falcão, L. F. M., Smith, V. C., de Sousa, J. R., Pagliari, C., Franco, E. C. S., Cruz, A. C. R., Chiang, J. O., Martins, L. C., Nunes, J. A. L., Vilacoert, F. S. d. S., Santos, L. C. d., Furlaneto, M. P., Fuzii, H. T., Bertonsin Filho, M. V., da Costa, L. D., Duarte, M. I. S., Furlaneto, I. P., Martins Filho, A. J., ... Quaresma, J. A. S. (2023). Comparative Analysis of Human Hepatic Lesions in Dengue, Yellow Fever, and Chikungunya: Revisiting Histopathological Changes in the Light of Modern Knowledge of Cell Pathology. Pathogens, 12(5), 680. https://doi.org/10.3390/pathogens12050680








