Effect of Temperature, Water Activity and Incubation Time on Trichothecene Production by Fusarium cerealis Isolated from Durum Wheat Grains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Medium
2.3. Inoculation, Incubation, and Growth Assessment
2.4. Nivalenol and Deoxynivalenol Analysis
2.5. Statistical Analysis
3. Results
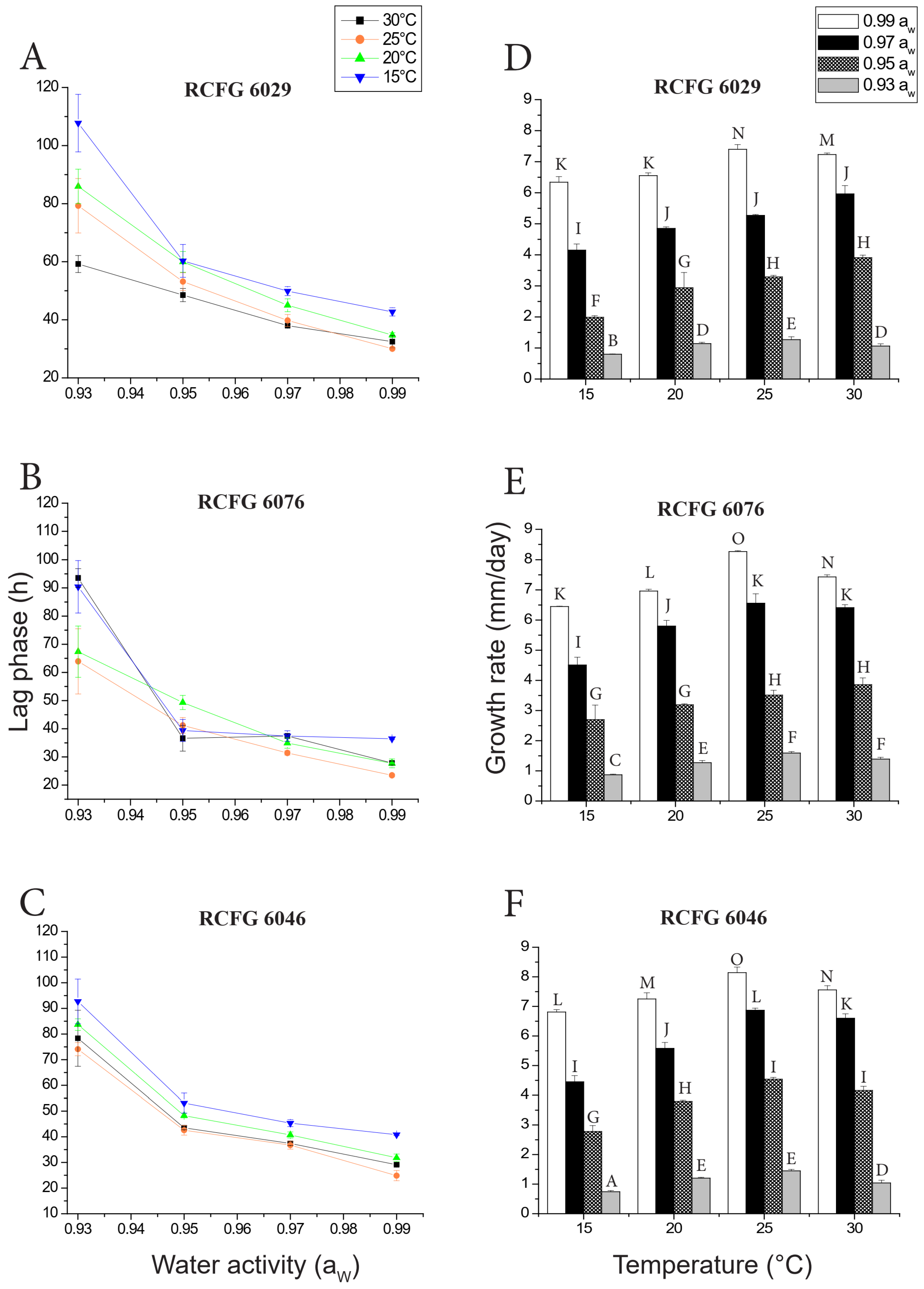
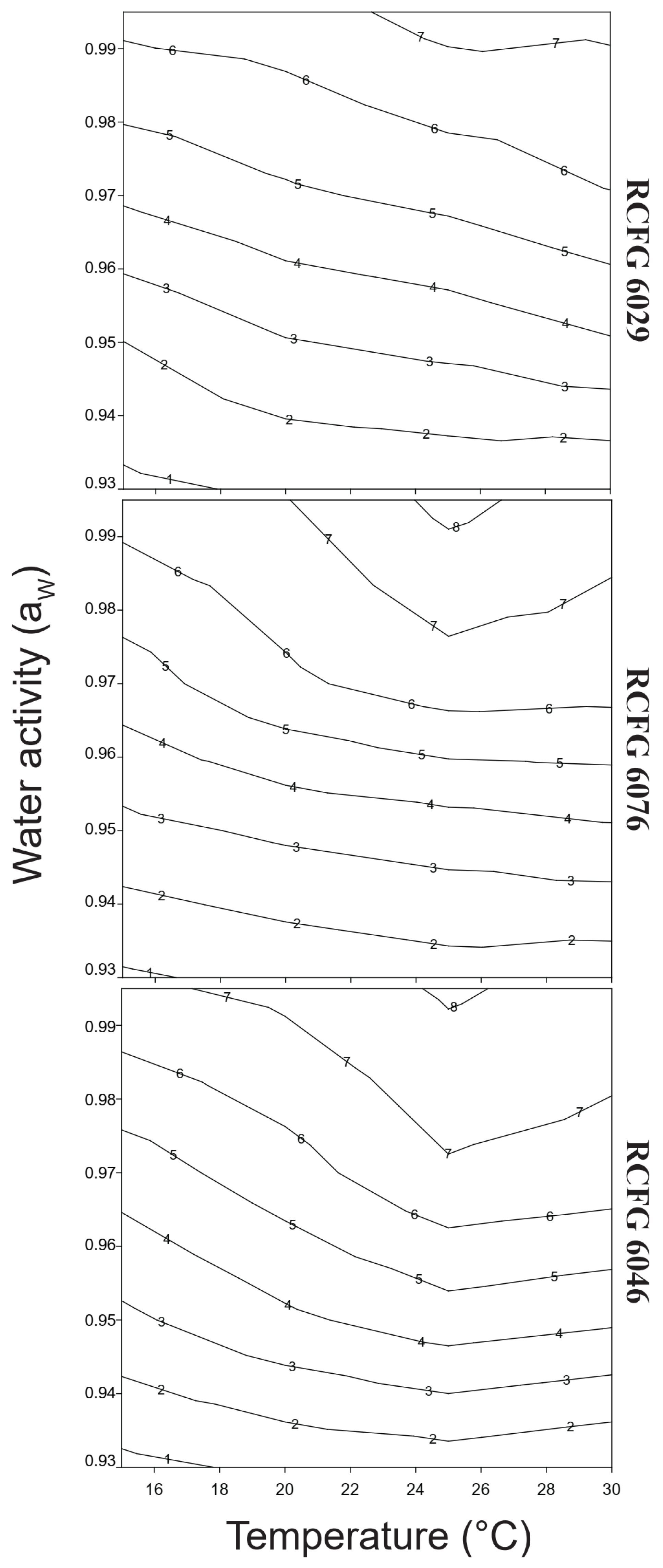
3.1. Effect of aW and Temperature on Lag Phase
3.2. Effect of aW and Temperature on Growth Rate
3.3. Effect of aW, Temperature, and Incubation Time on Nivalenol and Deoxynivalenol Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martínez, M.; Ramírez Albuquerque, L.; Arata, A.F.; Biganzoli, F.; Fernández Pinto, V.; Stenglein, S.A. Effects of Fusarium graminearum and Fusarium poae on Disease Parameters, Grain Quality and Mycotoxins Contamination in Bread Wheat (Part I). J. Sci. Food Agric. 2020, 100, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.M.; Palacios, S.A.; Yerkovich, N.; Palazzini, J.M.; Battilani, P.; Leslie, J.F.; Logrieco, A.F.; Chulze, S.N. Fusarium Head Blight and Mycotoxins in Wheat: Prevention and Control Strategies across the Food Chain. World Mycotoxin J. 2019, 12, 333–355. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Gupta, A.; Pandey, S.; Paul, V.; Saurabh, V.; Pandey, A.K.; Selvakumar, R.; Barua, S.; Kapri, M.; et al. Nivalenol Mycotoxin Concerns in Foods: An Overview on Occurrence, Impact on Human and Animal Health and Its Detection and Management Strategies. Toxins 2022, 14, 527. [Google Scholar] [CrossRef] [PubMed]
- Gab-Allah, M.A.; Choi, K.; Kim, B. Type B Trichothecenes in Cereal Grains and Their Products: Recent Advances on Occurrence, Toxicology, Analysis and Post-Harvest Decontamination Strategies. Toxins 2023, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Khaneghah, A.M.; Martins, L.M.; von Hertwig, A.M.; Bertoldo, R.; Sant’Ana, A.S. Deoxynivalenol and Its Masked Forms: Characteristics, Incidence, Control and Fate during Wheat and Wheat Based Products Processing—A Review. Trends Food Sci. Technol. 2018, 71, 13–24. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and Its Toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Toxicity, Mechanisms and Animal Health Risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. Trichothecenes in Cereal Grains—An Update. Toxins 2019, 11, 634. [Google Scholar] [CrossRef]
- Cheat, S.; Gerez, J.R.; Cognié, J.; Alassane-Kpembi, I.; Bracarense, A.P.F.L.; Raymond-Letron, I.; Oswald, I.P.; Kolf-Clauw, M. Nivalenol Has a Greater Impact than Deoxynivalenol on Pig Jejunum Mucosa in Vitro on Explants and in Vivo on Intestinal Loops. Toxins 2015, 7, 1945–1961. [Google Scholar] [CrossRef]
- Minervini, F.; Fornelli, F.; Flynn, K.M. Toxicity and Apoptosis Induced by the Mycotoxins Nivalenol, Deoxynivalenol and Fumonisin B1 in a Human Erythroleukemia Cell Line. Toxicol. Vitr. 2004, 18, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Hymery, N.; Troadec, S.; Pawtowski, A.; Coton, E.; Madec, S. Hepatotoxicity of Fusariotoxins, Alone and in Combination, towards the HepaRG Human Hepatocyte Cell Line. Food Chem. Toxicol. 2017, 109, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Yim, L.; Wan, M.; Turner, P.C.; El-nezami, H. Individual and Combined Cytotoxic Effects of Fusarium Toxins (Deoxynivalenol, Nivalenol, Zearalenone and Fumonisins B1) on Swine Jejunal Epithelial Cells. Food Chem. Toxicol. 2013, 57, 276–283. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, S.; Tan, Y.; Liu, N.; Wu, A. Individual and Combined Cytotoxic Effects of Co-Occurring Deoxynivalenol Family Mycotoxins on Human Gastric Epithelial Cells. Toxins 2017, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Puel, O.; Pinton, P.; Cossalter, A.M.; Chou, T.C.; Oswald, I.P. Co-Exposure to Low Doses of the Food Contaminants Deoxynivalenol and Nivalenol Has a Synergistic Inflammatory Effect on Intestinal Explants. Arch. Toxicol. 2017, 91, 2677–2687. [Google Scholar] [CrossRef]
- Alassane-kpembi, I.; Kolf-clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New Insights into Mycotoxin Mixtures: The Toxicity of Low Doses of Type B Trichothecenes on Intestinal Epithelial Cells Is Synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef]
- Castañares, E.; Dinolfo, M.I.; Moreno, M.V.; Berón, C.; Stenglein, S.A. Fusarium cerealis Associated with Barley Seeds in Argentina. J. Phytopathol. 2013, 161, 586–589. [Google Scholar] [CrossRef]
- Amarasinghe, C.C.; Tittlemier, S.A.; Fernando, W.G.D. Nivalenol-Producing Fusarium cerealis Associated with Fusarium Head Blight in Winter Wheat in Manitoba, Canada. Plant Pathol. 2015, 64, 988–995. [Google Scholar] [CrossRef]
- Osman, M.; He, X.; Benedettelli, S.; Singh, P.K. Trichothecene Genotypes of Fusarium graminearum Species Complex and F. Crookwellense Isolates from Mexican Cereals. J. Plant Pathol. 2016, 98, 331–336. [Google Scholar] [CrossRef]
- Cummings, J.A.; Myers, K.; Blachez, A.F.; Bergstrom, G.C. First Report of Fusarium Head Blight Caused by Fusarium cerealis in Barley in New York. Plant Dis. 2017, 101, 1955. [Google Scholar] [CrossRef]
- Minnaar-Ontong, A.; Herselman, L.; Kriel, W.M.; Leslie, J.F. Morphological Characterization and Trichothecene Genotype Analysis of a Fusarium Head Blight Population in South Africa. Eur. J. Plant Pathol. 2017, 148, 261–269. [Google Scholar] [CrossRef]
- Cerón-Bustamante, M.; Ward, T.J.; Kelly, A.; Vaughan, M.M.; McCormick, S.P.; Cowger, C.; Leyva-Mir, S.G.; Villaseñor-Mir, H.E.; Ayala-Escobar, V.; Nava-Díaz, C. Regional Differences in the Composition of Fusarium Head Blight Pathogens and Mycotoxins Associated with Wheat in Mexico. Int. J. Food Microbiol. 2018, 273, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gil-Serna, J.; Patiño, B.; Verheecke-Vaessen, C.; Vázquez, C.; Medina, Á. Searching for the Fusarium spp. Which Are Responsible for Trichothecene Contamination in Oats Using Metataxonomy to Compare the Distribution of Toxigenic Species in Fields from Spain and the UK. Toxins 2022, 14, 592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, W.Q.; Xu, J.; Xu, J.S.; Feng, J. First Report of Fusarium cerealis Causing Fusarium Head Blight on Barley in China. Plant Dis. 2011, 95, 774. [Google Scholar] [CrossRef] [PubMed]
- Alisaac, E.; Mahlein, A.-K. Fusarium Head Blight on Wheat: Biology, Modern Detection and Diagnosis and Integrated Disease Management. Toxins 2023, 15, 192. [Google Scholar] [CrossRef]
- Bouanaka, H.; Bellil, I.; Khelifi, D. First Report on Fusarium cerealis, Identification and Virulence as Causal Agents of Crown Rot on Wheat in Algeria. Arch. Phytopathol. Plant Prot. 2022, 55, 597–614. [Google Scholar] [CrossRef]
- Palacios, S.A.; Del Canto, A.; Erazo, J.; Torres, A.M. Fusarium cerealis Causing Fusarium Head Blight of Durum Wheat and Its Associated Mycotoxins. Int. J. Food Microbiol. 2021, 346, 109161. [Google Scholar] [CrossRef]
- Goertz, A.; Zuehlke, S.; Spiteller, M.; Steiner, U.; Dehne, H.W.; Waalwijk, C.; de Vries, I.; Oerke, E.C. Fusarium Species and Mycotoxin Profiles on Commercial Maize Hybrids in Germany. Eur. J. Plant Pathol. 2010, 128, 101–111. [Google Scholar] [CrossRef]
- Shan, L.; Zhang, J.; Ma, N.; Dai, X.; Guo, W. First Report of Maize Stalk Rot Disease Caused by Fusarium cerealis in Yunnan, China. Plant Dis. 2018, 102, 444. [Google Scholar] [CrossRef]
- Abdelmagid, A.; Hafez, M.; Lawley, Y.; Adam, L.R.; Daayf, F. First Report of Fusarium cerealis Causing Root Rot on Soybean. Plant Dis. 2018, 102, 2638. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Guan, Y.M.; Chen, C.Q. Fusarium cerealis, A New Pathogen Causing Ginseng (Panax ginseng) Root Rot in China. Plant Dis. 2014, 98, 1433. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.A.; Giaj Merlera, G.; Erazo, J.; Reynoso, M.M.; Farnochi, M.C.; Torres, A.M. Trichothecene Genotype and Genetic Variability of Fusarium graminearum and F. cerealis Isolated from Durum Wheat in Argentina. Eur. J. Plant Pathol. 2017, 149, 969–981. [Google Scholar] [CrossRef]
- Palacios, S.A.; Farnochi, M.C.; Ramirez, M.L.; Reynoso, M.M.; Zappacosta, D.; Soresi, D.; Torres, A.M. Natural contamination with nivalenol and deoxynivalenol in durum wheat germplasms in Argentina. In Proceedings of the VI Latin American Congress of Mycotoxicology and II International Symposium on Fungal and Algal Toxins for Industry, Mérida, Mexico, 27 June–1 July 2010; p. 245. [Google Scholar]
- Palacios, S.A.; Erazo, J.G.; Ciasca, B.; Lattanzio, V.M.T.; Reynoso, M.M.; Farnochi, M.C.; Torres, A.M. Occurrence of Deoxynivalenol and Deoxynivalenol-3-Glucoside in Durum Wheat from Argentina. Food Chem. 2017, 230, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- Kolawole, O.; Meneely, J.; Petchkongkaew, A.; Elliott, C. A Review of Mycotoxin Biosynthetic Pathways: Associated Genes and Their Expressions under the Influence of Climatic Factors. Fungal Biol. Rev. 2021, 37, 8–26. [Google Scholar] [CrossRef]
- Hope, R.; Magan, N. Two-Dimensional Environmental Profiles of Growth, Deoxynivalenol and Nivalenol Production by Fusarium culmorum on a Wheat-Based Substrate. Lett. Appl. Microbiol. 2003, 37, 70–74. [Google Scholar] [CrossRef]
- Hope, R.; Aldred, D.; Agan, N. Comparison of Environmental Profiles for Growth and Deoxynivalenol Production by Fusarium culmorum and F. graminearum on Wheat Grain. Lett. Appl. Microbiol. 2005, 40, 295–300. [Google Scholar] [CrossRef]
- Ramirez, M.L.; Chulze, S.; Magan, N. Temperature and Water Activity Effects on Growth and Temporal Deoxynivalenol Production by Two Argentinean Strains of Fusarium graminearum on Irradiated Wheat Grain. Int. J. Food Microbiol. 2006, 106, 291–296. [Google Scholar] [CrossRef]
- Belizán, M.M.E.; Gomez, A.d.l.A.; Terán Baptista, Z.P.; Jimenez, C.M.; Sánchez Matías, M.d.H.; Catalán, C.A.N.; Sampietro, D.A. Influence of Water Activity and Temperature on Growth and Production of Trichothecenes by Fusarium graminearum Sensu Stricto and Related Species in Maize Grains. Int. J. Food Microbiol. 2019, 305, 108242. [Google Scholar] [CrossRef]
- Yoder, W.T.; Christianson, L.M. Species-Specific Primers Resolve Members of Section Fusarium Taxonomic Status of the Edible “Quorn” Fungus Reevaluated. Fungal Genet. Biol. 1998, 80, 68–80. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing Professional: Ames, IA, USA, 2006; pp. 3–30. [Google Scholar] [CrossRef]
- Dallyn, H.; Fox, A. Spoilage of material of reduced water activity by xerophilic fungi. In Microbial Growth and Survival in Extreme Environments; Corry, J.E.L., Gould, G.H., Eds.; Academic Press: London, UK, 1980; pp. 129–139. [Google Scholar]
- Li, C.; Fan, S.; Wen, Y.; Tan, Z.; Liu, C. Enantioselective Effect of Flutriafol on Growth, Deoxynivalenol Production, and TRI Gene Transcript Levels in Fusarium graminearum. J. Agric. Food Chem. 2021, 69, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2018. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available online: http://www.infostat.com.ar (accessed on 20 March 2022).
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium Ear Blight (Scab) in Small Grain Cereals—A Review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Garcia-Cela, E.; Verheecke-Vaessen, C.; Ósk-Jónsdóttir, I.; Lawson, R.; Magan, N. Growth Kinetic Parameters and Prediction of Growth and Zearalenone and Deoxynivalenol Production Boundaries by Three Fusarium asiaticum Strains Isolated from Wheat. Fermentation 2022, 8, 577. [Google Scholar] [CrossRef]
- Nazari, L.; Pattori, E.; Manstretta, V.; Terzi, V.; Morcia, C.; Somma, S.; Moretti, A.; Ritieni, A.; Rossi, V. Effect of Temperature on Growth, Wheat Head Infection, and Nivalenol Production by Fusarium poae. Food Microbiol. 2018, 76, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. Modelling the Relationship between Environmental Factors, Transcriptional Genes and Deoxynivalenol Mycotoxin Production by Strains of Two Fusarium Species. J. R. Soc. Interface 2011, 8, 117–126. [Google Scholar] [CrossRef]
- Marín, P.; Jurado, M.; Magan, N.; Vázquez, C.; González-Jaén, M.T. Effect of Solute Stress and Temperature on Growth Rate and TRI5 Gene Expression Using Real Time RT-PCR in Fusarium graminearum from Spanish Wheat. Int. J. Food Microbiol. 2010, 140, 169–174. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J.W. Limiting Mycotoxins in Stored Wheat. Food Addit. Contam. Part A 2010, 27, 644–650. [Google Scholar] [CrossRef]
- Garcia-Cela, E.; Kiaitsi, E.; Medina, A.; Sulyok, M.; Krska, R.; Magan, N. Interacting Environmental Stress Factors Affects Targeted Metabolomic Profiles in Stored Natural Wheat and That Inoculated with F. graminearum. Toxins 2018, 10, 56. [Google Scholar] [CrossRef]
- Rabaaoui, A.; Dall’asta, C.; Righetti, L.; Susca, A.; Logrieco, A.F.; Namsi, A.; Gdoura, R.; Werbrouck, S.P.O.; Moretti, A.; Masiello, M. Phylogeny and Mycotoxin Profile of Pathogenic Fusarium Species Isolated from Sudden Decline Syndrome and Leaf Wilt Symptoms on Date Palms (Phoenix Dactylifera) in Tunisia. Toxins 2021, 13, 463. [Google Scholar] [CrossRef]



| Source of Variation | Lag Phase (h) | Growth Rate (mm/day) | ||||
|---|---|---|---|---|---|---|
| MS | F | p | MS | F | p | |
| S | 771.72 | 39.32 | <0.0001 | 3.85 | 143.67 | <0.0001 |
| T° | 1135.92 | 57.88 | <0.0001 | 11.92 | 444.85 | <0.0001 |
| aW | 16,896.93 | 860.95 | <0.0001 | 244.77 | 9136.71 | <0.0001 |
| T° × aW | 150.71 | 7.68 | <0.0001 | 0.90 | 33.77 | <0.0001 |
| T° × S | 210.78 | 10.74 | <0.0001 | 0.26 | 9.6 | <0.0001 |
| aW × S | 58.92 | 3 | <0.01 | 0.53 | 19.97 | <0.0001 |
| T° × aW × S | 125.84 | 6.41 | <0.0001 | 0.12 | 4.36 | <0.0001 |
| Source of Variation | Nivalenol | Deoxynivalenol | ||
|---|---|---|---|---|
| F | p | F | p | |
| S | 232.68 | <0.0001 | 40.46 | <0.0001 |
| T° | 53.86 | <0.0001 | 6.55 | 0.0003 |
| aW | 197.17 | <0.0001 | 94.07 | <0.0001 |
| Incubation time | 14.38 | <0.0001 | 165.91 | <0.0001 |
| T° × S | 12.93 | <0.0001 | 9.68 | <0.0001 |
| aW × S | 14.04 | <0.0001 | 10.02 | <0.0001 |
| S x incubation time | 7.76 | <0.0001 | 9.32 | <0.0001 |
| T° × aW | 4.58 | <0.0001 | 13.82 | <0.0001 |
| T° × incubation time | 5.54 | <0.0001 | 2.42 | 0.0678 |
| aW × incubation time | 14.19 | <0.0001 | 26.63 | <0.0001 |
| S × T° × aW | 4.03 | <0.0001 | 5.31 | <0.0001 |
| S × T° × incubation time | 3.18 | <0.0001 | 11.9 | <0.0001 |
| S × aW × incubation time | 4.46 | <0.0001 | 5.07 | <0.0001 |
| T° × aW × incubation time | 2.99 | <0.0001 | 10.75 | <0.0001 |
| S × T° × aW × incubation time | 2.90 | <0.0001 | 5.59 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erazo, J.G.; Palacios, S.A.; Veliz, N.A.; Del Canto, A.; Plem, S.; Ramirez, M.L.; Torres, A.M. Effect of Temperature, Water Activity and Incubation Time on Trichothecene Production by Fusarium cerealis Isolated from Durum Wheat Grains. Pathogens 2023, 12, 736. https://doi.org/10.3390/pathogens12050736
Erazo JG, Palacios SA, Veliz NA, Del Canto A, Plem S, Ramirez ML, Torres AM. Effect of Temperature, Water Activity and Incubation Time on Trichothecene Production by Fusarium cerealis Isolated from Durum Wheat Grains. Pathogens. 2023; 12(5):736. https://doi.org/10.3390/pathogens12050736
Chicago/Turabian StyleErazo, Jessica G., Sofía A. Palacios, Nuria A. Veliz, Agostina Del Canto, Silvana Plem, María L. Ramirez, and Adriana M. Torres. 2023. "Effect of Temperature, Water Activity and Incubation Time on Trichothecene Production by Fusarium cerealis Isolated from Durum Wheat Grains" Pathogens 12, no. 5: 736. https://doi.org/10.3390/pathogens12050736







