Host Cells of Leucocytozoon (Haemosporida, Leucocytozoidae) Gametocytes, with Remarks on the Phylogenetic Importance of This Character
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Selection and Microscopical Analysis
2.2. DNA Extraction, PCR, Sequencing, and Parasites Lineage Identification
2.3. Phylogenetic Analysis
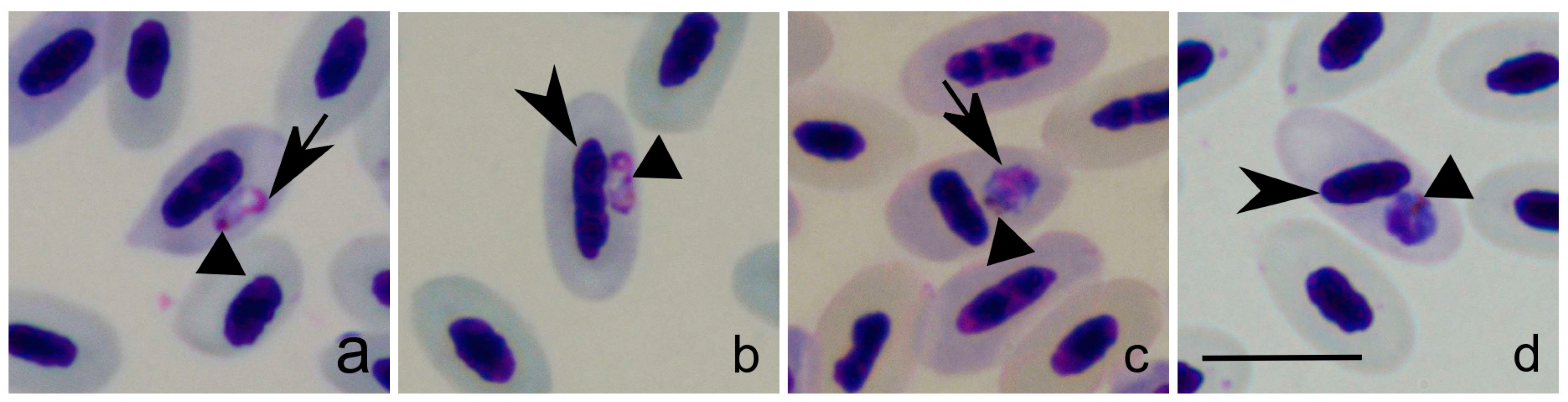
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fecchio, A.; Chagas, C.R.F.; Bell, J.A.; Kirchgatter, K. Evolutionary Ecology, Taxonomy, and Systematics of Avian Malaria and Related Parasites. Acta Tropica 2020, 204, 105364. [Google Scholar] [CrossRef] [PubMed]
- Groff, T.C.; Lorenz, T.J.; Iezhova, T.A.; Valkiūnas, G.; Sehgal, R.N.M. Description and Molecular Characterization of Novel Leucocytozoon Parasite (Apicomplexa: Haemosporida: Leucocytozoidae), Leucocytozoon polynuclearis n. sp. Found in North American Woodpeckers. Syst. Parasitol. 2022, 99, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A Public Database of Malaria Parasites and Related Haemosporidians in Avian Hosts Based on Mitochondrial Cytochrome b Lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Harl, J.; Himmel, T.; Valkiūnas, G.; Ilgūnas, M.; Bakonyi, T.; Weissenböck, H. Geographic and Host Distribution of Haemosporidian Parasite Lineages from Birds of the Family Turdidae. Malar. J. 2020, 19, 335. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Lotta, I.A.; Pacheco, M.A.; Escalante, A.A.; González, A.D.; Mantilla, J.S.; Moncada, L.I.; Adler, P.H.; Matta, N.E. Leucocytozoon Diversity and Possible Vectors in the Neotropical Highlands of Colombia. Protist 2016, 167, 185–204. [Google Scholar] [CrossRef]
- Santiago-Alarcon, D.; Palinauskas, V.; Schaefer, H.M. Diptera Vectors of Avian Haemosporidian Parasites: Untangling Parasite Life Cycles and Their Taxonomy. Biol. Rev. 2012, 87, 928–964. [Google Scholar] [CrossRef]
- Žiegytė, R.; Bernotienė, R. Contribution to the Knowledge on Black Flies (Diptera: Simuliidae) as Vectors of Leucocytozoon (Haemosporida) Parasites in Lithuania. Parasitol. Int. 2022, 87, 102515. [Google Scholar] [CrossRef]
- Himmel, T.; Harl, J.; Matt, J.; Weissenböck, H. A Citizen Science-Based Survey of Avian Mortality Focusing on Haemosporidian Infections in Wild Passerine Birds. Malar. J. 2021, 20, 417. [Google Scholar] [CrossRef]
- Himmel, T.; Harl, J.; Pfanner, S.; Nedorost, N.; Nowotny, N.; Weissenböck, H. Haemosporidioses in Wild Eurasian Blackbirds (Turdus merula) and Song Thrushes (T. philomelos): An In Situ Hybridization Study with Emphasis on Exo-Erythrocytic Parasite Burden. Malar. J. 2020, 19, 69. [Google Scholar] [CrossRef]
- Himmel, T.; Harl, J.; Kübber-Heiss, A.; Konicek, C.; Fernández, N.; Juan-Sallés, C.; Ilgūnas, M.; Valkiūnas, G.; Weissenböck, H. Molecular Probes for the Identification of Avian Haemoproteus and Leucocytozoon Parasites in Tissue Sections by Chromogenic in Situ Hybridization. Parasites Vectors 2019, 12, 282. [Google Scholar] [CrossRef]
- Ilgūnas, M.; Himmel, T.; Harl, J.; Dagys, M.; Valkiūnas, G.; Weissenböck, H. Exo-Erythrocytic Development of Avian Haemosporidian Parasites in European Owls. Animals 2022, 12, 2212. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Iezhova, T.A. Exo-Erythrocytic Development of Avian Malaria and Related Haemosporidian Parasites. Malar. J. 2017, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.C.; Santiago-Alarcon, D.; Braga, É.M. Diptera Vectors of Avian Haemosporidians: With Emphasis on Tropical Regions. In Avian Malaria and Related Parasites in the Tropics; Santiago-Alarcon, D., Marzal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 185–250. ISBN 978-3-030-51632-1. [Google Scholar]
- Zhao, W.; Liu, J.; Xu, R.; Zhang, C.; Pang, Q.; Chen, X.; Liu, S.; Hong, L.; Yuan, J.; Li, X.; et al. The Gametocytes of Leucocytozoon sabrazesi Infect Chicken Thrombocytes, Not Other Blood Cells. PLoS ONE 2015, 10, e0133478. [Google Scholar] [CrossRef] [PubMed]
- Peirce, M.A.; Adlard, R.D.; Lederer, R. A New Species of Leucocytozoon Berestneff, 1904 (Apicomplexa: Leucocytozoidae) from the Avian Family Artamidae. Syst. Parasitol. 2005, 60, 151–154. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A.; Križanauskienė, A.; Palinauskas, V.; Sehgal, R.N.M.; Bensch, S. A Comparative Analysis of Microscopy and PCR-Based Detection Methods for Blood Parasites. J. Parasitol. 2008, 94, 1395–1401. [Google Scholar] [CrossRef]
- Clark, P.; Boardman, W.S.J.; Raidal, S. Atlas of Clinical Avian Hematology; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Sambrook, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbour Laboratory Press: Cold Spring Harbour, NY, USA, 2001. [Google Scholar]
- Hellgren, O.; Waldenström, J.; Bensch, S. A New PCR Assay for Simultaneous Studies of Leucocytozoon, Plasmodium and Haemoproteus from Avian Blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), IEEE, New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree: Tree Figure Drawing Tool Version 1.4.0.; Institute of Evolutionary Biology, University of Edinburg: Edinburg, UK, 2006. [Google Scholar]
- Ramisz, A. Protozoa from the genus Leukocytozoon in birds from the vicinity of Wroclaw. Wiad. Parazytol. 1961, 7, 203–206. [Google Scholar]
- Barraclough, R.K.; Robert, V.; Peirce, M.A. New Species of Haematozoa from the Avian Families Campephagidae and Apodidae. Parasite 2008, 15, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.I.; Sehgal, R.N.M.; Smith, T.B. Leucocytozoon (Apicomplexa: Leucocytozoidae) from West African Birds, with Description of Two Species. J. Parasitol. 2005, 91, 397–401. [Google Scholar] [CrossRef]
- Lotta, I.A.; Valkiūnas, G.; Pacheco, M.A.; Escalante, A.A.; Hernández, S.R.; Matta, N.E. Disentangling Leucocytozoon Parasite Diversity in the Neotropics: Descriptions of Two New Species and Shortcomings of Molecular Diagnostics for Leucocytozoids. Int. J. Parasitol. Parasites Wildl. 2019, 9, 159–173. [Google Scholar] [CrossRef]
- Lotta, I.A.; Gonzalez, A.D.; Pacheco, M.A.; Escalante, A.A.; Valkiūnas, G.; Moncada, L.I.; Matta, N.E. Leucocytozoon pterotenuis sp. nov. (Haemosporida, Leucocytozoidae): Description of the Morphologically Unique Species from the Grallariidae Birds, with Remarks on the Distribution of Leucocytozoon Parasites in the Neotropics. Parasitol. Res. 2015, 114, 1031–1044. [Google Scholar] [CrossRef]
- Lotta, I.A.; Matta, N.E.; Torres, R.D.; Sandino, M.M.; Moncada, L.I. Leucocytozoon fringillinarum and Leucocytozoon dubreuili in Turdus fuscater from a Colombian Páramo Ecosystem. J. Parasitol. 2013, 99, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Matta, N.E.; Lotta, I.A.; Valkiūnas, G.; González, A.D.; Pacheco, M.A.; Escalante, A.A.; Moncada, L.I.; Rodríguez-Fandiño, O.A. Description of Leucocytozoon quynzae sp. nov. (Haemosporida, Leucocytozoidae) from Hummingbirds, with Remarks on Distribution and Possible Vectors of Leucocytozoids in South America. Parasitol. Res. 2014, 113, 457–468. [Google Scholar] [CrossRef]
- Parsons, N.J.; Peirce, M.A.; Strauss, V. New Species of Haematozoa in Phalacrocoracidae and Stercorariidae in South Africa. Ostrich 2010, 81, 103–108. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Sehgal, R.N.M.; Iezhova, T.A.; Hull, A.C. Identification of Leucocytozoon toddi Group (Haemosporida: Leucocytozoidae), with Remarks on the Species Taxonomy of Leucocytozoids. J. Parasitol. 2010, 96, 170–177. [Google Scholar] [CrossRef]
- Walther, E.; Valkiūnas, G.; Wommack, E.A.; Bowie, R.C.K.; Iezhova, T.A.; Sehgal, R.N.M. Description and Molecular Characterization of a New Leucocytozoon Parasite (Haemosporida: Leucocytozoidae), Leucocytozoon californicus sp. nov., Found in American Kestrels (Falco sparverius sparverius). Parasitol. Res. 2016, 115, 1853–1862. [Google Scholar] [CrossRef]
- Savage, A.F.; Ariey, F.; Greiner, E.C. Leucocytozoon atkinsoni n. sp. (Apicomplexa: Leucocytozoidae) from the Avian Family Timaliidae. Syst. Parasitol. 2006, 64, 105–109. [Google Scholar] [CrossRef]
- Fecchio, A.; Silveira, P.; Weckstein, J.D.; Dispoto, J.H.; Anciães, M.; Bosholn, M.; Tkach, V.V.; Bell, J.A. First Record of Leucocytozoon (Haemosporida: Leucocytozoidae) in Amazonia: Evidence for Rarity in Neotropical Lowlands or Lack of Sampling for This Parasite Genus? J. Parasitol. 2018, 104, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quevedo, C.; Pabón, A.; Rivera-Gutierrez, H.F. Prevalence of Haemosporidians in a Neotropical Endemic Bird Area. ACE 2016, 11, art7. [Google Scholar] [CrossRef]
- Hellgren, O.; Križanauskienė, A.; Hasselquist, D.; Bensch, S. Low Haemosporidian Diversity and One Key-Host Species in a Bird Malaria Community on a Mid-Atlantic Island (São Miguel, Azores). J. Wildl. Dis. 2011, 47, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, F.; Rao, M.; Huang, X.; Bensch, S. Estimating Prevalence of Avian Haemosporidians in Natural Populations: A Comparative Study on Screening Protocols. Parasites Vectors 2017, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Alarcon, D.; MacGregor-Fors, I.; Kühnert, K.; Segelbacher, G.; Schaefer, H.M. Avian Haemosporidian Parasites in an Urban Forest and Their Relationship to Bird Size and Abundance. Urban Ecosyst. 2016, 19, 331–346. [Google Scholar] [CrossRef]
- Van Hemert, C.; Meixell, B.W.; Smith, M.M.; Handel, C.M. Prevalence and Diversity of Avian Blood Parasites in a Resident Northern Passerine. Parasites Vectors 2019, 12, 292. [Google Scholar] [CrossRef]
- Bradbury, R.S.; Sapp, S.G.H.; Potters, I.; Mathison, B.A.; Frean, J.; Mewara, A.; Sheorey, H.; Tamarozzi, F.; Couturier, M.R.; Chiodini, P.; et al. Where Have All the Diagnostic Morphological Parasitologists Gone? J. Clin. Microbiol. 2022, 60, e00986-22. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Bensch, S.; Iezhova, T.A.; Križanauskienė, A.; Hellgren, O.; Bolshakov, C.V. Nested Cytochrome B Polymerase Chain Reaction Diagnostics Underestimate Mixed Infections of Avian Blood Haemosporidian Parasites: Microscopy Is Still Essential. J. Parasitol. 2006, 92, 418–422. [Google Scholar] [CrossRef]
- Zehtindjiev, P.; Križanauskienė, A.; Bensch, S.; Palinauskas, V.; Asghar, M.; Dimitrov, D.; Scebba, S.; Valkiūnas, G. A New Morphologically Distinct Avian Malaria Parasite That Fails Detection by Established Polymerase Chain Reaction–Based Protocols for Amplification of the Cytochrome B Gene. J. Parasitol. 2012, 98, 657–665. [Google Scholar] [CrossRef]
- Galen, S.C.; Nunes, R.; Sweet, P.R.; Perkins, S.L. Integrating Coalescent Species Delimitation with Analysis of Host Specificity Reveals Extensive Cryptic Diversity despite Minimal Mitochondrial Divergence in the Malaria Parasite Genus Leucocytozoon. BMC Evol. Biol. 2018, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Bensch, S.; Hellgren, O. The Use of Molecular Methods in Studies of Avian Haemosporidians. In Avian Malaria and Related Parasites in the Tropics; Santiago-Alarcon, D., Marzal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 113–135. ISBN 978-3-030-51632-1. [Google Scholar]
- Perkins, S.L. Molecular Systematics of the Three Mitochondrial Protein-Coding Genes of Malaria Parasites: Corroborative and New Evidence for the Origins of Human Malaria: Full-Length Research Article. DNA Seq. 2008, 19, 471–478. [Google Scholar] [CrossRef]
- Atkinson, C.T. Avian Malaria. In Parasitic Diseases of Wild Birds; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 35–53. [Google Scholar]
- Palinauskas, V.; Valkiūnas, G.; Bolshakov, C.V.; Bensch, S. Plasmodium relictum (Lineage P-SGS1): Effects on Experimentally Infected Passerine Birds. Exp. Parasitol. 2008, 120, 372–380. [Google Scholar] [CrossRef]
- Campbell, T.W. Hematology. In Avian Medicine: Principles and Application; Wingers Pub: Lake Worth, FL, USA, 1994; pp. 176–198. [Google Scholar]
- Walton, R.M.; Siegel, A. Avian Inflammatory Markers. Vet. Clin. North Am. Exot. Anim. Pract. 2022, 25, 679–695. [Google Scholar] [CrossRef]
- Wobeser, G.A. Parasitism: Costs and Effects. In Parasitic Diseases of Wild Birds; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 3–9. [Google Scholar]
- Palinauskas, V.; Žiegytė, R.; Iezhova, T.A.; Ilgūnas, M.; Bernotienė, R.; Valkiūnas, G. Description, Molecular Characterisation, Diagnostics and Life Cycle of Plasmodium elongatum (Lineage PERIRUB01), the Virulent Avian Malaria Parasite. Int. J. Parasitol. 2016, 46, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Olias, P.; Wegelin, M.; Zenker, W.; Freter, S.; Gruber, A.D.; Klopfleisch, R. Avian Malaria Deaths in Parrots, Europe. Emerg. Infect. Dis. 2011, 17, 950–952. [Google Scholar] [CrossRef]
- Ortiz-Catedral, L.; Brunton, D.; Stidworthy, M.F.; Elsheikha, H.M.; Pennycott, T.; Schulze, C.; Braun, M.; Wink, M.; Gerlach, H.; Pendl, H.; et al. Haemoproteus minutus Is Highly Virulent for Australasian and South American Parrots. Parasites Vectors 2019, 12, 40. [Google Scholar] [CrossRef]
- Martín-Maldonado, B.; Mencía-Gutiérrez, A.; Andreu-Vázquez, C.; Fernández, R.; Pastor-Tiburón, N.; Alvarado, A.; Carrero, A.; Fernández-Novo, A.; Esperón, F.; González, F. A Four-Year Survey of Hemoparasites from Nocturnal Raptors (Strigiformes) Confirms a Relation between Leucocytozoon and Low Hematocrit and Body Condition Scores of Parasitized Birds. Vet. Sci. 2023, 10, 54. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The Use of Leukocyte Profiles to Measure Stress in Vertebrates: A Review for Ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Townsend, A.K.; Wheeler, S.S.; Freund, D.; Sehgal, R.N.M.; Boyce, W.M. Links between Blood Parasites, Blood Chemistry, and the Survival of Nestling American Crows. Ecol. Evol. 2018, 8, 8779–8790. [Google Scholar] [CrossRef] [PubMed]
- Shutler, D.; Ankney, C.D.; Dennis, D.G. Could the Blood Parasite Leucocytozoon Deter Mallard Range Expansion? J. Wildl. Manag. 1996, 60, 569. [Google Scholar] [CrossRef]
- Fudge, A.M. Laboratory Medicine: Avian and Exotic Pets; W. B. Saunders: Philadelphia, PA, USA, 2000; ISBN 978-0-7216-7679-1. [Google Scholar]


| Bird Species (Common Name) | Leucocytozoon cytb Lineage | Avian Host Cell | Co-infections with Other Haemosporidian Parasites (cytb Lineage) |
|---|---|---|---|
| T. philomelos (Song thrush) | STUR1 | erythrocytes | Plasmodium circumflexum (TURDUS1) |
| T. merula (Blackbird) | NEVE01 + TURMER15 | erythrocytes | Plasmodium matutinum (LINN1) Haemoproteus minutus (TURDUS2) |
| S. borin (Garden warbler) | - a | erythrocytes | - |
| C. caeruleus (Blue tit) | PARUS4 | lymphocytes | P. circumflexum (TURDUS1) |
| P. sibilatrix (Wood warbler) | WW6 | thrombocytes | Haemoproteus homopalloris (PHSIB2) |
| P. collybita (Common chiffchaff) | AFR205 | thrombocytes | Haemoproteus asymmetricus (TUPHI01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chagas, C.R.F.; Duc, M.; Gutiérrez-Liberato, G.A.; Valkiūnas, G. Host Cells of Leucocytozoon (Haemosporida, Leucocytozoidae) Gametocytes, with Remarks on the Phylogenetic Importance of This Character. Pathogens 2023, 12, 712. https://doi.org/10.3390/pathogens12050712
Chagas CRF, Duc M, Gutiérrez-Liberato GA, Valkiūnas G. Host Cells of Leucocytozoon (Haemosporida, Leucocytozoidae) Gametocytes, with Remarks on the Phylogenetic Importance of This Character. Pathogens. 2023; 12(5):712. https://doi.org/10.3390/pathogens12050712
Chicago/Turabian StyleChagas, Carolina Romeiro Fernandes, Mélanie Duc, Germán Alfredo Gutiérrez-Liberato, and Gediminas Valkiūnas. 2023. "Host Cells of Leucocytozoon (Haemosporida, Leucocytozoidae) Gametocytes, with Remarks on the Phylogenetic Importance of This Character" Pathogens 12, no. 5: 712. https://doi.org/10.3390/pathogens12050712
APA StyleChagas, C. R. F., Duc, M., Gutiérrez-Liberato, G. A., & Valkiūnas, G. (2023). Host Cells of Leucocytozoon (Haemosporida, Leucocytozoidae) Gametocytes, with Remarks on the Phylogenetic Importance of This Character. Pathogens, 12(5), 712. https://doi.org/10.3390/pathogens12050712








