Anthelmintic-Like Activity and Ultrastructure Changes Produced by Two Polyphenolic Combinations against Cooperia punctata Adult Worms and Infective Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Allocation
2.2. Polyphenolic Compounds and Combinations
2.3. Biological Material
2.3.1. Infective Larvae
2.3.2. Adult Worm Recovery
2.4. Evaluation of Polyphenolic Compounds Combinations (PCC)
2.4.1. Larval Motility Inhibition Assay (LMIA)
2.4.2. Adult Motility Inhibition Assay (AMIA)
2.4.3. Polyphenolic Combinations Effect over the Ultrastructure of C. punctata Infective Larvae and Adult Stages
2.5. Scanning and Transmission Electron Microscopy (TEM and SEM)
2.6. Statistical Analyses
3. Results
3.1. Effect of Polyphenolic Combinations on the Motility of C. punctata Infective Larvae
3.2. Effect of PCs-Combinations on the Motility of C. punctata Adult Worms
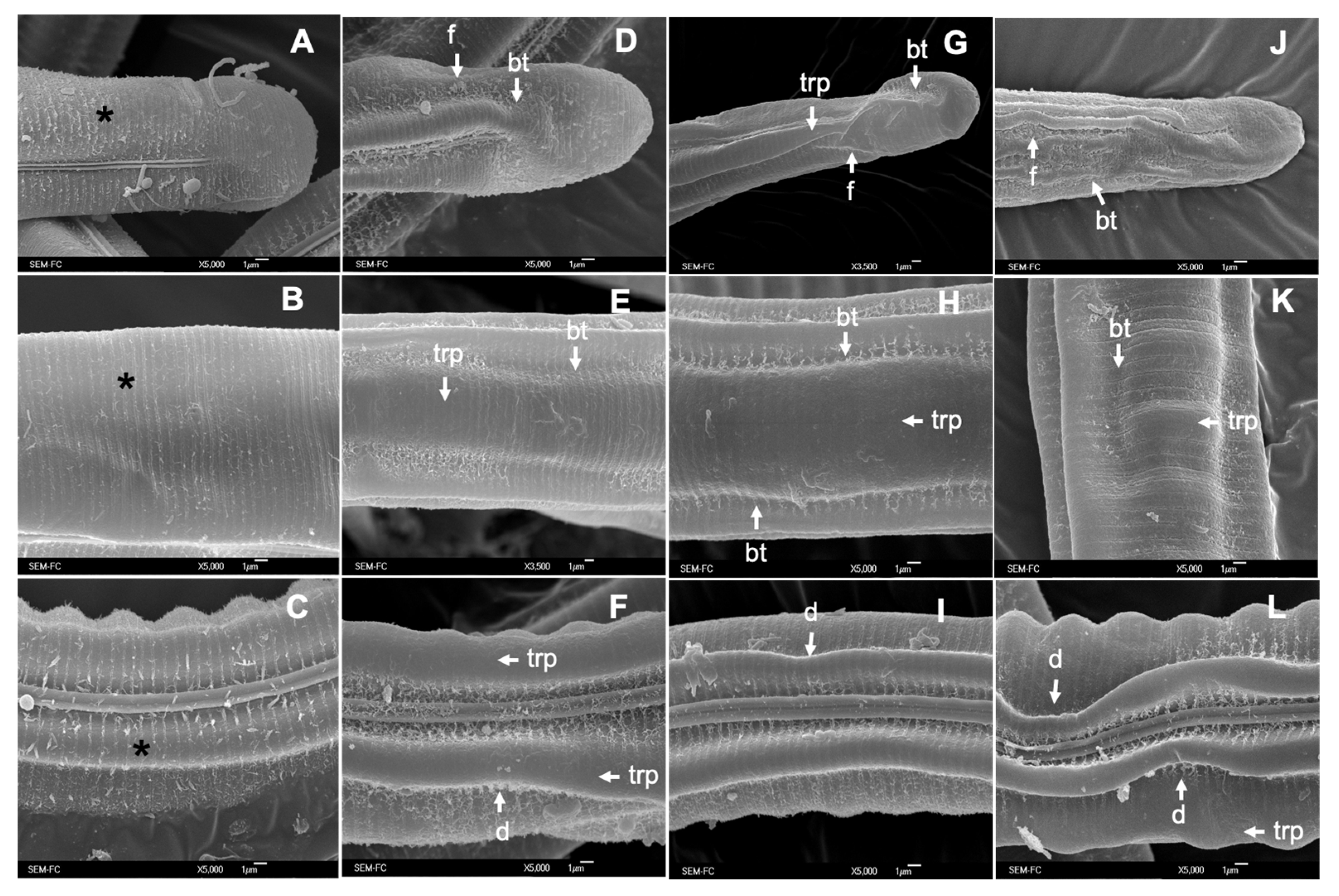
3.3. Scanning Electron Microscopy (SEM)
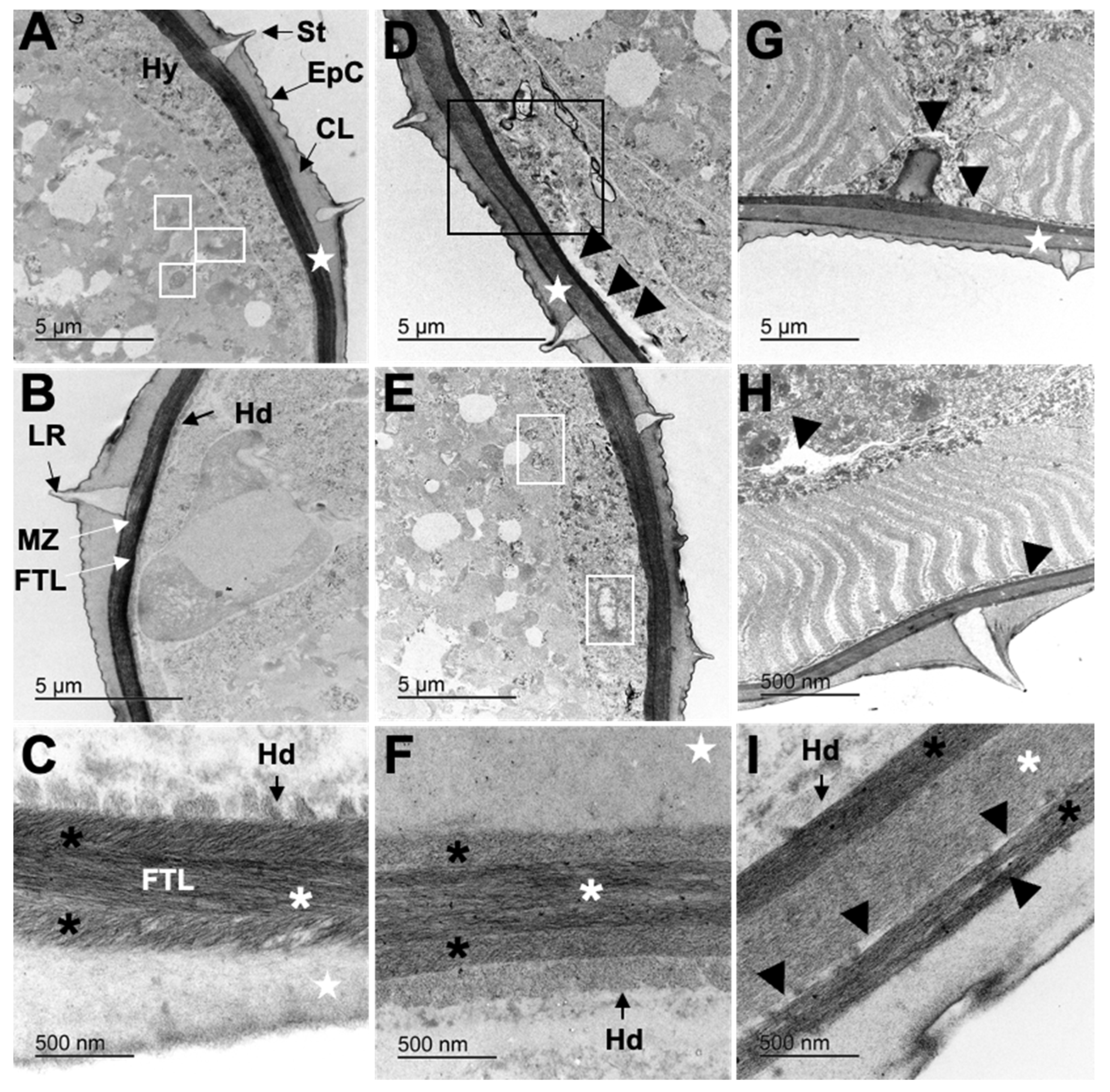
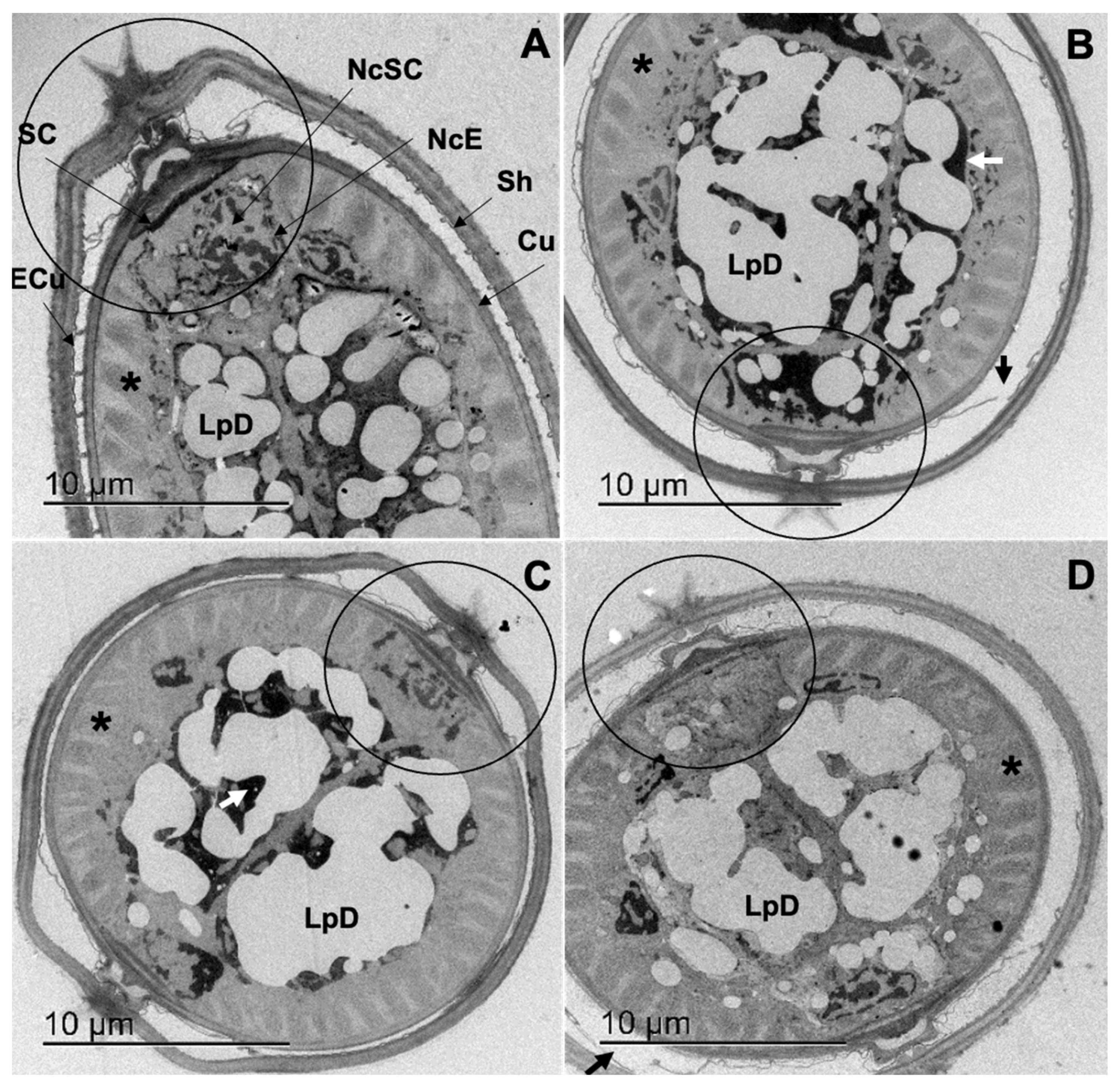
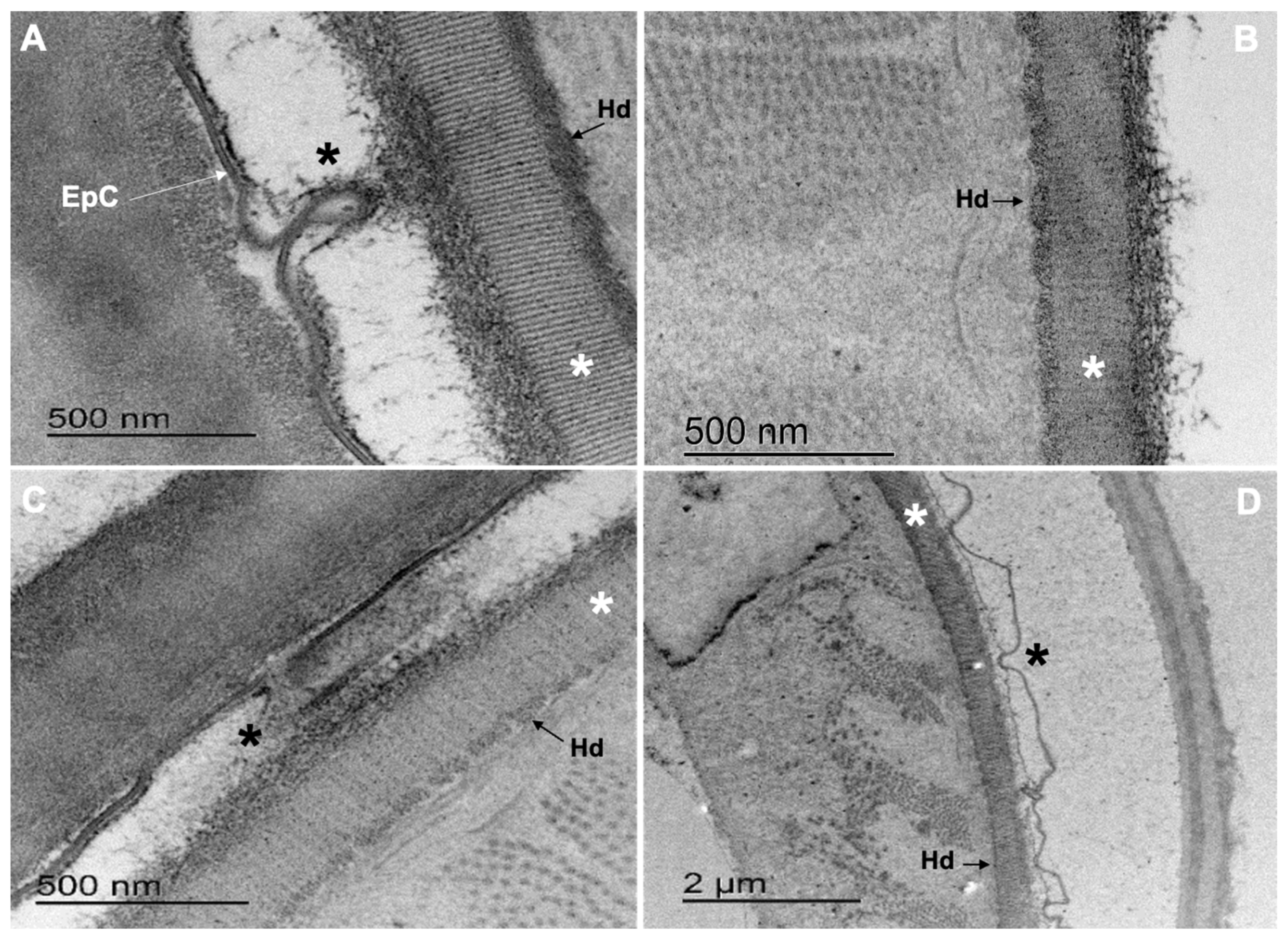
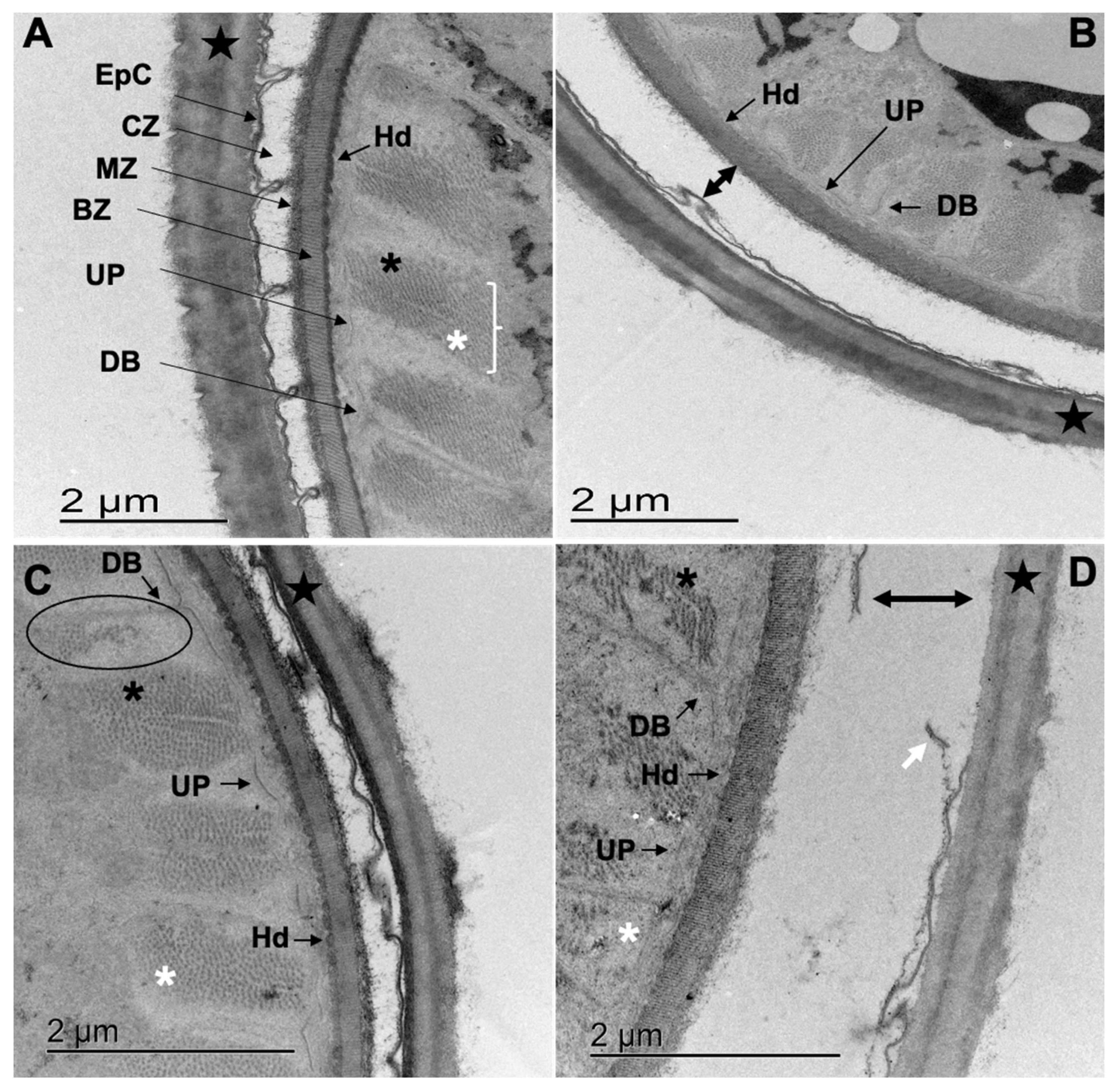
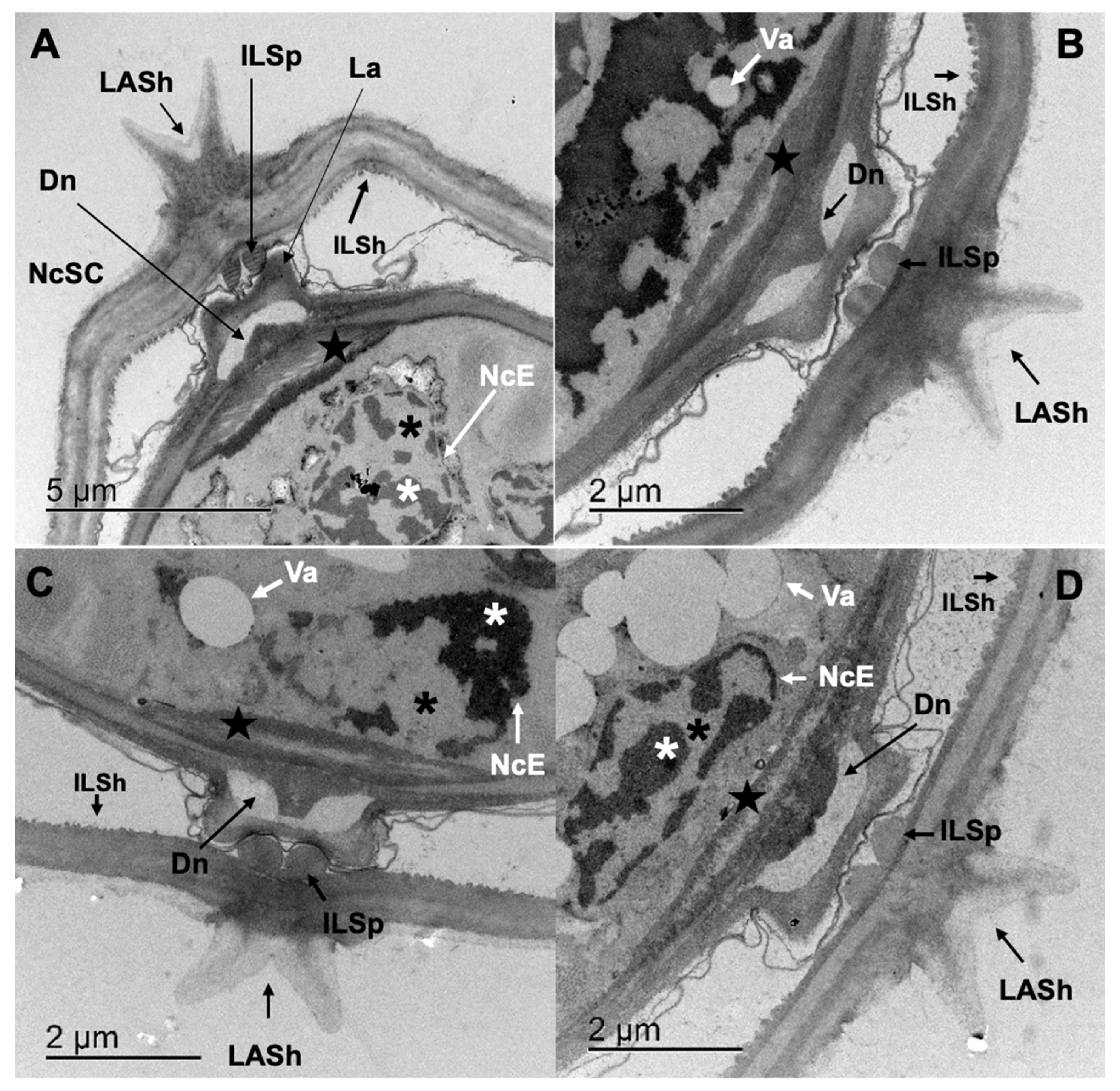
3.4. Transmission Electron Microscopy (TEM)
3.4.1. Cooperia punctata Infective Larvae L3
3.4.2. Adult Worms
4. Discussion
4.1. Motility Inhibition of Polyphenolic Compounds Combinations (PCs-Combinations)
4.2. Structural and Ultrastructural Alterations Observed through SEM and TEM in Both L3 and Adult Worms of C. punctata
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stromberg, B.E.; Gasbarre, L.C.; Waite, A.; Bechtol, D.T.; Brown, M.S.; Robinson, N.A.; Olson, E.J.; Newcomb, H. Cooperia punctata: Effect on cattle productivity? Vet. Parasitol. 2012, 183, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D. Anticholinesterase activity of selected phenolic acids and flavonoids–interaction testing in model solutions. Ann. Agric. Environ. Med. 2015, 22, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Handique, J.G.; Baruah, J.B. Polyphenolic compounds: An overview. React. Funct. Polym. 2002, 52, 163–188. [Google Scholar] [CrossRef]
- Wawrosch, C.; Zotchev, S.B. Production of bioactive plant secondary metabolites through in vitro technologies—Status and outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649–6668. [Google Scholar] [CrossRef]
- Smout, M.J.; Kotze, A.C.; McCarthy, J.S.; Loukas, A. A Novel High Throughput Assay for Anthelmintic Drug Screening and Resistance Diagnosis by RealTime Monitoring of Parasite Motility. PLoS Negl. Trop. Dis. 2010, 4, e885. [Google Scholar] [CrossRef]
- Geary, T.G.; Sakanari, J.A.; Caffrey, C.R. Anthelmintic Drug Discovery: Into the Future. J. Parasitol. 2015, 101, 125–133. [Google Scholar] [CrossRef]
- Escareño-Díaz, S.; Alonso-Díaz, M.; de Gives, P.M.; Castillo-Gallegos, E.; Fernex, E.V.S.-D. Anthelmintic-Like Activity Of Polyphenolic Compounds And Their interactions Against The Cattle Nematode Cooperia Punctata. Vet. Parasitol. 2019, 274, 108909. [Google Scholar] [CrossRef]
- Fernex, E.V.S.-D.; Alonso-Díaz, M.; Gives, P.M.-D.; la Mora, B.V.-D.; González-Cortazar, M.; Zamilpa, A.; Gallegos, E.C. Elucidation of Leucaena leucocephala anthelmintic-like phytochemicals and the ultrastructural damage generated to eggs of Cooperia spp. Vet. Parasitol. 2015, 214, 89–95. [Google Scholar] [CrossRef]
- von Son-de Fernex, E.; Alonso-Díaz, M.Á.; Valles-de la Mora, B.; Mendoza-de Gives, P.; González-Cortazar, M.; Zamilpa, A. Anthelmintic effect of 2H-chromen-2-one isolated from Gliricidia sepium against Cooperia punctata. Exp. Parasitol. 2017, 178, 1–6. [Google Scholar] [CrossRef]
- Rabel, B.; Mcgregor, R.; Douch, P.G.C. Improved bioassay for estimation of inhibitory effects of ovine gastrointestinal mucus and anthelmintics on nematode larval migration. Int. J. Parasitol. 1994, 24, 671–676. [Google Scholar] [CrossRef]
- Peña-Espinoza, M.; Williams, A.R.; Thamsborg, S.M.; Simonsen, H.T.; Enemark, H.L. Anthelmintic effects of forage chicory (Cichorium intybus) against free-living and parasitic stages of Cooperia oncophora. Vet. Parasitol. 2017, 243, 204–207. [Google Scholar] [CrossRef]
- Martínez-Ortiz-de-Montellano, C.; de Jesús Torres-Acosta, J.F.; Fourquaux, I.; Sandoval-Castro, C.A.; Hoste, H. Ultrastructural study of adult Haemonchus contortus exposed to polyphenol-rich materials under in vivo conditions in goats. Parasite 2019, 26, 65. [Google Scholar] [CrossRef]
- Vázquez, N.G.; Echeverría, O. Introducción a la microscopía electrónica aplicada a las ciencias biológicas. México: Fondo de Cultura Económica. Cienc. UANL IV 2001, 1, 91–92. [Google Scholar]
- Paolini, V.; Frayssines, A.; De la Farge, F.; Dorchies, P.; Hoste, H. Effects of condensed tannins on established populations and on incoming larvae of Trichostrongylus colubriformis and Teladorsagia circumcincta in goats. Vet. Res. 2003, 34, 331–339. [Google Scholar] [CrossRef]
- Page, A.P.; Stepek, G.; Winter, A.D.; Pertab, D. Enzymology of the nematode cuticle: A potential drug target? Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 133–141. [Google Scholar] [CrossRef]
- von Son-de Fernex, E.; Alonso-Díaz, M.A.; Valles-de la Mora, B.; Capetillo-Leal, C.M. In vitro anthelmintic activity of five tropical legumes on the exsheathment and motility of Haemonchus contortus infective larvae. Exp. Parasitol. 2012, 131, 413–418. [Google Scholar] [CrossRef]
- Alonso-Díaz, M.A.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Aguilar-Caballero, A.J.; Hoste, H. In vitro larval migration and kinetics of exsheathment of Haemonchus contortus larvae exposed to four tropical tanniniferous plant extracts. Vet. Parasitol. 2008, 153, 313–319. [Google Scholar] [CrossRef]
- Wharton, D.A. Movement and Co-Ordination. In A Functional Biology of Nematodes; Functional Biology Series; Springer: Boston, MA, USA, 1986. [Google Scholar] [CrossRef]
- Martínez-Ortiz-de-Montellano, C.; Arroyo-López, C.; Fourquaux, I.; Torres-Acosta, J.F.; Sandoval-Castro, C.A.; Hoste, H. Scanning electron microscopy of Haemonchus contortus exposed to tannin-rich plants under in vivo and in vitro conditions. Exp. Parasitol. 2013, 133, 281–286. [Google Scholar] [CrossRef]
- Demeler, J.; Küttler, U.; von Samson-Himmelstjerna, G. Adaptation and evaluation of three different in vitro tests for the detection of resistance to anthelmintics in gastrointestinal nematodes of cattle. Vet. Parasitol. 2010, 170, 61–70. [Google Scholar] [CrossRef]
- Neuhaus, B.; Bresciani, J.; Christensen, C.M.; Frandsen, F. Ultrastructure and Development of the Body Cuticle of Oesophagostomum dentatum (Strongylida, Nematoda). J. Parasitol. 1996, 82, 820–828. [Google Scholar] [CrossRef]
- Bae, Y.K.; Barr, M.M. Sensory roles of neuronal cilia: Cilia development, morphogenesis, and function in C. elegans. Front. Biosci. 2011, 13, 5959–5974. [Google Scholar] [CrossRef] [PubMed]
- Sulston, J.E.; Horvitz, H.R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 1977, 56, 110–156. [Google Scholar] [CrossRef]
- Abuzar, M.E.; Rosline, H.; Zamzuri, I.; Zulkifli, M.; Wan-Arfah, N.; Wan-Zaidah, A. Fibrinolytic activity of caffeic acid phenethyl ester (cape): In vitro study on whole blood clot. Int. J. Pharm. Pharm. Sci. 2013, 5, 459–462. [Google Scholar]
- Engström, M.T.; Pälijärvi, M.; Salminen, J.P. Rapid fingerprint analysis of plant extracts for ellagitannins, gallic acid, and quinic acid derivatives and quercetin, kaempferol and myrecitin-based flavonol glycosides by UPLC-QqQ-MS/MS. J. Agric. Food Chem. 2015, 63, 4068–4079. [Google Scholar] [CrossRef] [PubMed]
- Selkirk, M.E.; Lazari, O.; Matthews, B. Functional genomics of nematode acetylcholinesterases. Parasitology 2005, 131, S3–S18. [Google Scholar] [CrossRef]
- Blanchard, A.; Guégnard, F.; Charvet, C.L.; Crisford, A.; Courtot, E.; Sauvé, C.; Harmache, A.; Duguet, T.; O’Connor, V.; Castagnone-Sereno, P.; et al. Deciphering the molecular determinants of cholinergic anthelmintic sensitivity in nematodes: When novel functional validation approaches highlight major differences between the model Caenorhabditis elegans and parasitic species. PLoS Pathog. 2018, 14, e1006996. [Google Scholar] [CrossRef]
- Narayanaswamy, V.K.; Gleiser, R.M.; Ksumbwe, K.; Aldhubiab, B.E.; Attimarad, M.V.; Odhav, B. Evaluation of Halogenated Coumarins for Antimosquito Properties. Sci. World J. 2014, 2014, 189824. [Google Scholar] [CrossRef]
- Anwar, J.; Spanevello, R.M.; Thomé, G.; Stefanello, N.; Schmatz, R.; Gutierres, J.; Vieira, J.; Baldissarelli, J.; Barbosa, F.C.; Melgarejo da Rosa, M.; et al. Effects of caffeic acid on behavioral parameters and on the activity of acetylcholinesterase in different tissues from adult rats. Pharmacol. Biochem. Behav. 2012, 103, 386–394. [Google Scholar] [CrossRef]
- Komuniecki, R.W.; Hobson, R.J.; Rex, E.B.; Hapiak, V.M.; Komuniecki, P.R. Biogenic amine receptors in parasitic nematodes: What can be learned from Caenorhabditis elegans? Mol. Biochem. Parasitol. 2004, 137, 1–11. [Google Scholar] [CrossRef]
- Kier, W.M. The diversity of hydrostatic skeletons. J. Exp. Biol. 2012, 215, 1247–1257. [Google Scholar] [CrossRef]
- Clark, R.B.; Cowey, J.B. Factors controlling the change of shape of certain nemertean and turbellarian worms. J. Exp. Biol. 1958, 35, 731–748. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.546.6039&rep=rep1&type=pdf (accessed on 22 February 2023). [CrossRef]
- Petzold, B.C.; Park, S.J.; Ponce, P.; Roozeboom, C.; Powell, C.; Goodman, M.B.; and Pruitt, B.L. Caenorhabditis elegans body mechanics are regulated by body wall muscle tone. Biophys. J. 2011, 100, 1977–1985. [Google Scholar] [CrossRef]
- Albrechtová, M.; Langrová, I.; Vadlejch, J.; Špakulová, M. A Revised Checklist of Cooperia Nematodes (Trichostrogyloidea), Common Parasites of Wild and Domestic Ruminants. Helminthologia 2020, 57, 280–287. [Google Scholar] [CrossRef]
- Lee, D.L.; Atkinson, H.J. Locomotion. In Physiology of Nematodes, 2nd ed.; Palgrave Macmillan: London, UK, 1976; p. 228. ISBN-13: 978-0333186008. [Google Scholar] [CrossRef]
- Irazoqui, J.E. Why Worms Watch Their Hemidesmosomes and Why You Should, Too. Immunity 2015, 42, 206–208. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, M.; Liu, X. Insight into the conformational and functional properties of myofibrillar protein modified by mulberry polyphenols. Food Chem. 2020, 308, 125592. [Google Scholar] [CrossRef]
- Cavaliere, V.; Papademetrio, D.L.; Lombardo, T.; Costantino, S.N.; Blanco, G.A.; Alvarez, E.M. Caffeic acid phenylethyl ester and MG132, two novel nonconventional chemotherapeutic agents, induce apoptosis of human leukemic cells by disrupting mitochondrial function. Target. Oncol. 2014, 9, 25–42. [Google Scholar] [CrossRef]
- Jin, U.; Song, K.; Motomura, M.; Suzuki, I.; Gu, Y.; Kang, Y.; Moon, T.; Kim, C. Caffeic acid phenethyl ester induces mitochondria-mediated apoptosis in human myeloid leukemia U937 cells. Mol. Cell. Biochem. 2008, 310, 43–48. [Google Scholar] [CrossRef]
- Brenzan, A.M.; Vataru, C.; Prado, B.; Ueda-Nakamura, T.; Young, M.C.M.; García, D.A. Antileishmanial activity of crude extract and coumarin from Calophyllum brasiliense leaves against Leishmania amazonensis. Parasitol. Res. 2007, 101, 715–722. [Google Scholar] [CrossRef]


| Treatment | Concentration (mg mL−1) | Motility Inhibition% (Mean ± SE) |
|---|---|---|
| Thiabendazole | 10 | 88.52 ± 6.59 a |
| Coumarin: quercetin | 0.8 | 3.82 ± 1.87 b |
| Caffeic acid: Rutin | 0.84 | 13.95 ± 4.84 c |
| Combination | Time Lapse (h) | EE | Limits IC 95% | R2 | ||
|---|---|---|---|---|---|---|
| EC50 (mg mL−1) | Inferior | Superior | ||||
| Coumarin: Quercetin | 6 | 1.621 | 0.154 | 0.944 | 12.41 | 0.87 |
| 12 | 0.398 | 0.064 | 0.267 | 0.677 | 0.93 | |
| 24 | 0.073 | 0.071 | 0.045 | 0.117 | 0.94 | |
| Caffeic acid: Rutin | 6 | NE | NE | NE | NE | NE |
| 12 | 0.192 | 0.061 | 0.129 | 0.288 | 0.95 | |
| 24 | 0.051 | 0.164 | 0.0008 | 0.125 | 0.75 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Son-de Fernex, E.; Zúñiga-Olivos, E.; Jiménez-García, L.F.; Mendoza-de Gives, P. Anthelmintic-Like Activity and Ultrastructure Changes Produced by Two Polyphenolic Combinations against Cooperia punctata Adult Worms and Infective Larvae. Pathogens 2023, 12, 744. https://doi.org/10.3390/pathogens12050744
von Son-de Fernex E, Zúñiga-Olivos E, Jiménez-García LF, Mendoza-de Gives P. Anthelmintic-Like Activity and Ultrastructure Changes Produced by Two Polyphenolic Combinations against Cooperia punctata Adult Worms and Infective Larvae. Pathogens. 2023; 12(5):744. https://doi.org/10.3390/pathogens12050744
Chicago/Turabian Stylevon Son-de Fernex, Elke, Estefanía Zúñiga-Olivos, Luis Felipe Jiménez-García, and Pedro Mendoza-de Gives. 2023. "Anthelmintic-Like Activity and Ultrastructure Changes Produced by Two Polyphenolic Combinations against Cooperia punctata Adult Worms and Infective Larvae" Pathogens 12, no. 5: 744. https://doi.org/10.3390/pathogens12050744
APA Stylevon Son-de Fernex, E., Zúñiga-Olivos, E., Jiménez-García, L. F., & Mendoza-de Gives, P. (2023). Anthelmintic-Like Activity and Ultrastructure Changes Produced by Two Polyphenolic Combinations against Cooperia punctata Adult Worms and Infective Larvae. Pathogens, 12(5), 744. https://doi.org/10.3390/pathogens12050744