Molecular Characterization of Porcine Reproductive and Respiratory Syndrome Virus in Korea from 2018 to 2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. RNA Extraction and PCR Amplication
2.3. Phylognetic Analysis Based on ORF5
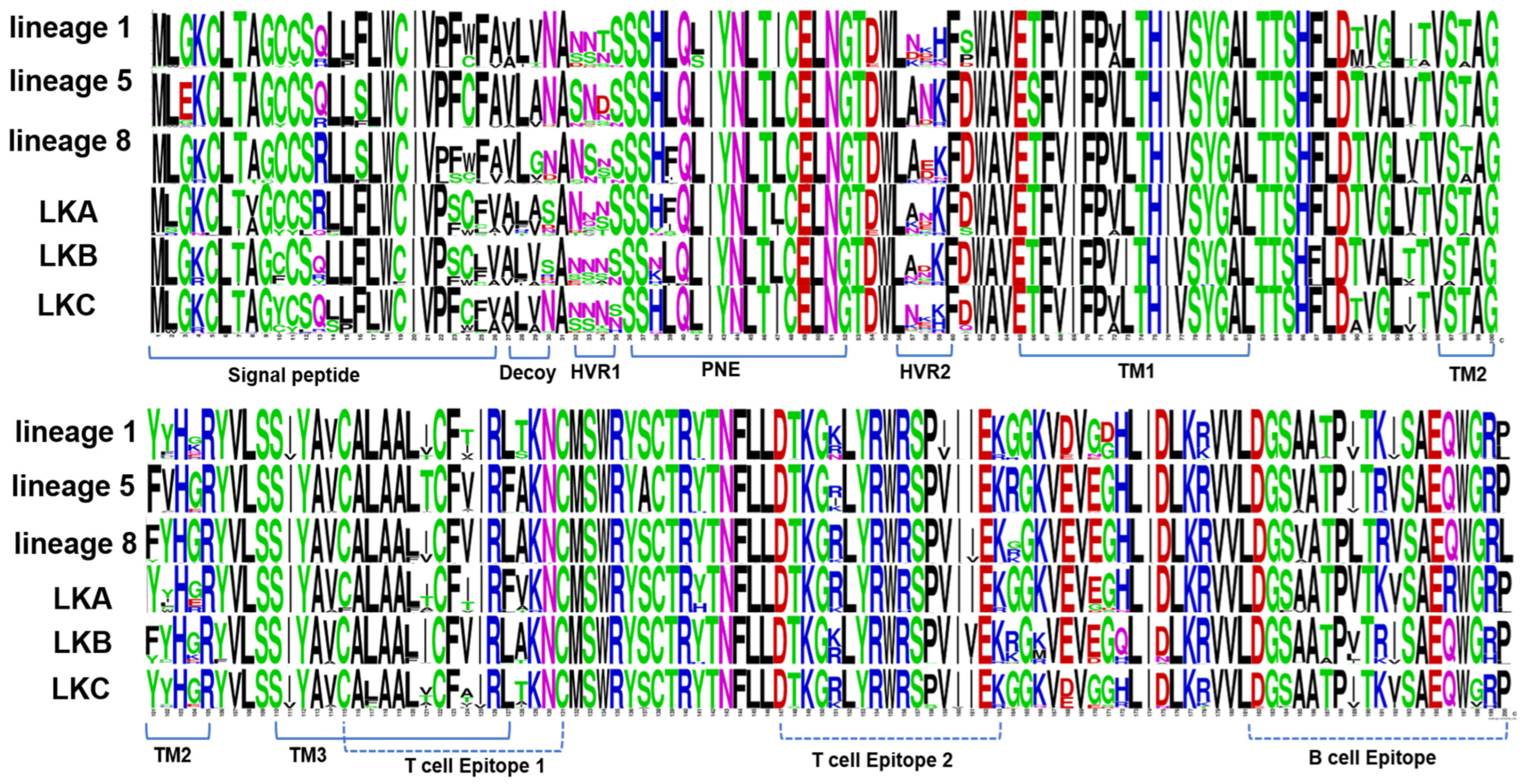
2.4. Analysis of Amino Acids of PRRSV
2.5. Analysis NSP2 Gene
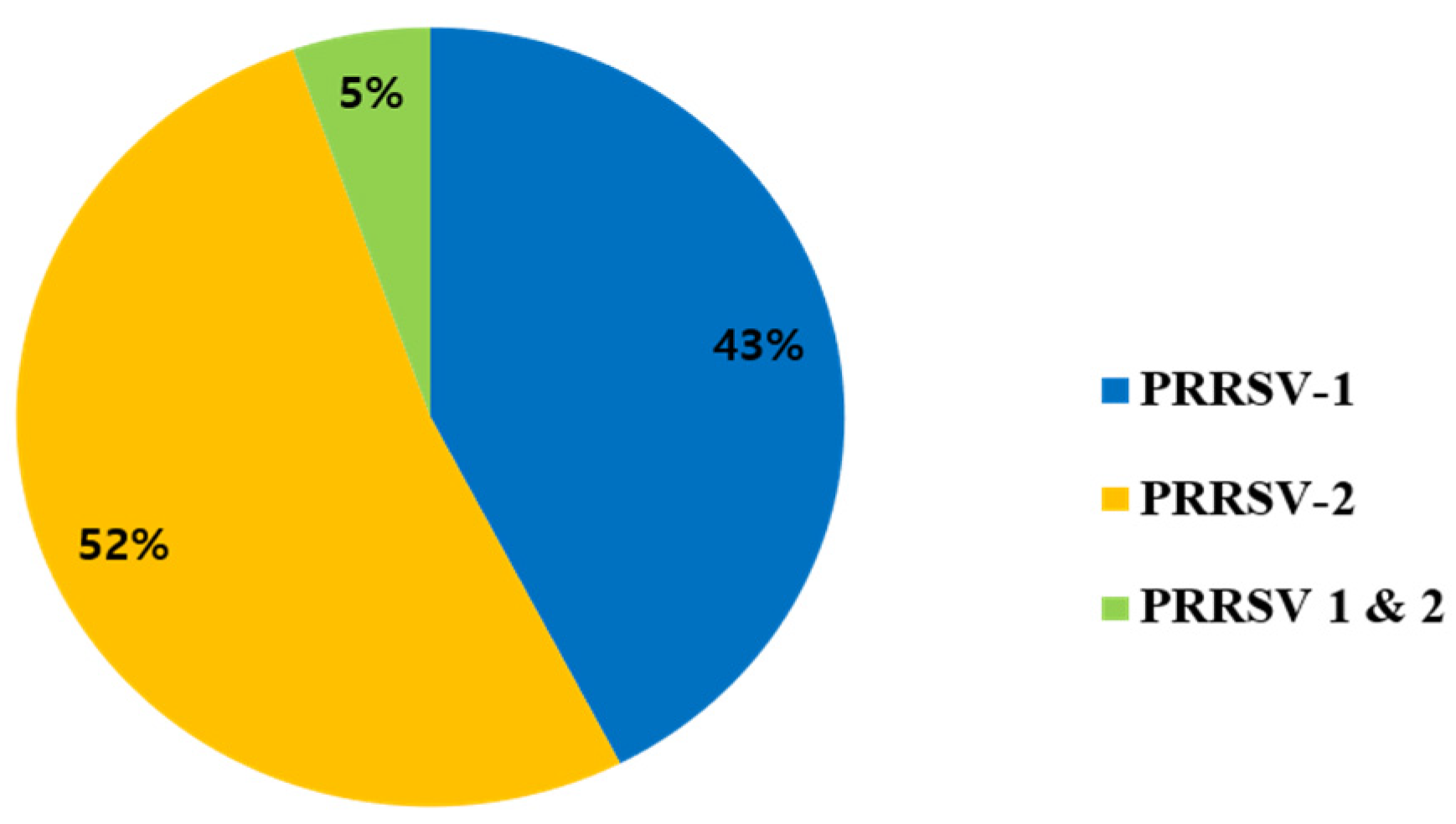
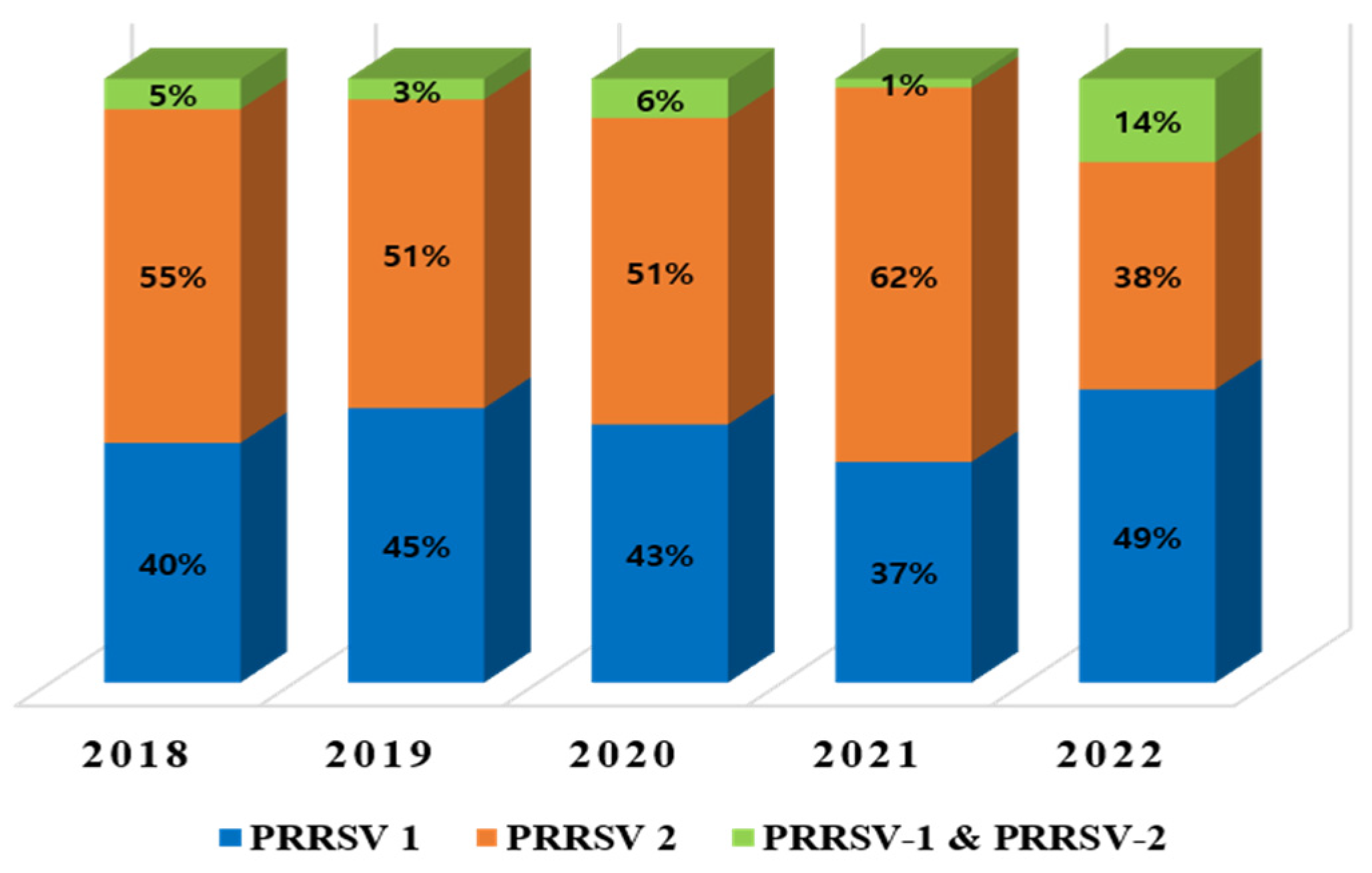
3. Results
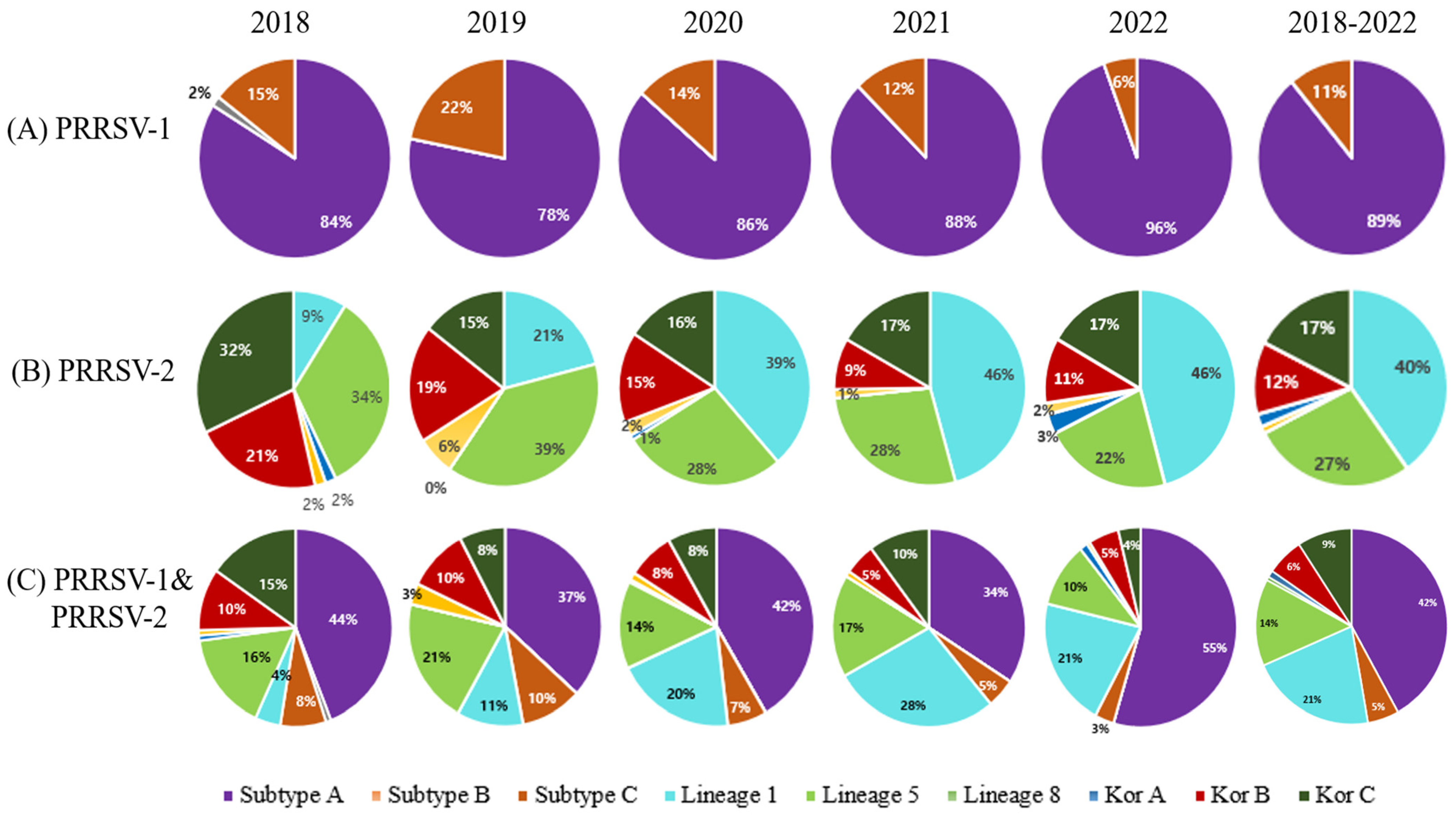
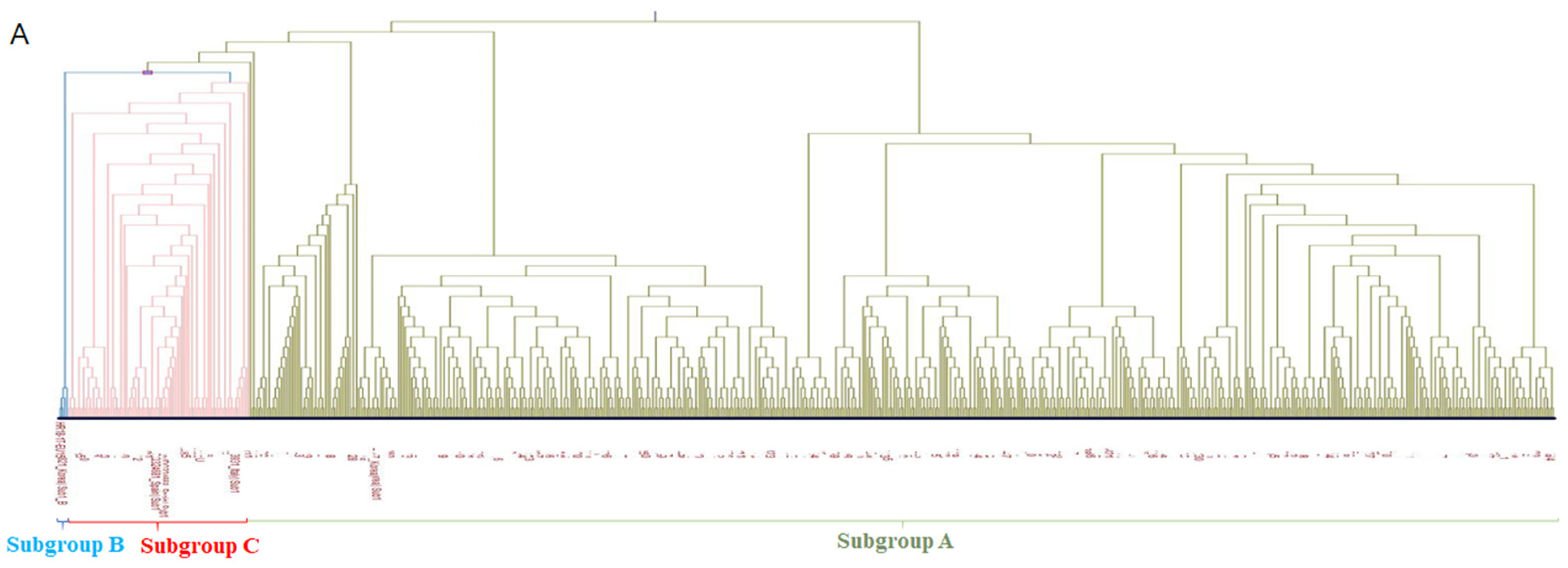
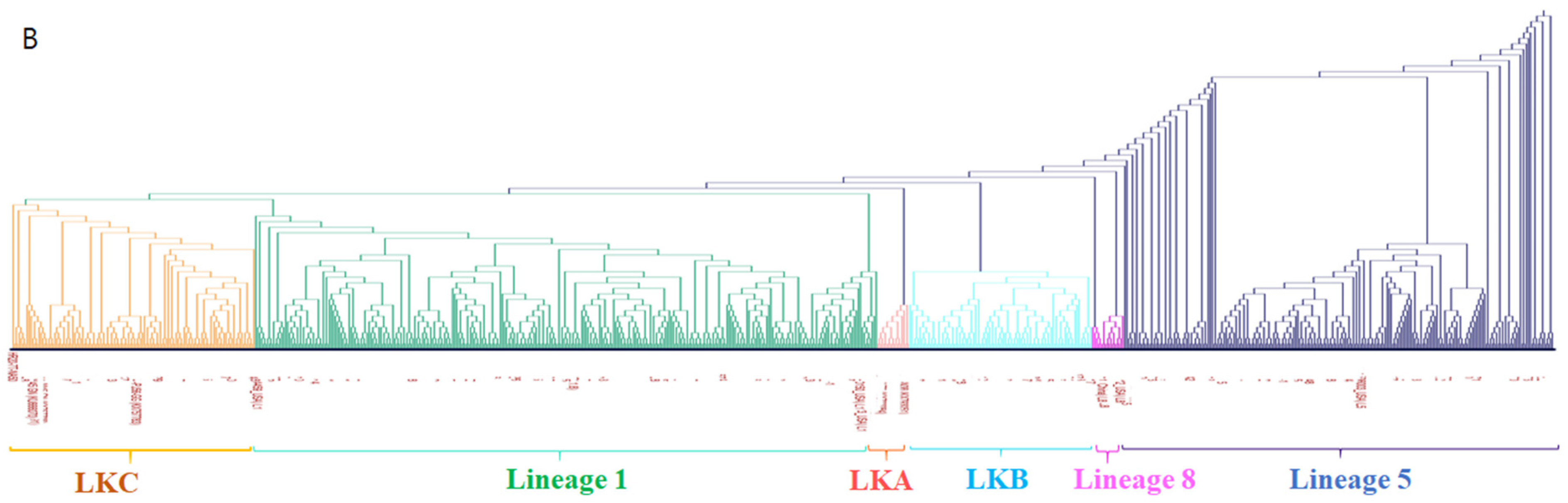
3.1. Phylogenetic Analysis
3.2. Amino Acids Analysis of PRRSV-1 Subgroup A and Subgroup C ORF5 Gene
3.3. Analysis of Amino Acids of PRRSV-2 GP5 (ORF5 Gene) Protein
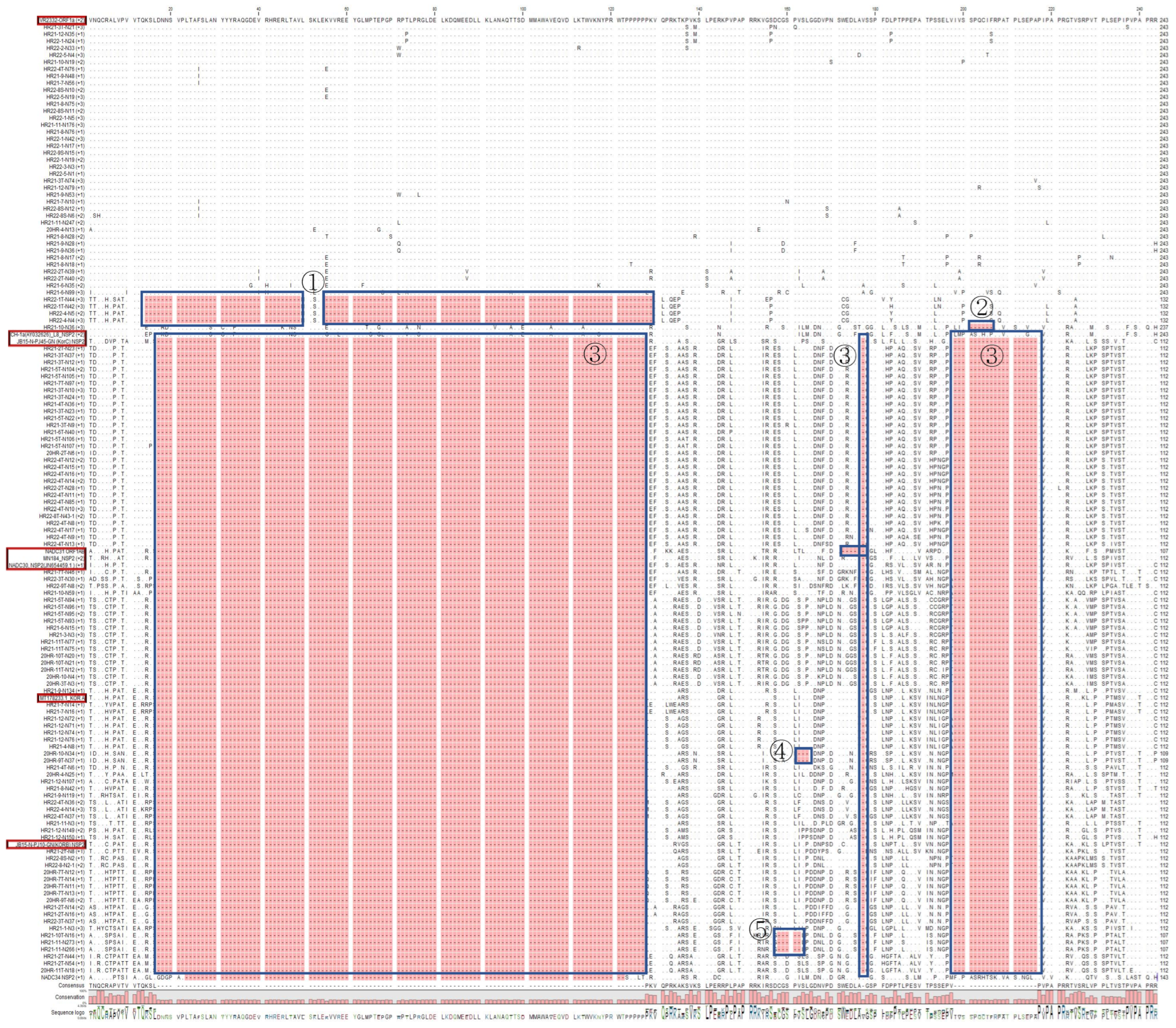
3.4. Non-Structural Protein (NSP2) Deletion Pattern of PRRSV-1
3.5. Non-Structural Protein (NSP2) Deletion Pattern of PRRSV-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keffaber, K.K. Reproductive failure of unknown etiology. Am. Assoc. Swine Pract. Newsl. 1989, 1, 1–9. [Google Scholar]
- Andreyev, V.G.; Wesley, R.D.; Mengeling, W.L.; Vorwald, A.C.; Lager, K.M. Genetic variation and phylogenetic relationships of 22 porcine reproductive and respiratory syndrome virus (PRRSV) field strains based on sequence analysis of open reading frame 5. Arch. Virol. 1997, 142, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Christopher-Hennings, J.; Nelson, E.A.; Nelson, J.K.; Hines, R.J.; Swenson, S.L.; Hill, H.T.; Zimmerman, J.J.; Katz, J.B.; Yeager, M.J.; Chase, C.C. Detection of porcine reproductive and respiratory syndrome virus in boar semen by PCR. J. Clin. Microbiol. 1995, 33, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; Junglen, S.; et al. Changes to virus taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 2019, 164, 2417–2429. [Google Scholar] [CrossRef]
- Kim, W.I.; Yoon, K.J. Molecular assessment of the role of envelope-associated structural proteins in cross neutralization among different PRRS viruses. Virus Genes 2008, 37, 380–391. [Google Scholar] [CrossRef]
- Meng, X.J. Heterogeneity of porcine reproductive and respiratory syndrome virus: Implications for current vaccine efficacy and future vaccine development. Vet. Microbiol. 2000, 74, 309–329. [Google Scholar] [CrossRef]
- Bautista, E.M.; Faaberg, K.S.; Mickelson, D.; McGruder, E.D. Functional properties of the predicted helicase of porcine reproductive and respiratory syndrome virus. Virology 2002, 298, 258–270. [Google Scholar] [CrossRef]
- Fang, Y.; Snijder, E.J. The PRRSV replicase: Exploring the multifunctionality of an intriguing set of nonstructural proteins. Virus Res. 2010, 154, 61–76. [Google Scholar] [CrossRef]
- Fang, Y.; Treffers, E.E.; Li, Y.; Tas, A.; Sun, Z.; Van Der Meer, Y.; De Ru, A.H.; Van Veelen, P.A.; Atkins, J.F.; Snijder, E.J.; et al. Efficient−2 frameshifting by mammalian ribosomes to synthesize an additional arterivirus protein. Proc. Natl. Acad. Sci. USA 2012, 109, E2920–E2928. [Google Scholar] [CrossRef]
- Snijder, E.J.; Spaan, W.J.M. Arteriviruses. In Field’s Virology, 5th ed.; Knipe, D.M., Howley, P.M., Griffin, D.E., Lamb, R.A., Martin, M.A., Roizman, B., Straus, S.E., Eds.; Lippincott Williams and Wilkins Publishers: Philadelphia, PA, USA, 2007; pp. 1337–1355. [Google Scholar]
- van Aken, D.; Zevenhoven-Dobbe, J.; Gorbalenya, A.E.; Snijder, E.J. Proteolytic maturation of replicase polyprotein pp1a by the nsp4 main proteinase is essential for equine arteritis virus replication and includes internal cleavage of nsp7. J. Gen. Virol. 2006, 87, 3473–3482. [Google Scholar] [CrossRef]
- Li, B.; Fang, L.; Guo, X.; Gao, J.; Song, T.; Bi, J.; He, K.; Chen, H.; Xiao, S. Epidemiology and evolutionary characteristics of the porcine reproductive and respiratory syndrome virus in China between 2006 and 2010. J. Clin. Microbiol. 2011, 49, 3175–3183. [Google Scholar] [CrossRef]
- Wang, P.P.; Dong, J.G.; Zhang, L.Y.; Liang, P.S.; Liu, Y.L.; Wang, L.; Fan, F.H.; Song, C.X. Sequence and phylogenetic analyses of the Nsp2 and ORF5 genes of porcine reproductive and respiratory syndrome virus in boars from South China in 2015. Transbound. Emerg. Dis. 2017, 64, 1953–1964. [Google Scholar] [CrossRef]
- Kang, H.; Yu, J.E.; Shin, J.E.; Kang, A.; Kim, W.I.; Lee, C.; Lee, J.; Cho, I.S.; Choe, S.E.; Cha, S.H. Geographic distribution and molecular analysis of porcine reproductive and respiratory syndrome viruses circulating in swine farms in the Republic of Korea between 2013 and 2016. BMC Vet. Res. 2018, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.A.; Torremorell, M.; Rossow, K.; Mahlum, C.; Otake, S.; Faaberg, K. Identification of genetically diverse sequences (ORF 5) of porcine reproductive and respiratory syndrome virus in a swine herd. Can. J. Vet. Res. 2001, 65, 254. [Google Scholar] [PubMed]
- Pirzadeh, B.; Gagnon, C.A.; Dea, S. Genomic and antigenic variations of porcine reproductive and respiratory syndrome virus major envelope GP5 glycoprotein. Can. J. Vet. Res. 1998, 62, 170. [Google Scholar] [PubMed]
- Wissink, E.H.J.; Kroese, M.V.; Van Wijk, H.A.R.; Rijsewijk, F.A.M.; Meulenberg, J.J.M.; Rottier, P.J.M. Envelope protein requirements for the assembly of infectious virions of porcine reproductive and respiratory syndrome virus. J. Virol. 2005, 79, 12495–12506. [Google Scholar] [CrossRef]
- Nilubol, D.; Tripipat, T.; Hoonsuwan, T.; Tipsombatboon, P.; Piriyapongsa, J. Genetic diversity of the ORF5 gene of porcine reproductive and respiratory syndrome virus (PRRSV) genotypes I and II in Thailand. Arch. Virol. 2013, 158, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.Z.; Wang, Z.; Ha, Z.; Zhang, Y.; Xie, Y.B.; Zhang, H.; Nan, F.L.; Zhang, J.Y.; Zhao, G.Y.; Li, Z.X.; et al. Genetic characterization of a new NSP2-deletion porcine reproductive and Respiratory Syndrome Virus in China. Microb. Pathog. 2021, 150, 104729. [Google Scholar] [CrossRef]
- Jakab, S.; Kaszab, E.; Marton, S.; Bányai, K.; Bálint, Á.; Nemes, I.; Szabó, I. Genetic diversity of imported PRRSV-2 strains, 2005–2020, Hungary. Front. Vet. Sci. 2022, 9, 986850. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lam, T.T.Y.; Hon, C.C.; Murtaugh, M.P.; Davies, P.R.; Hui, R.K.H.; Li, J.; Wong, L.T.W.; Yip, C.W.; Jiang, J.W.; et al. Phylogeny-based evolutionary, demographical, and geographical dissection of North American type 2 porcine reproductive and respiratory syndrome viruses. J. Virol. 2010, 84, 8700–8711. [Google Scholar] [CrossRef]
- Kim, S.H.; Roh, I.S.; Choi, E.J.; Lee, C.; Lee, C.H.; Lee, K.H.; Lee, K.K.; Song, Y.K.; Lee, O.S.; Park, C.K. A molecular analysis of European porcine reproductive and respiratory syndrome virus isolated in South Korea. Vet. Microbiol. 2010, 143, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Moon, S.H.; Jeong, C.G.; Park, G.S.; Park, J.Y.; Jeoung, H.Y.; Shin, G.E.; Ko, M.K.; Kim, S.H.; Lee, K.K.; et al. Whole-genome sequencing and genetic characteristics of representative porcine reproductive and respiratory syndrome virus (PRRSV) isolates in Korea. Virol. J. 2022, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.Y.; Campler, M.R.; Schroeder, D.C.; Yang, M.; Mor, S.K.; Ferreira, J.B.; Arruda, A.G. Detection of multiple lineages of PRRSV in breeding and growing swine farms. Front. Vet. Sci. 2022, 9, 750. [Google Scholar] [CrossRef]
- Shin, J.H.; Kang, Y.B.; Kim, Y.J.; Yeom, S.H.; Kweon, C.H.; Lee, W.Y.; Jean, Y.H.; Hwang, E.K.; Rhee, J.C.; An, S.H.; et al. Sero-epidemiological studies on porcine reproductive and respiratory syndrome in Korea-(1)-Detection of indirect fluorescent antibodies. RDA J. Agric. Sci. 1993, 35, 572–576. [Google Scholar]
- Cho, J.G.; Dee, S.A. Porcine reproductive and respiratory syndrome virus. Theriogenology 2006, 66, 655–662. [Google Scholar] [CrossRef]
- Kim, S.C.; Jeong, C.G.; Park, G.S.; Park, J.Y.; Jeoung, H.Y.; Shin, G.E.; Ko, M.K.; Kim, S.H.; Lee, K.K.; Kim, W.I. Temporal lineage dynamics of the ORF5 gene of porcine reproductive and respiratory syndrome virus in Korea in 2014–2019. Arch. Virol. 2021, 166, 2803–2815. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.Y.; Sur, J.H.; Lyoo, Y.S. Serological and genetic characterization of the European strain of the porcine reproductive and respiratory syndrome virus isolated in Korea. Korean J. Vet. Res. 2006, 46, 363–370. [Google Scholar]
- Nam, E.; Park, C.K.; Kim, S.H.; Joo, Y.S.; Yeo, S.G.; Lee, C. Complete genomic characterization of a European type 1 porcine reproductive and respiratory syndrome virus isolate in Korea. Arch. Virol. 2009, 154, 629–638. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.; Kang, B.; Yeom, M.; Han, S.; Moon, H.; Park, S.; Kim, H.; Song, D.; Park, B. Prevalence and phylogenetic analysis of the isolated type I porcine reproductive and respiratory syndrome virus from 2007 to 2008 in Korea. Virus Genes 2010, 40, 225–230. [Google Scholar] [CrossRef]
- Kwon, T.; Yoo, S.J.; Park, J.W.; Kang, S.C.; Park, C.K.; Lyoo, Y.S. Genomic characteristics and pathogenicity of natural recombinant porcine reproductive and respiratory syndrome virus 2 harboring genes of a Korean field strain and VR-2332-like strain. Virology 2019, 530, 89–98. [Google Scholar] [CrossRef]
- Yin, B.; Qi, S.; Sha, W.; Qin, H.; Liu, L.; Yun, J.; Zhu, J.; Li, G.; Sun, D. Molecular characterization of the Nsp2 and ORF5 (ORF5a) genes of PRRSV strains in nine provinces of China during 2016–2018. Front. Vet. Sci. 2021, 8, 605832. [Google Scholar] [CrossRef]
- Ramos, N.; Betancour, G.; Puig, J.; Arbiza, J. An update on genetic analysis of porcine reproductive and respiratory syndrome virus type 2 (PRRSV-2) in South America: Identification of ORF5 sequences of lineage 1A, 1C and 1G. Arch. Microbiol. 2022, 204, 367. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Cao, Z.; Tang, Y.D.; Xia, D.; Wang, G.; Shan, H. Recent advances in porcine reproductive and respiratory syndrome virus NADC30-like research in China: Molecular characterization, pathogenicity, and control. Front. Microbiol. 2021, 12, 791313. [Google Scholar] [CrossRef] [PubMed]
- Brockmeier, S.L.; Loving, C.L.; Vorwald, A.C.; Kehrli, M.E., Jr.; Baker, R.B.; Nicholson, T.L.; Lager, K.M.; Miller, L.C.; Faaberg, K.S. Genomic sequence and virulence comparison of four type 2 porcine reproductive and respiratory syndrome virus strains. Virus Res. 2012, 169, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Van Geelen, A.G.M.; Anderson, T.K.; Lager, K.M.; Das, P.B.; Otis, N.J.; Montiel, N.A.; Miller, L.C.; Kulshreshtha, V.; Buckley, A.C.; Brockmeier, S.L.; et al. Porcine reproductive and respiratory disease virus: Evolution and recombination yields distinct ORF5 RFLP 1-7-4 viruses with individual pathogenicity. Virology 2018, 513, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kim, H.J.; Moon, S.H.; Cho, H.S.; Kim, W.I. First identification and Genomic Characterization of NADC34-like PRRSV Strains Isolated from MLV-Vaccinated Pigs in Korea. Transbound. Emerg. Dis. 2023, 2013, 9995433. [Google Scholar] [CrossRef]
- Zhao, K.; Ye, C.; Chang, X.B.; Jiang, C.G.; Wang, S.J.; Cai, X.H.; Cai, X.H.; Tong, G.Z.; Tian, Z.J.; Shi, M.; et al. Importation and recombination are responsible for the latest emergence of highly pathogenic porcine reproductive and respiratory syndrome virus in China. J. Virol. 2015, 89, 10712–10716. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Kang, R.; Zhang, Y.; Ding, M.; Xie, B.; Tian, Y.; Wu, X.; Zuo, L.; Yang, X.; Wang, H. Whole genome analysis of two novel type 2 porcine reproductive and respiratory syndrome viruses with complex genome recombination between lineage 8, 3, and 1 strains identified in southwestern China. Viruses 2018, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Yu, X.; Zhao, T.; Feng, Y.; Cao, Z.; Wang, C.; Hu, Y.; Chen, X.; Hu, D.; Tian, X.; et al. Emergence of fatal PRRSV variants: Unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS ONE 2007, 2, e526. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, X.X.; Li, R.; Qiao, S.; Zhang, G. The prevalent status and genetic diversity of porcine reproductive and respiratory syndrome virus in China: A molecular epidemiological perspective. Virol. J. 2018, 15, 2. [Google Scholar] [CrossRef]
- Wenhui, L.; Zhongyan, W.; Guanqun, Z.; Zhili, L.; JingYun, M.; Qingmei, X.; Baoli, S.; Yingzuo, B. Complete genome sequence of a novel variant porcine reproductive and respiratory syndrome virus (PRRSV) strain: Evidence for recombination between vaccine and wild-type PRRSV strains. J. Virol. 2012, 86, 9543. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.H.; Tun, H.M.; Sun, B.L.; Mo, J.; Zhou, Q.F.; Deng, Y.X.; Xie, Q.M.; Bi, Y.Z.; Leung, F.C.; Ma, J.Y. Re-emerging of porcine respiratory and reproductive syndrome virus (lineage 3) and increased pathogenicity after genomic recombination with vaccine variant. Vet. Microbiol. 2015, 175, 332–340. [Google Scholar] [CrossRef]
- Chen, N.; Liu, Q.; Qiao, M.; Deng, X.; Chen, X.; Sun, M. Whole genome characterization of a novel porcine reproductive and respiratory syndrome virus 1 isolate: Genetic evidence for recombination between Amervac vaccine and circulating strains in mainland China. Infect. Genet. Evol. 2017, 54, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.H.; Kaewprom, K.; Wang, S.Y.; Lin, C.F.; Yang, C.Y.; Chiou, M.T.; Lin, C.N. Outbreak of porcine reproductive and respiratory syndrome virus 1 in Taiwan. Viruses 2020, 12, 316. [Google Scholar] [CrossRef]
- Lin, W.H.; Shih, H.C.; Wang, S.Y.; Lin, C.F.; Yang, C.Y.; Chiou, M.T.; Lin, C.N. Emergence of a virulent porcine reproductive and respiratory syndrome virus in Taiwan in 2018. Transbound. Emerg. Dis. 2019, 66, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, X.; Lunney, J.K.; Lawson, S.; Sun, Z.; Brown, E.; Christopher-Hennings, J.; Knudsen, D.; Nelson, E.; Fang, Y. Immunodominant epitopes in nsp2 of porcine reproductive and respiratory syndrome virus are dispensable for replication, but play an important role in modulation of the host immune response. J. Gen. Virol. 2010, 91, 1047–1057. [Google Scholar] [CrossRef]
- Nan, Y.; Wu, C.; Gu, G.; Sun, W.; Zhang, Y.J.; Zhou, E.M. Improved vaccine against PRRSV: Current progress and future perspective. Front. Microbiol. 2017, 8, 1635. [Google Scholar] [CrossRef]
- Wang, X. Immunological Selection as a Driver of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Evolution. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2016. [Google Scholar]
- Lee, J.A.; Lee, N.H.; Lee, J.B.; Park, S.Y.; Song, C.S.; Choi, I.S.; Lee, S.W. Genetic diversity of the Korean field strains of porcine reproductive and respiratory syndrome virus. Infect. Genet. Evol. 2016, 40, 288–294. [Google Scholar] [CrossRef]
- Martínez-Lobo, F.J.; Díez-Fuertes, F.; Segalés, J.; García-Artiga, C.; Simarro, I.; Castro, J.M.; Prieto, C. Comparative pathogenicity of type 1 and type 2 isolates of porcine reproductive and respiratory syndrome virus (PRRSV) in a young pig infection model. Vet. Microbiol. 2011, 154, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhuang, J.; Wang, J.; Han, L.; Sun, Z.; Xiao, Y.; Ji, G.; Li, Y.; Tan, F.; Li, X.; et al. Outbreak Investigation of NADC 30-Like PRRSV in South-East China. Transbound. Emerg. Dis. 2016, 63, 474–479. [Google Scholar] [CrossRef]
- Ramírez, M.; Bauermann, F.V.; Navarro, D.; Rojas, M.; Manchego, A.; Nelson, E.A.; Diel, D.G.; Rivera, H. Detection of porcine reproductive and respiratory syndrome virus (PRRSV) 1-7-4-type strains in Peru. Transbound. Emerg. Dis. 2019, 66, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, C.; Li, W.; Zhao, J.; Gong, B.; Sun, Q.; Tang, Y.D.; Xiang, L.; Leng, C.; Peng, J.; et al. Novel characteristics of Chinese NADC34-like PRRSV during 2020–2021. Transbound. Emerg. Dis. 2022, 69, e3215–e3224. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Lee, C.H.; Song, J.Y.; Song, H.J.; Park, C.K.; Kim, B.; Shin, Y.K. Genetic diversity of porcine reproductive and respiratory syndrome virus in Korea. J. Vet. Sci. 2013, 14, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Choi, E.J.; Park, J.H.; Yoon, S.R.; Song, J.Y.; Kwon, J.H.; Song, H.J.; Yoon, K.J. Molecular characterization of recent Korean porcine reproductive and respiratory syndrome (PRRS) viruses and comparison to other Asian PRRS viruses. Vet. Microbiol. 2006, 117, 248–257. [Google Scholar] [CrossRef]
- Kim, H.K.; Nguyen, V.G.; Kim, I.O.; Park, J.H.; Park, S.J.; Rho, S.M.; Han, J.Y.; Park, B.K. Epidemiologic and phylogenetic characteristics of porcine reproductive and respiratory syndrome viruses in conventional swine farms of Jeju Island as a candidate region for PRRSV eradication. Transbound. Emerg. Dis. 2012, 59, 62–71. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, X.; Tian, Y.; Yin, S.; Geng, G.; Ge, X.; Guo, X.; Yang, H. Genetic diversity analysis of genotype 2 porcine reproductive and respiratory syndrome viruses emerging in recent years in China. BioMed Res. Int. 2014, 2014, 748068. [Google Scholar] [CrossRef]
- Zhao, H.; Han, Q.; Zhang, L.; Zhang, Z.; Wu, Y.; Shen, H.; Jiang, P. Emergence of mosaic recombinant strains potentially associated with vaccine JXA1-R and predominant circulating strains of porcine reproductive and respiratory syndrome virus in different provinces of China. Virol. J. 2017, 14, 67. [Google Scholar] [CrossRef]
- Zhou, L.; Kang, R.; Ji, G.; Tian, Y.; Ge, M.; Xie, B.; Yang, X.; Wang, H. Molecular characterization and recombination analysis of porcine reproductive and respiratory syndrome virus emerged in southwestern China during 2012–2016. Virus Genes 2018, 54, 98–110. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, X.; Zhang, H.; Tang, X.; Bian, T.; Sun, Y.; Zhou, M.; Ren, F.; Wu, P. Evolutionary and recombination analysis of porcine reproductive and respiratory syndrome isolates in China. Virus Genes 2020, 56, 354–360. [Google Scholar] [CrossRef]
- Fang, K.; Liu, S.; Li, X.; Chen, H.; Qian, P. Epidemiological and genetic characteristics of porcine reproductive and respiratory syndrome virus in South China between 2017 and 2021. Front. Vet. Sci. 2022, 9, 853044. [Google Scholar] [CrossRef]
- Kapur, V.; Elam, M.R.; Pawlovich, T.M.; Murtaugh, M.P. Genetic variation in porcine reproductive and respiratory syndrome virus isolates in the midwestern United States. J. Gen. Virol. 1996, 77, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lemey, P.; Brar, M.S.; Suchard, M.A.; Murtaugh, M.P.; Carman, S.; D’Allaire, S.; Delisle, B.; Lambert, M.È.; Gagnon, C.A.; et al. The spread of type 2 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in North America: A phylogeographic approach. Virology 2013, 447, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Tian, K. Suppl-1, M3: NADC30-like porcine reproductive and respiratory syndrome in China. Open Virol. J. 2017, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Renson, P.; Touzain, F.; Lebret, A.; Le Dimna, M.; Quenault, H.; Normand, V.; Claude, J.B.; Pez, F.; Rose, N.; Blanchard, Y.; et al. Complete genome sequence of a recombinant porcine reproductive and respiratory syndrome virus strain from two genotype 1 modified live virus vaccine strains. Genome Announc. 2017, 5, e00454-17. [Google Scholar] [CrossRef] [PubMed]


| Target Gene | Genotype | Primer | Sequence (5′–3′) | Size (bp) |
|---|---|---|---|---|
| ORF5 | PRRSV-1 | EU F | 5′AATGAGGTGGGCYACAACC3′ | 754 |
| EU R | 5′GCGTGACACCTTAAGGGC3′ | |||
| PRRSV-2 | NA F | 5′CCATTCTGGTGGCAATTTGA3′ | 716 | |
| NA R | 5′GGCATATATCATCACTGGCG3′ | |||
| NSP2 | PRRSV-1 | F | CGGCACTGTTGTYGYCCTGC | 505/562 |
| R | AGACGCGGTGGACTTCACTG | |||
| PRRSV-2 | F | GTGATTGAGGACTGCTGCTGTTC | 907/1236 | |
| R | GTCGATGATGGCTTGAGCTGA |
| Year | Serum | Tissue | Total |
|---|---|---|---|
| 2018 | 243 | 250 | 493 |
| 2019 | 275 | 270 | 545 |
| 2020 | 432 | 444 | 876 |
| 2021 | 1307 | 830 | 2137 |
| 2022 | 337 | 674 | 1011 |
| Genotype | Reference | Subgroup/Lineage | Sequence Similarity | |
|---|---|---|---|---|
| Nucleotide (%) | Amino Acid (%) | |||
| PRRSV-1 | VP-046 | Subgroup C | 90.26–99.83 | 88.61–99.50 |
| Subgroup A | 79.87–88.78 | 69.31–90.0 | ||
| DV | Subgroup C | 86.14–100 | 84.16–100 | |
| Subgroup A | 82.54–88.12 | 68.32–88.56 | ||
| PRRSV-2 | VR2332 | L1 | 79.06–87.91 | 78.00–87.06 |
| L5 | 88.89–99.34 | 85.81–100 | ||
| L8 | 88.23–91.71 | 86.90–90.45 | ||
| LKA | 82.92–88.50 | 81.82–88.07 | ||
| LKB | 77.00–88.02 | 78.11–87.06 | ||
| LKC | 78.25–85.29 | 78.71–86.07 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-A.; Jayaramaiah, U.; You, S.-H.; Shin, E.-G.; Song, S.-M.; Ju, L.; Kang, S.-J.; Hyun, B.-H.; Lee, H.-S. Molecular Characterization of Porcine Reproductive and Respiratory Syndrome Virus in Korea from 2018 to 2022. Pathogens 2023, 12, 757. https://doi.org/10.3390/pathogens12060757
Lee M-A, Jayaramaiah U, You S-H, Shin E-G, Song S-M, Ju L, Kang S-J, Hyun B-H, Lee H-S. Molecular Characterization of Porcine Reproductive and Respiratory Syndrome Virus in Korea from 2018 to 2022. Pathogens. 2023; 12(6):757. https://doi.org/10.3390/pathogens12060757
Chicago/Turabian StyleLee, Min-A, Usharani Jayaramaiah, Su-Hwa You, Eun-Gyeong Shin, Seung-Min Song, Lanjeong Ju, Seok-Jin Kang, Bang-Hun Hyun, and Hyang-Sim Lee. 2023. "Molecular Characterization of Porcine Reproductive and Respiratory Syndrome Virus in Korea from 2018 to 2022" Pathogens 12, no. 6: 757. https://doi.org/10.3390/pathogens12060757




