Longitudinal Study of the Occurrence of Usutu Virus and West Nile Virus Infections in Birds in a Zoological Garden in Northern Germany
Abstract
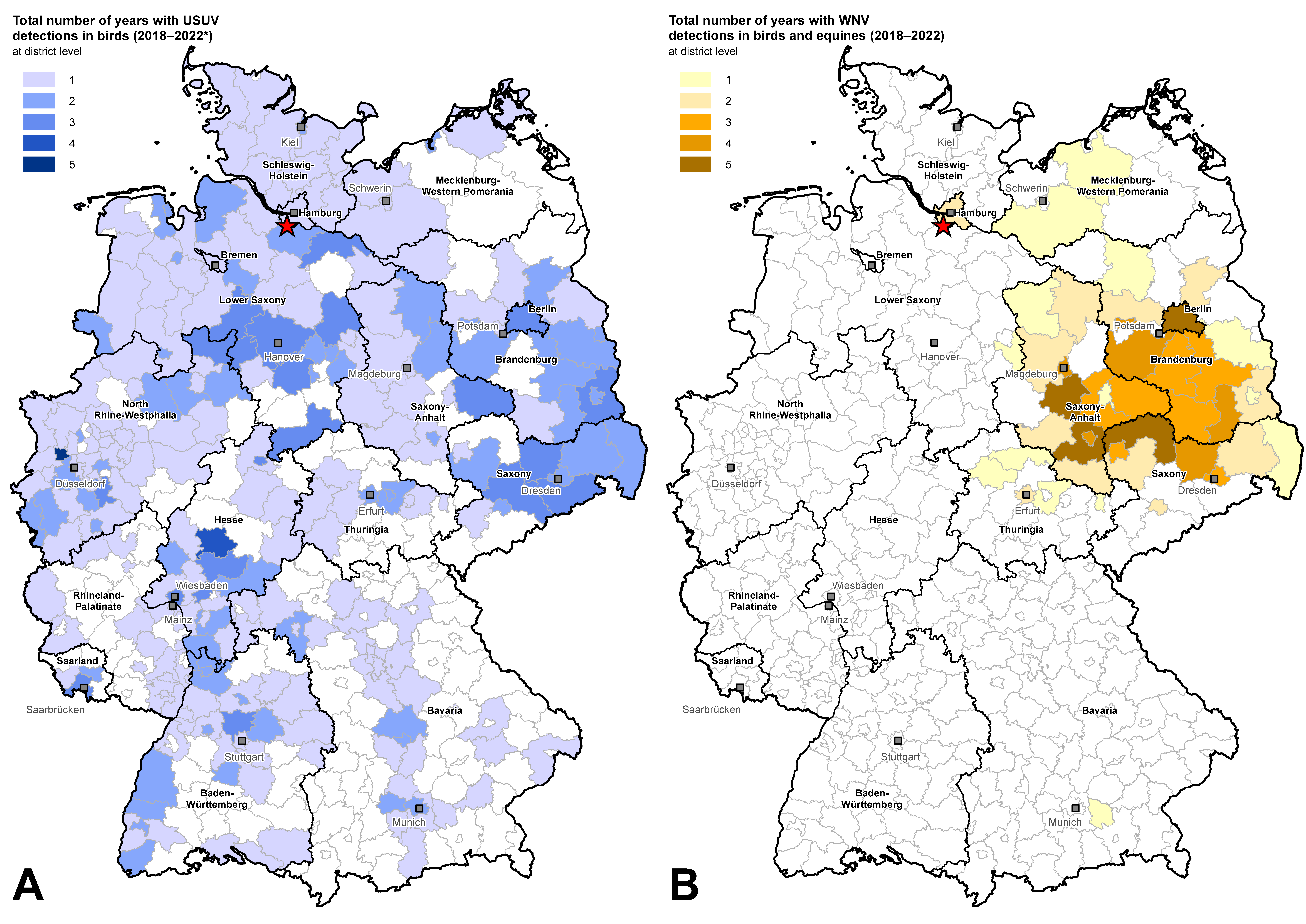
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Molecular Investigation
2.3. Whole Genome Sequencing and Phylogenetic Analysis
2.4. Serological Investigation
2.5. Pathology and Immunohistochemistry
2.6. Ethical Statement
3. Results
3.1. Molecular Results
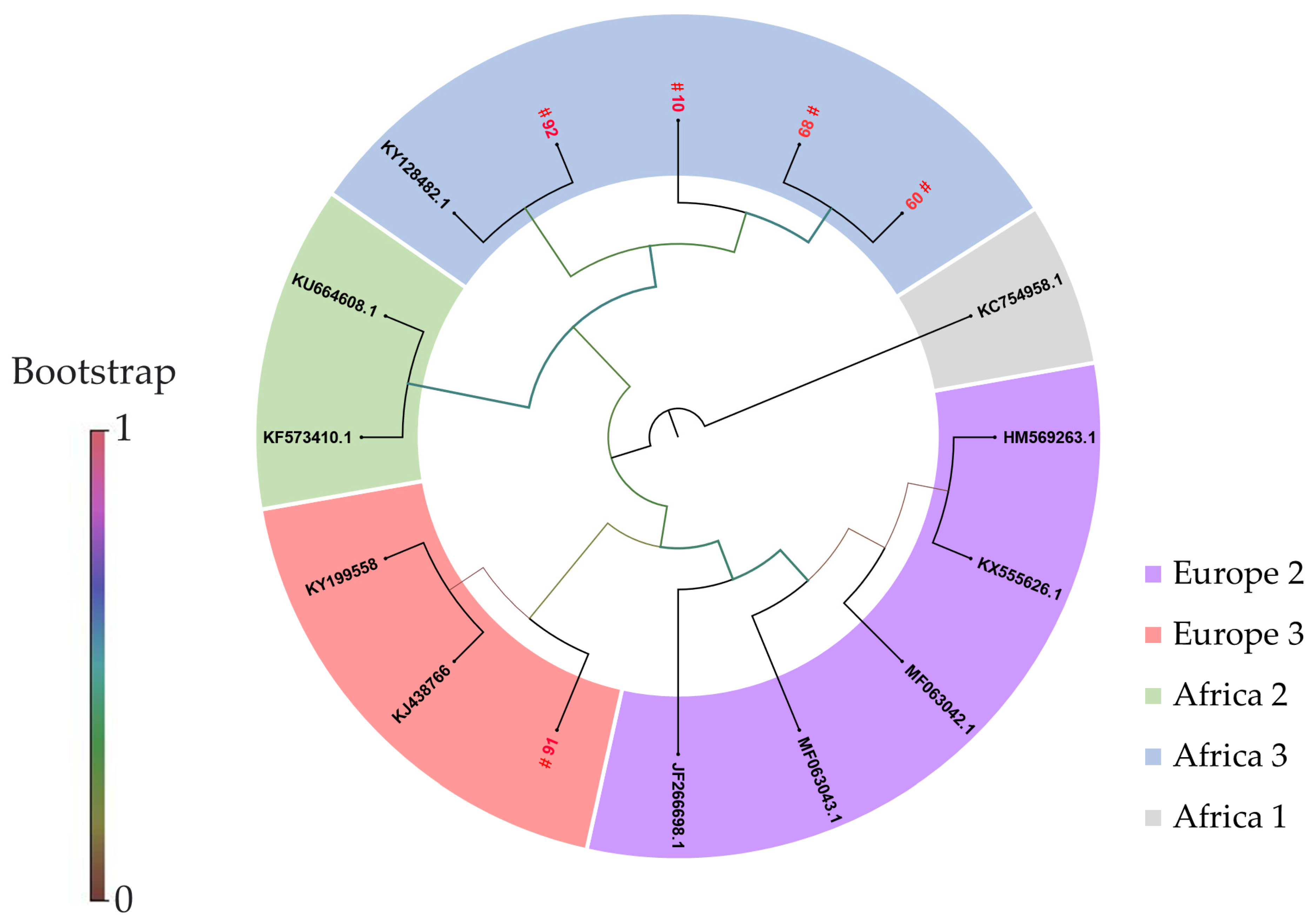
3.2. Phylogenetic Analyses
3.3. Serological Results
3.3.1. Serological Results Obtained by Blocking ELISA (bELISA)
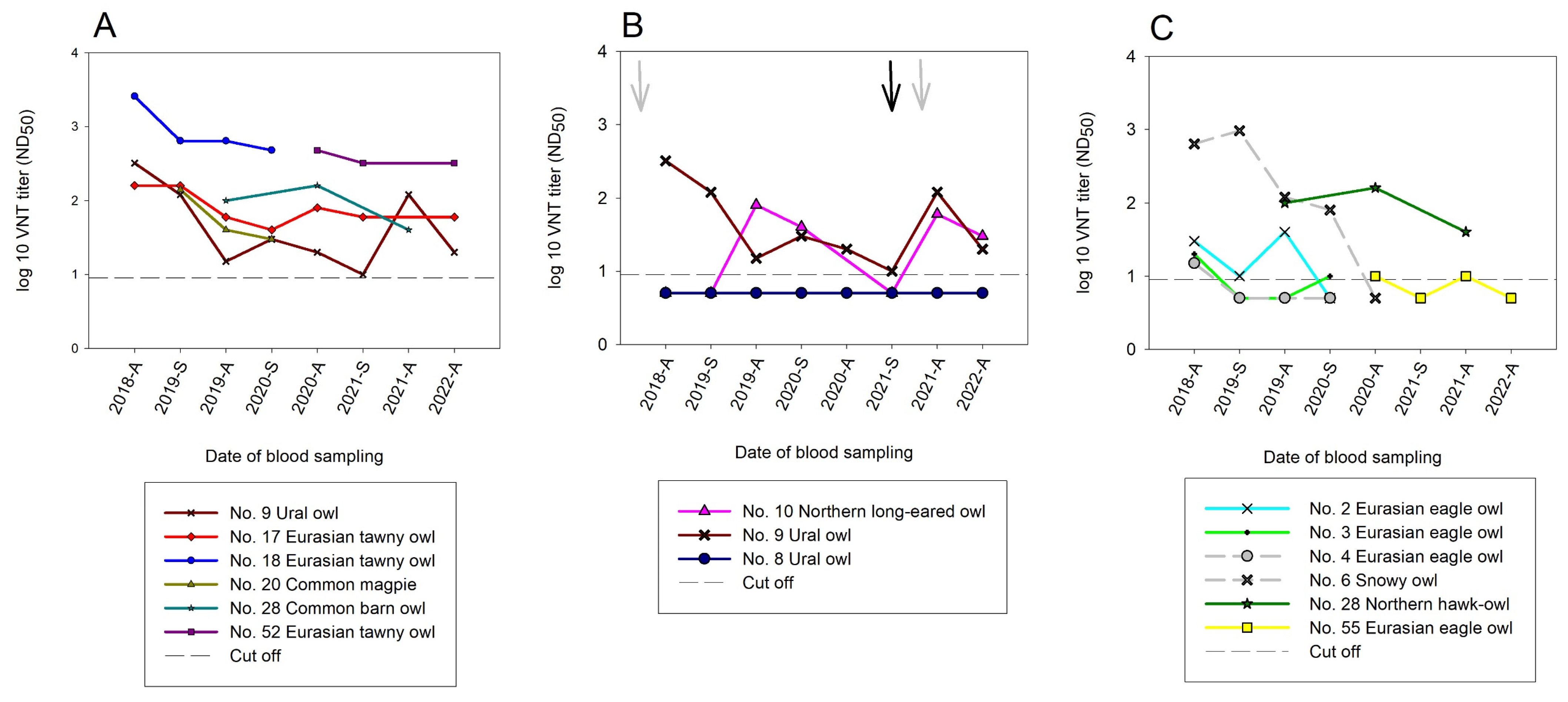
3.3.2. Serological Results with Virus Neutralization Assays (VNTs)
3.3.3. Serological Results in the Temporal Context of this Longitudinal Study
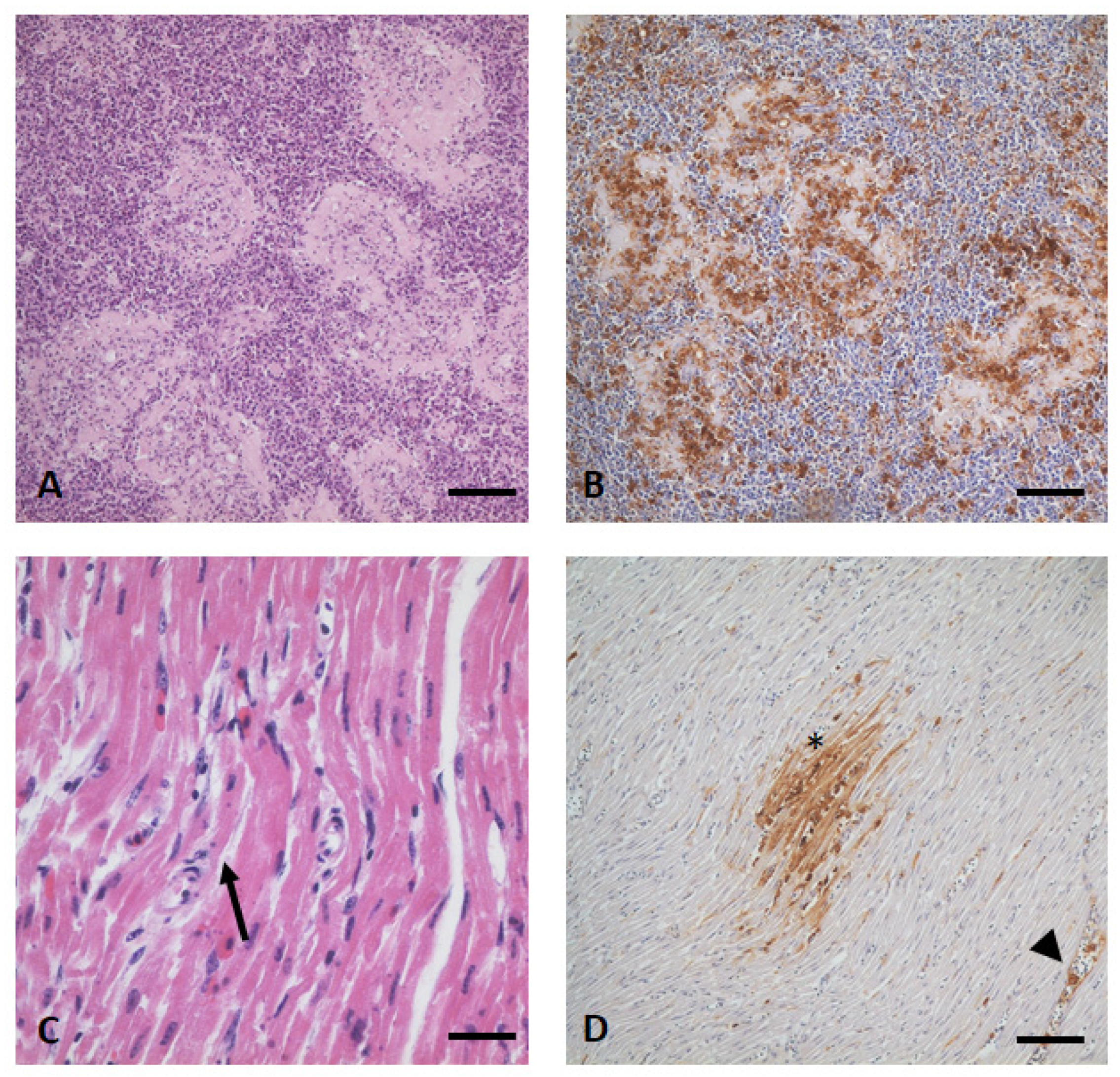
3.4. Gross Examination, Histopathology, and Immunohistochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolay, B. A review of West Nile and Usutu virus co-circulation in Europe: How much do transmission cycles overlap? Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Zannoli, S.; Sambri, V. West Nile Virus and Usutu Virus Co-Circulation in Europe: Epidemiology and Implications. Microorganisms 2019, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Clé, M.; Beck, C.; Salinas, S.; Lecollinet, S.; Gutierrez, S.; Van de Perre, P.; Baldet, T.; Foulongne, V.; Simonin, Y. Usutu virus: A new threat? Epidemiol. Infect. 2019, 147, e232. [Google Scholar] [CrossRef]
- Ziegler, U.; Bergmann, F.; Fischer, D.; Müller, K.; Holicki, C.M.; Sadeghi, B.; Sieg, M.; Keller, M.; Schwehn, R.; Reuschel, M.; et al. Spread of West Nile Virus and Usutu Virus in the German Bird Population, 2019–2020. Microorganisms 2022, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.; Fischer, D.; Eiden, M.; Fast, C.; Reuschel, M.; Müller, K.; Rinder, M.; Urbaniak, S.; Brandes, F.; Schwehn, R.; et al. West Nile Virus and Usutu Virus Monitoring of Wild Birds in Germany. Int. J. Environ. Res. Public Health 2018, 15, 171. [Google Scholar] [CrossRef] [PubMed]
- McNamara, T. The role of zoos in biosurveillance. Int. Zoo Yearb. 2007, 41, 12–15. [Google Scholar] [CrossRef]
- Ziegler, U.; Santos, P.D.; Groschup, M.H.; Hattendorf, C.; Eiden, M.; Höper, D.; Eisermann, P.; Keller, M.; Michel, F.; Klopfleisch, R.; et al. West Nile Virus Epidemic in Germany Triggered by Epizootic Emergence, 2019. Viruses 2020, 12, 448. [Google Scholar] [CrossRef]
- Weissenböck, H.; Kolodziejek, J.; Url, A.; Lussy, H.; Rebel-Bauder, B.; Nowotny, N. Emergence of Usutu virus, an African Mosquito-Borne Flavivirus of the Japanese Encephalitis Virus Group, Central Europe. Emerg. Infect. Dis. 2002, 8, 652–656. [Google Scholar] [CrossRef]
- Buchebner, N.; Zenker, W.; Wenker, C.; Steinmetz, H.W.; Sós, E.; Lussy, H.; Nowotny, N. Low Usutu virusseroprevalence in four zoological gardens in central Europe. BMC Vet. Res. 2013, 9, 153. [Google Scholar] [CrossRef]
- Manarolla, G.; Bakonyi, T.; Gallazzi, D.; Crosta, L.; Weissenböck, H.; Dorrestein, G.M.; Nowotny, N. Usutu virus in wild birds in northern Italy. Vet. Microbiol. 2010, 141, 159–163. [Google Scholar] [CrossRef]
- Steinmetz, H.W.; Bakonyi, T.; Weissenböck, H.; Hatt, J.-M.; Eulenberger, U.; Robert, N.; Hoop, R.; Nowotny, N. Emergence and establishment of Usutu virus infection in wild and captive avian species in and around Zurich, Switzerland—Genomic and pathologic comparison to other central European outbreaks. Vet. Microbiol. 2011, 148, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Jöst, H.; Bialonski, A.; Maus, D.; Sambri, V.; Eiden, M.; Groschup, M.H.; Günther, S.; Becker, N.; Schmidt-Chanasit, J. Isolation of Usutu virus in Germany. Am. J. Trop. Med. Hyg. 2011, 85, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Jöst, H.; Ziegler, U.; Eiden, M.; Höper, D.; Emmerich, P.; Fichet-Calvet, E.; Ehichioya, D.U.; Czajka, C.; Gabriel, M.; et al. Epizootic Emergence of Usutu Virus in Wild and Captive Birds in Germany. PLoS ONE 2012, 7, e32604. [Google Scholar] [CrossRef]
- Ziegler, U.; Lühken, R.; Keller, M.; Cadar, D.; van der Grinten, E.; Michel, F.; Albrecht, K.; Eiden, M.; Rinder, M.; Lachmann, L.; et al. West Nile virus epizootic in Germany, 2018. Antivir. Res. 2019, 162, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.; Sieg, M.; Fischer, D.; Keller, M.; Eiden, M.; Reuschel, M.; Schmidt, V.; Schwehn, R.; Rinder, M.; Urbaniak, S.; et al. Evidence for West Nile Virus and Usutu Virus Infections in Wild and Resident Birds in Germany, 2017 and 2018. Viruses 2019, 11, 674. [Google Scholar] [CrossRef]
- Friedrich-Loeffler-Institut. Das West-Nil-Virus War Auch im Jahr 2022 Wieder Deutlich Sichtbar. Available online: https://www.fli.de/de/aktuelles/kurznachrichten/neues-einzelansicht/das-west-nil-virus-war-auch-im-jahr-2022-wieder-deutlich-sichtbar/ (accessed on 18 January 2023).
- Eiden, M.; Vina-Rodriguez, A.; Hoffmann, B.; Ziegler, U.; Groschup, M.H. Two New Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction Assays with Unique Target Sites for the Specific and Sensitive Detection of Lineages 1 and 2 West Nile Virus Strains. J. Vet. Diagn. Investig. 2010, 22, 748–753. [Google Scholar] [CrossRef]
- Cavrini, F.; Della Pepa, M.E.; Gaibani, P.; Pierro, A.M.; Rossini, G.; Landini, M.P.; Sambri, V. A rapid and specific real-time RT-PCR assay to identify Usutu virus in human plasma, serum, and cerebrospinal fluid. J. Clin. Virol. 2011, 50, 221–223. [Google Scholar] [CrossRef]
- Hoffmann, B.; Depner, K.; Schirrmeier, H.; Beer, M. A universal heterologous internal control system for duplex real-time RT-PCR assays used in a detection system for pestiviruses. J. Virol. Methods 2006, 136, 200–209. [Google Scholar] [CrossRef]
- Holicki, C.M.; Bergmann, F.; Stoek, F.; Schulz, A.; Groschup, M.H.; Ziegler, U.; Sadeghi, B. Expedited retrieval of high-quality Usutu virus genomes via Nanopore sequencing with and without target enrichment. Front. Microbiol. 2022, 13, 1044316. [Google Scholar] [CrossRef]
- Quick, J.; Grubaugh, N.D.; Pullan, S.T.; Claro, I.M.; Smith, A.D.; Gangavarapu, K.; Oliveira, G.; Robles-Sikisaka, R.; Rogers, T.F.; Beutler, N.A.; et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat. Protoc. 2017, 12, 1261–1276. [Google Scholar] [CrossRef]
- Oude Munnink, B.B.; Kik, M.; de Bruijn, N.D.; Kohl, R.; van der Linden, A.; Reusken, C.B.E.M.; Koopmans, M. Towards high quality real-time whole genome sequencing during outbreaks using Usutu virus as example. Infect. Genet. Evol. 2019, 73, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine—National Center for Biotechnology Information. Basic Local Alignment Search Tool (BLAST): Version 2.13.0. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 25 January 2023).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree, Version 1.4.3.; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2012. [Google Scholar]
- Seidowski, D.; Ziegler, U.; von Rönn, J.A.; Müller, K.; Hüppop, K.; Müller, T.; Freuling, C.; Mühle, R.-U.; Nowotny, N.; Ulrich, R.G.; et al. West Nile Virus Monitoring of Migratory and Resident Birds in Germany. Vector-Borne Zoonotic Dis. 2010, 10, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Mayr, A.; Bachmann, P.A.; Bibrack, B.; Wittmann, G. Neutralisationstest: Virologische Arbeitsmethoden (Serologie), 1st ed.; Gustav Fischer Verlag: Jena, Germany, 1977. [Google Scholar]
- Bergmann, F.; Holicki, C.M.; Michel, F.; Bock, S.; Scuda, N.; Priemer, G.; Kenklies, S.; Siempelkamp, T.; Skuballa, J.; Sauerwald, C.; et al. Reconstruction of the molecular evolution of Usutu virus in Germany: Insights into virus emersion and circulation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Roehrig, J.T.; Deubel, V.; Smith, J.; Parker, M.; Steele, K.; Crise, B.; Volpe, K.E.; Crabtree, M.B.; Scherret, J.H.; et al. Origin of the West Nile Virus Responsible for an Outbreak of Encephalitis in the Northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Benzarti, E.; Rivas, J.; Sarlet, M.; Franssen, M.; Desmecht, D.; Schmidt-Chanasit, J.; Savini, G.; Lorusso, A.; Van Laere, A.-S.; Garigliany, M.-M. Experimental Usutu Virus Infection in Domestic Canaries Serinus canaria. Viruses 2020, 12, 164. [Google Scholar] [CrossRef]
- Clé, M.; Constant, O.; Barthelemy, J.; Desmetz, C.; Martin, M.F.; Lapeyre, L.; Cadar, D.; Savini, G.; Teodori, L.; Monaco, F.; et al. Differential neurovirulence of Usutu virus lineages in mice and neuronal cells. J. Neuroinflamm. 2021, 18, 11. [Google Scholar] [CrossRef]
- Kuchinsky, S.C.; Hawks, S.A.; Mossel, E.C.; Coutermarsh-Ott, S.; Duggal, N.K. Differential pathogenesis of Usutu virus isolates in mice. PLoS Neglected Trop. Dis. 2020, 14, e0008765. [Google Scholar] [CrossRef]
- Rijks, J.M.; Kik, M.L.; Slaterus, R.; Foppen, R.; Stroo, A.; Ijzer, J.; Stahl, J.; Gröne, A.; Koopmans, M.; van der Jeugd, H.P.; et al. Widespread Usutu virus outbreak in birds in the Netherlands, 2016. Euro Surveill. 2016, 21, 30391. [Google Scholar] [CrossRef]
- Ziegler, U.; Fast, C.; Eiden, M.; Bock, S.; Schulze, C.; Hoeper, D.; Ochs, A.; Schlieben, P.; Keller, M.; Zielke, D.E.; et al. Evidence for an independent third Usutu virus introduction into Germany. Vet. Microbiol. 2016, 192, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Chvala, S.; Kolodziejek, J.; Nowotny, N.; Weissenböck, H. Pathology and Viral Distribution in Fatal Usutu Virus Infections of Birds from the 2001 and 2002 Outbreaks in Austria. J. Comp. Pathol. 2004, 131, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Garigliany, M.-M.; Marlier, D.; Tenner-Racz, K.; Eiden, M.; Cassart, D.; Gandar, F.; Beer, M.; Schmidt-Chanasit, J.; Desmecht, D. Detection of Usutu virus in a bullfinch (Pyrrhula pyrrhula) and a great spotted woodpecker (Dendrocopos major) in north-west Europe. Vet. J. 2014, 199, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Störk, T.; de le Roi, M.; Haverkamp, A.-K.; Jesse, S.T.; Peters, M.; Fast, C.; Gregor, K.M.; Könenkamp, L.; Steffen, I.; Ludlow, M.; et al. Analysis of avian Usutu virus infections in Germany from 2011 to 2018 with focus on dsRNA detection to demonstrate viral infections. Sci. Rep. 2021, 11, 24191. [Google Scholar] [CrossRef] [PubMed]
- Agliani, G.; Giglia, G.; Marshall, E.M.; Gröne, A.; Rockx, B.H.; van den Brand, J.M. Pathological features of West Nile and Usutu virus natural infections in wild and domestic animals and in humans: A comparative review. One Health 2023, 16, 100525. [Google Scholar] [CrossRef]
- Constant, O.; Bollore, K.; Clé, M.; Barthelemy, J.; Foulongne, V.; Chenet, B.; Gomis, D.; Virolle, L.; Gutierrez, S.; Desmetz, C.; et al. Evidence of Exposure to USUV and WNV in Zoo Animals in France. Pathogens 2020, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Peter, N.; Holicki, C.M.; Schantz, A.V.; Ziegler, U.; Eiden, M.; Dörge, D.D.; Vilcinskas, A.; Groschup, M.H.; Klimpel, S. SARS-CoV-2 and West Nile Virus Prevalence Studies in Raccoons and Raccoon Dogs from Germany. Viruses 2022, 14, 2559. [Google Scholar] [CrossRef]
- Holicki, C.M.; Michel, F.; Vasić, A.; Fast, C.; Eiden, M.; Răileanu, C.; Kampen, H.; Werner, D.; Groschup, M.H.; Ziegler, U. Pathogenicity of West Nile Virus Lineage 1 to German Poultry. Vaccines 2020, 8, 507. [Google Scholar] [CrossRef]
- Kvapil, P.; Račnik, J.; Kastelic, M.; Bártová, E.; Korva, M.; Jelovšek, M.; Avšič-Županc, T. A Sentinel Serological Study in Selected Zoo Animals to Assess Early Detection of West Nile and Usutu Virus Circulation in Slovenia. Viruses 2021, 13, 626. [Google Scholar] [CrossRef]


| Species Order | Common Name | Scientific Name | USUV Positive/ Birds Tested | WNV Positive/ Birds Tested |
|---|---|---|---|---|
| Anseriformes | Eurasian teal | Anas crecca | 0/2 | 0/2 |
| Canada goose | Branta canadensis | 0/1 | 0/1 | |
| Graylag goose | Anser anser | 0/1 | 0/1 | |
| Barnacle goose | Branta leucosis | 0/1 | 0/1 | |
| Muscovy duck | Cairina moschata | 0/2 | 0/2 | |
| Ruddy shelduck | Tadorna ferruginea | 0/1 | 0/1 | |
| Mallard | Anas platyrhyn. | 0/1 | 0/1 | |
| Lesser white-fronted goose | Anser erythropus | 0/2 | 0/2 | |
| Ciconiiformes | Black stork | Ciconia nigra | 0/1 | 0/1 |
| White stork | Ciconia ciconia | 0/4 | 0/4 | |
| Coraciiformes | Laughing kookaburra | Dacelo novaeguineae | 0/1 | 0/1 |
| Galliformes | Golden pheasant | Chrysolophus pictus | 0/3 | 0/3 |
| Common pheasant | Phasianus colchicus | 0/4 | 0/4 | |
| Hamburg chicken | Gallus gallus domesticus | 0/2 | 0/2 | |
| Gruiformes | Common crane | Grus grus | 0/1 | 0/1 |
| Passeriformes | Common magpie | Pica pica | 0/1 | 0/1 |
| Common raven | Corvus corax | 0/3 | 0/3 | |
| Strigiformes | Great grey owl | Strix nebulosa | 4/5 | 0/5 |
| Ural owl | Strix uralensis | 2/2 | 0/2 | |
| Common barn owl | Tyto alba | 1/11 | 0/11 | |
| Snowy owl | Bubo scandiacus | 0/8 | 0/8 | |
| Northern hawk-owl | Surnia ulula | 0/7 | 0/7 | |
| Eurasian eagle owl | Bubo bubo | 0/11 | 0/11 | |
| Eurasian tawny owl | Strix aluco | 0/7 | 0/7 | |
| Northern long-eared owl | Asio otus | 1/8 | 0/8 | |
| Eurasian scops owl | Otus scops | 0/2 | 0/2 | |
| 8/92 | 0/92 |
| Bird’s ID | Species Order | Common Name | Scientific Name | Ct-Value ¶ | Tissue | Date of Sampling | USUV Lineage | GenBank Accession No. |
|---|---|---|---|---|---|---|---|---|
| 91 *,# | Strigiformes | Great grey owl | Strix nebulosa | 15.75 | Brain | 08-2018 | Europe 3 | OQ630904 |
| 15.32 | Liver | |||||||
| 92 *,# | Strigiformes | Great grey owl | Strix nebulosa | 21.26 | Brain | 09-2018 | Africa 3 | OQ630905 |
| 18.30 | Liver | |||||||
| 8 | Strigiformes | Ural owl | Strix uralensis | 37.12 | Blood coagulum | 03-2021 | nt | nt |
| 9 | Strigiformes | Ural owl | Strix uralensis | 33.23 | Blood coagulum | 03-2021 | nt | nt |
| 10 # | Strigiformes | Northern long-eared owl | Asio otus | 32.79 | Blood coagulum | 03-2021 | Africa 3 | OQ630908 |
| 49 | Strigiformes | Common barn owl | Tyto alba | 35.77 | Blood coagulum | 03-2021 | nt | nt |
| 60 # | Strigiformes | Great grey owl | Strix nebulosa | 23.88 | Brain | 08-2021 | Africa 3 | OQ630907 |
| 20.48 | Liver | |||||||
| 22.44 | Spleen | |||||||
| 68 # | Strigiformes | Great grey owl | Strix nebulosa | 25.73 | Brain | 08-2021 | Africa 3 | OQ630906 |
| 20.08 | Liver | |||||||
| 21.47 | Spleen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergmann, F.; Schmoock-Wellhausen, M.; Fast, C.; Holicki, C.M.; Michel, F.; Wysocki, P.; Sadeghi, B.; Groschup, M.H.; Ziegler, U. Longitudinal Study of the Occurrence of Usutu Virus and West Nile Virus Infections in Birds in a Zoological Garden in Northern Germany. Pathogens 2023, 12, 753. https://doi.org/10.3390/pathogens12060753
Bergmann F, Schmoock-Wellhausen M, Fast C, Holicki CM, Michel F, Wysocki P, Sadeghi B, Groschup MH, Ziegler U. Longitudinal Study of the Occurrence of Usutu Virus and West Nile Virus Infections in Birds in a Zoological Garden in Northern Germany. Pathogens. 2023; 12(6):753. https://doi.org/10.3390/pathogens12060753
Chicago/Turabian StyleBergmann, Felicitas, Martina Schmoock-Wellhausen, Christine Fast, Cora M. Holicki, Friederike Michel, Patrick Wysocki, Balal Sadeghi, Martin H. Groschup, and Ute Ziegler. 2023. "Longitudinal Study of the Occurrence of Usutu Virus and West Nile Virus Infections in Birds in a Zoological Garden in Northern Germany" Pathogens 12, no. 6: 753. https://doi.org/10.3390/pathogens12060753
APA StyleBergmann, F., Schmoock-Wellhausen, M., Fast, C., Holicki, C. M., Michel, F., Wysocki, P., Sadeghi, B., Groschup, M. H., & Ziegler, U. (2023). Longitudinal Study of the Occurrence of Usutu Virus and West Nile Virus Infections in Birds in a Zoological Garden in Northern Germany. Pathogens, 12(6), 753. https://doi.org/10.3390/pathogens12060753






