Molecular Detection of Anaplasma, Ehrlichia and Rickettsia Pathogens in Ticks Collected from Humans in the Republic of Korea, 2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Collection and Identification
2.2. DNA Extraction
2.3. Molecular Detection of TBPs
2.4. Sequencing and Phylogenetic Analysis
2.5. Statistical Analyses
3. Results
3.1. Tick Collecction and Identification
3.2. Molecular Detection of TBPs
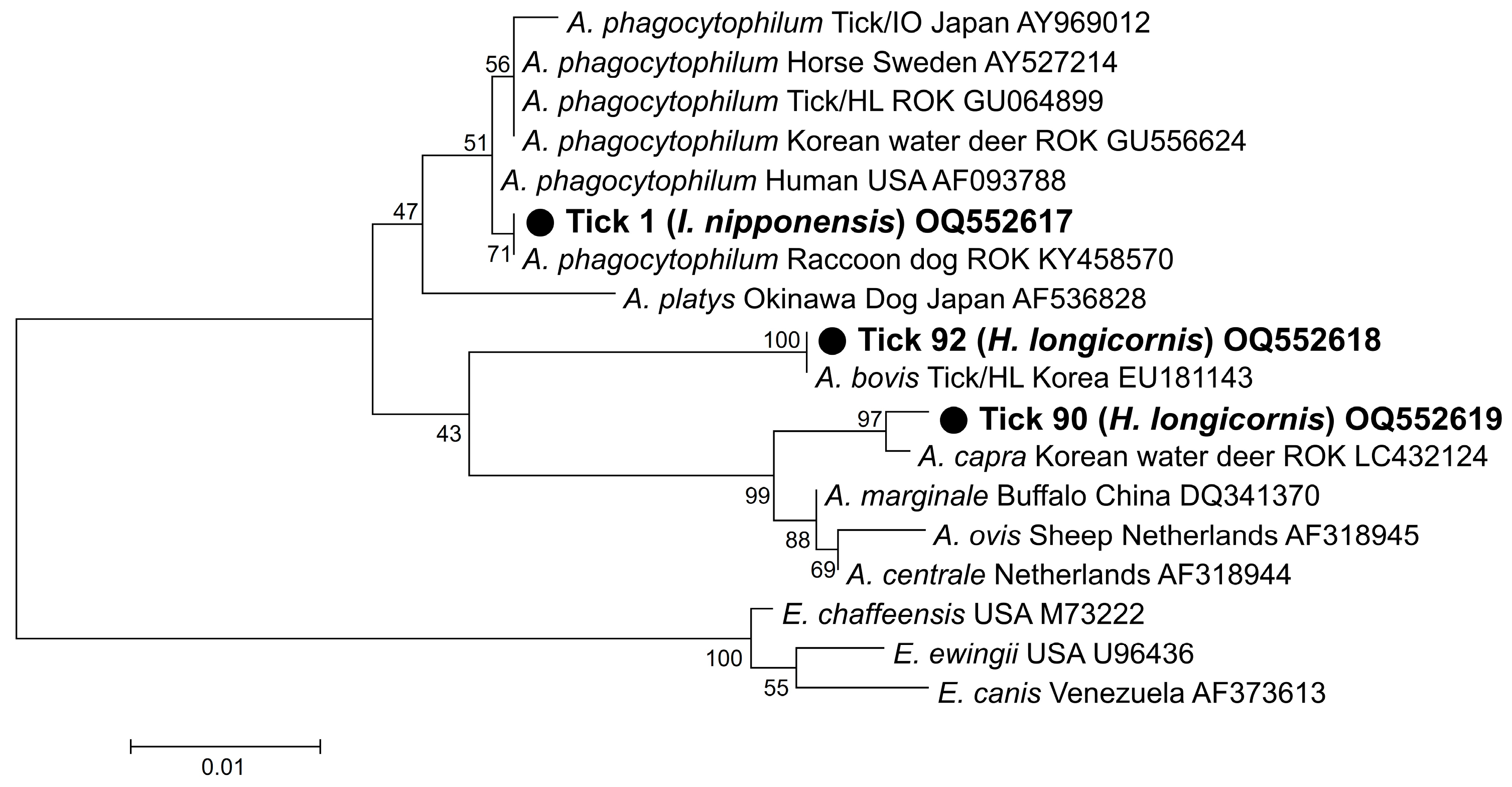
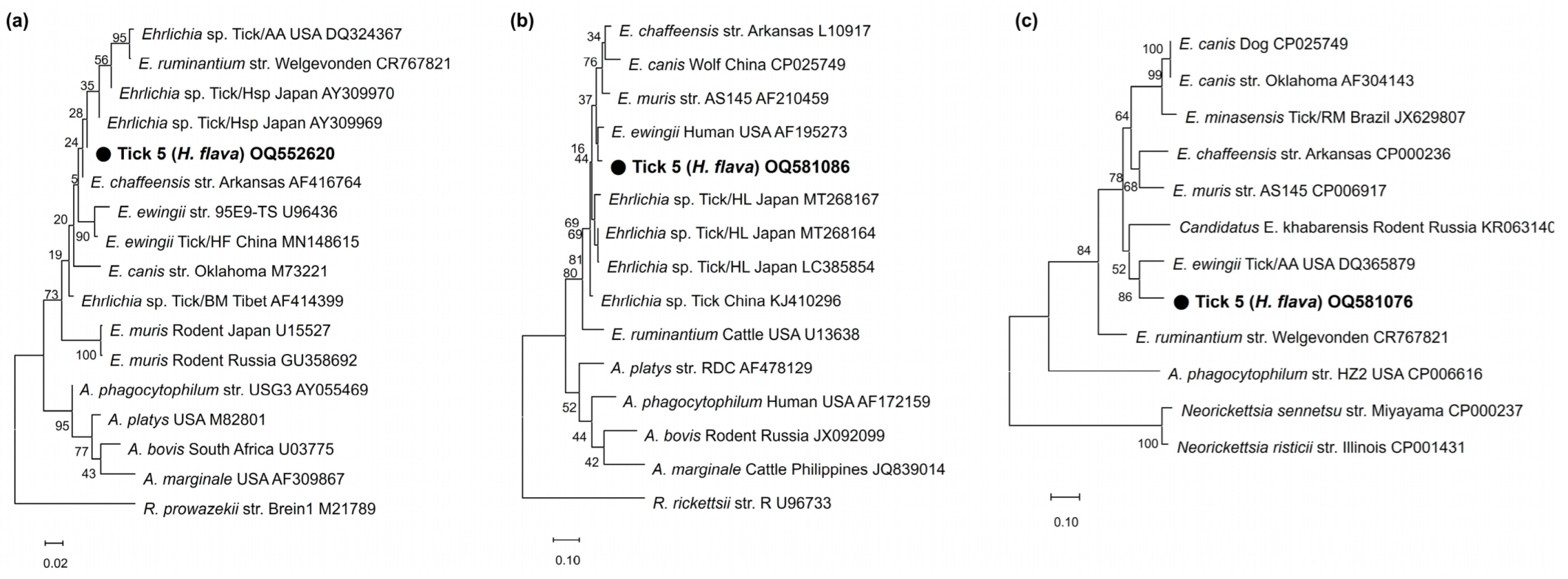
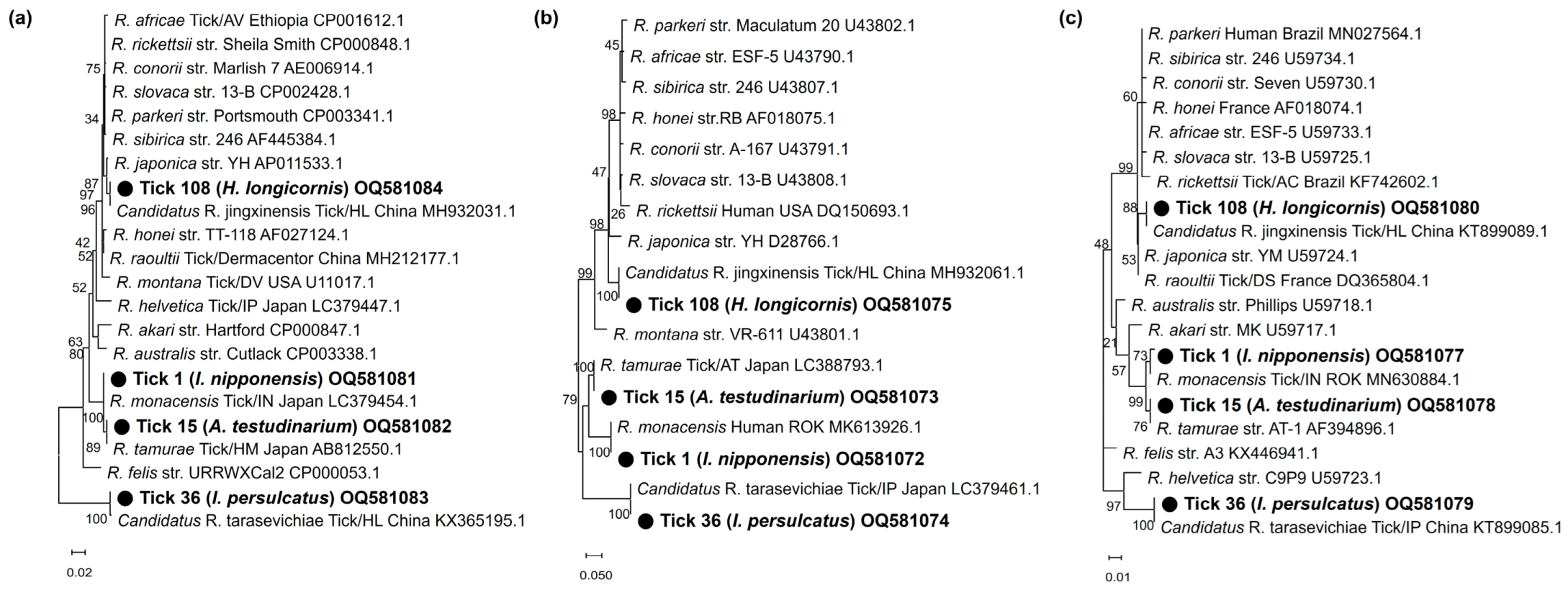
3.3. Sequencing and Phylogenetic Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef]
- Černý, J.; Lynn, G.; Hrnková, J.; Golovchenko, M.; Rudenko, N.; Grubhoffer, L. Management options for Ixodes ricinus-associated pathogens: A review of prevention strategies. Int. J. Environ. Res. Public Health 2020, 17, 1830. [Google Scholar] [CrossRef]
- Rossati, A. Global warming and its health impact. Int. J. Occup. Environ. Med. 2017, 8, 7–20. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jang, J.-Y. Correlations between climate change-related infectious diseases and meteorological factors in Korea. J. Prev. Med. Public Health 2010, 43, 436–444. [Google Scholar] [CrossRef]
- Medlock, J.M.; Leach, S.A. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect. Dis. 2015, 15, 721–730. [Google Scholar] [CrossRef]
- Bouchard, C.; Dibernardo, A.; Koffi, J.; Wood, H.; Leighton, P.A.; Lindsay, L.R. Increased risk of tick-borne diseases with climate and environmental changes. Can. Commun. Dis. Rep. 2019, 45, 83–89. [Google Scholar] [CrossRef]
- Im, J.H.; Baek, J.; Durey, A.; Kwon, H.Y.; Chung, M.-H.; Lee, J.-S. Current status of tick-borne diseases in South Korea. Vector-Borne Zoonotic Dis. 2019, 19, 225–233. [Google Scholar] [CrossRef]
- Sul, H.; Dong, M.K. Present state and future of tick-borne infectious diseases in Korea. J. Korean Med. Assoc. 2017, 60, 475–483. [Google Scholar] [CrossRef]
- Rogers, R.; O’Brien, T.; Aridi, J.; Beckwith, C.G. The COVID-19 diagnostic dilemma: A clinician’s perspective. J. Clin. Micobiol. 2020, 58, e01287-20. [Google Scholar] [CrossRef]
- Chen, S.M.; Dumler, J.S.; Bakken, J.S.; Walker, D.H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 1994, 32, 589–595. [Google Scholar] [CrossRef]
- Mowla, S.J.; Drexler, N.A.; Cherry, C.C.; Annambholta, P.D.; Kracalik, I.T.; Basavaraju, S.V. Ehrlichiosis and Anaplasmosis among Transfusion and Transplant Recipients in the United States. Emerg. Infect. Dis. 2021, 27, 2768–2775. [Google Scholar] [CrossRef] [PubMed]
- Centers of Disease Control and Prevention. Epidemiology and Statistics. Number of Reported Cases of Anaplasmosis in US. 2000–2019. Available online: https://www.cdc.gov/anaplasmosis/ (accessed on 12 December 2022).
- Stuen, S. Anaplasma phagocytophilum—The most widespread tick-borne infection in animals in Europe. Vet. Res. Commun. 2007, 31, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.C.; Zhao, Q.M.; Zhang, P.H.; Dumler, J.S.; Zhang, X.T.; Fang, L.Q.; Yang, H. Granulocytic Ehrlichiae in Ixodes persulcatus ticks from an area in China where Lyme disease is endemic. J. Clin. Microbiol. 2000, 38, 4208–4210. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, A.N.; Dubinina, H.V.; Van De Pol, I.; Schouls, L.M. Identification of Ehrlichia spp. and Borrelia burgdorferi in Ixodes ticks in the Baltic regions of Russia. J. Clin. Microbiol. 2001, 39, 2237–2242. [Google Scholar] [CrossRef]
- Kim, K.-H.; Yi, J.; Oh, W.S.; Kim, N.-H.; Choi, S.J.; Choe, P.G.; Kim, N.-J.; Lee, J.-K.; Oh, M.-d. Human granulocytic anaplasmosis, South Korea, 2013. Emerg. Infect. Dis. 2014, 20, 1708–1711. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, H.H.; Kim, J.Y.; Yoo, J.E.; Gill, B.C. Laboratory-based diagnostic test results for human granulocytic anaplasmosis in 2020. Public Health Wkly. Rep. PHWR 2021, 14, 2773–2780. [Google Scholar]
- Kim, C.-M.; Yi, Y.-H.; Yu, D.-H.; Lee, M.-J.; Cho, M.-R.; Desai, A.R.; Shringi, S.; Klein, T.A.; Kim, H.-C.; Song, J.-W.; et al. Tick-borne rickettsial pathogens in ticks and small mammals in Korea. Appl. Environ. Microbiol. 2006, 72, 5766–5776. [Google Scholar] [CrossRef]
- Heitman, K.N.; Dahlgren, F.S.; Drexler, N.A.; Massung, R.F.; Behravesh, C.B. Increasing incidence of ehrlichiosis in the United States: A summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am. J. Trop. Med. Hyg. 2016, 94, 52–60. [Google Scholar] [CrossRef]
- Centers of Disease Control and Prevention. Epidemiology and Statistics. Number of Reported Cases of Ehrlichiosis in US. 2000–2019. Available online: https://www.cdc.gov/ehrlichiosis/ (accessed on 12 December 2022).
- Buller, R.S.; Arens, M.; Hmiel, S.P.; Paddock, C.D.; Sumner, J.W.; Rikihisa, Y.; Storch, G.A. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 1999, 341, 148–155. [Google Scholar] [CrossRef]
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging tick-borne diseases. Clin. Microbiol. Rev. 2020, 33, e00083-18. [Google Scholar] [CrossRef]
- Sachar, D. Ehrlichia chaffeensis infection in an active duty soldier stationed in Korea. Med. Surveill. Mon. Rep. 2000, 6, 9–11. [Google Scholar]
- Cowan, G. Rickettsial diseases: The typhus group of fevers—A review. Postgrad. Med. J. 2000, 76, 269–272. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Centers of Disease Control and Prevention. Epidemiology and Statistics. Number of Reported Cases of Spotted Fever Group Rickettsioses in US. 2000–2019. Available online: https://www.cdc.gov/otherspottedfever/ (accessed on 12 December 2022).
- Ericsson, C.D.; Jensenius, M.; Fournier, P.E.; Raoult, D. Rickettsioses and the international traveler. Clin. Infect. Dis. 2004, 39, 1493–1499. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.S.; Jung, K.D.; Jang, W.J.; Koh, S.E.; Kang, S.S.; Lee, I.Y.; Lee, W.J.; Kim, B.J.; Kook, Y.H.; et al. Identification of the spotted fever group rickettsiae detected from Haemaphysalis longicornis in Korea. Microbiol. Immunol. 2003, 47, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Choi, Y.J.; Kim, J.; Kim, H.C.; Klein, T.A.; Chong, S.T.; Jang, W.J. Distribution of Rickettsia spp. in ticks from northwestern and southwestern provinces, Republic of Korea. Korean J. Parasitol. 2019, 57, 161–166. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, Y.J.; Lee, K.M.; Ahn, K.J.; Kim, H.C.; Klein, T.; Jiang, J.; Richards, A.; Park, K.H.; Jang, W.J. First isolation of Rickettsia monacensis from a patient in South Korea. Microbiol. Immunol. 2017, 61, 258–263. [Google Scholar] [CrossRef]
- Nadelman, R.B.; Wormser, G.P. Erythema migrans and early Lyme disease. Am. J. Med. 1995, 98, 15S–24S. [Google Scholar] [CrossRef]
- Centers of Disease Control and Prevention. Epidemiology and Statistics. Number of Reported Cases of Lyme in US. 2000–2019. Available online: https://www.cdc.gov/lyme/ (accessed on 12 December 2022).
- Korea Disease Control and Prevention Agency. Number of Reported Cases of Lyme in the ROK. 2011–2020. Available online: https://www.kdca.go.kr (accessed on 12 December 2022).
- Kim, S.Y.; Kim, T.-K.; Kim, T.Y.; Lee, H.I. Geographical distribution of Borrelia burgdorferi sensu lato in ticks collected from wild rodents in the Republic of Korea. Pathogens 2020, 9, 866. [Google Scholar] [CrossRef]
- Bang, M.S.; Kim, C.-M.; Pyun, S.-H.; Kim, D.-M.; Yun, N.R. Molecular investigation of tick-borne pathogens in ticks removed from tick-bitten humans in the southwestern region of the Republic of Korea. PLoS ONE 2021, 16, e0252992. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Seo, J.Y.; Kim, S.Y.; Lee, H.I. Molecular detection of Anaplasma phagocytophilum and Ehrlichia species in ticks removed from humans in the Republic of Korea. Microorganisms 2022, 10, 1224. [Google Scholar] [CrossRef] [PubMed]
- Yamaguti, N.; Tipton, V.J.; Keegan, H.L.; Toshioka, S. Ticks of Japan, Korea, and the Ryukyu islands. Brigham Young Univ. Sci. Bull. Biol. Ser. 1971, 15, 1. [Google Scholar]
- Barlough, J.E.; Madigan, J.E.; DeRock, E.; Bigornia, L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus). Vet. Parasitol. 1996, 63, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Massung, R.F.; Levin, M.L.; Munderloh, U.G.; Silverman, D.J.; Lynch, M.J.; Gaywee, J.K.; Kurtti, T.J. Isolation and propagation of the Ap-Variant 1 strain of Anaplasma phagocytophilum in a tick cell line. J. Clin. Microbiol. 2007, 45, 2138–2143. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z.; Niu, Q.; Liu, J.; Xie, J.; Chen, Q.; Chen, Z.; Guan, G.; Liu, G.; Luo, J.; et al. Evaluation of different nested PCRs for detection of Anaplasma phagocytophilum in ruminants and ticks. BMC Vet. Res. 2016, 12, 35. [Google Scholar] [CrossRef]
- Guo, W.-P.; Wang, X.; Li, Y.-N.; Xu, G.; Wang, Y.-H.; Zhou, E.-M. GroEL gene typing and genetic diversity of Anaplasma bovis in ticks in Shaanxi, China. Infect. Genet. Evol. 2019, 74, 103927. [Google Scholar] [CrossRef]
- Oh, J.Y.; Moon, B.-C.; Bae, B.K.; Shin, E.-H.; Ko, Y.H.; Kim, Y.-J.; Park, Y.H.; Chae, J.S. Genetic identification and phylogenetic analysis of Anaplasma and Ehrlichia species in Haemaphysalis longicornis collected from Jeju Island, Korea. J. Bacteriol. Virol. 2009, 39, 257–267. [Google Scholar] [CrossRef]
- Lee, S.O.; Na, D.K.; Kim, C.M.; Li, Y.H.; Cho, Y.H.; Park, J.H.; Lee, J.H.; Eo, S.K.; Klein, T.A.; Chae, J.S. Identification and prevalence of Ehrlichia chaffeensis infection in Haemaphysalis longicornis ticks from Korea by PCR, sequencing and phylogenetic analysis based on 16S rRNA gene. J. Vet. Sci. 2005, 6, 151–155. [Google Scholar] [CrossRef]
- Pritt, B.S.; Sloan, L.M.; Johnson, D.K.H.; Munderloh, U.G.; Paskewitz, S.M.; McElroy, K.M.; McFadden, J.D.; Binnicker, M.J.; Neitzel, D.F.; Liu, G.; et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N. Engl. J. Med. 2011, 365, 422–429. [Google Scholar] [CrossRef]
- Su, H.; Onoda, E.; Tai, H.; Fujita, H.; Sakabe, S.; Azuma, K.; Akachi, S.; Oishi, S.; Abe, F.; Ando, S.; et al. Diversity unearthed by the estimated molecular phylogeny and ecologically quantitative characteristics of uncultured Ehrlichia bacteria in Haemaphysalis ticks, Japan. Sci. Rep. 2021, 11, 687. [Google Scholar] [CrossRef]
- Arai, R.; Sato, M.; Kato, M.; Aoki, J.; Nishida, A.; Watanabe, K.; Hirokawa, C.; Ikeda, S.; Watanabe, K.; Regilme, M.A.F.; et al. Spotted fever group rickettsiae (SFGR) detection in ticks following reported human case of Japanese spotted fever in Niigata Prefecture, Japan. Sci. Rep. 2021, 11, 2595. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Konishi, T.; Hashimoto, Y.; Takahashi, H.; Nakaya, K.; Fukunaga, M.; Nakao, M. Rapid diagnosis of lyme disease: Flagellin gene-based nested polymerase chain reaction for identification of causative Borrelia species. Int. J. Infect. Dis. 1997, 2, 64–73. [Google Scholar] [CrossRef]
- Yun, S.-M.; Lee, W.-G.; Ryou, J.; Yang, S.-C.; Park, S.-W.; Roh, J.Y.; Lee, Y.-J.; Park, C.; Han, M.G. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg. Infect. Dis. 2014, 20, 1358–1361. [Google Scholar] [CrossRef]
- Suh, J.-H.; Kim, H.-C.; Yun, S.-M.; Lim, J.-W.; Kim, J.-H.; Chong, S.-T.; Kim, D.-H.; Kim, H.-T.; Kim, H.; Klein, T.A.; et al. Detection of SFTS virus in Ixodes nipponensis and Amblyomma testudinarium (Ixodida: Ixodidae) collected from reptiles in the Republic of Korea. J. Med. Entomol. 2016, 53, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-G.; Kwon, O.-D.; Kwak, D. Molecular detection of Rickettsia raoultii, Rickettsia tamurae, and associated pathogens from ticks parasitizing water deer (Hydropotes inermis argyropus) in South Korea. Ticks Tick-Borne Dis. 2021, 12, 101712. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, K.; Kjær, L.J.; Isbrand, A.; Hansen, M.F.; Bødker, R. Multiple infections in questing nymphs and adult female Ixodes ricinus ticks collected in a recreational forest in Denmark. Ticks Tick-Borne Dis. 2019, 10, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-pathogen interactions and vector competence: Identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; de la Fuente, J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antivir. Res. 2014, 108, 104–128. [Google Scholar] [CrossRef]
- Merhej, V.; Angelakis, E.; Socolovschi, C.; Raoult, D. Genotyping, evolution and epidemiological findings of Rickettsia species. Infect. Genet. Evol. 2014, 25, 122–137. [Google Scholar] [CrossRef]
- Raoult, D.; Roux, V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 1997, 10, 694–719. [Google Scholar] [CrossRef]
- Paddock, C.D.; Sumner, J.W.; Comer, J.A.; Zaki, S.R.; Goldsmith, C.S.; Goddard, J.; McLellan, S.L.F.; Tamminga, C.L.; Ohl, C.A. Rickettsia parkeri: A newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 2004, 38, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Ren, Q.; Jian, R.; Xie, G.-C.; Chen, Y.; Wang, J.; Du, L.; Guo, W.-P. Molecular detection of “Candidatus Rickettsia tarasevichiae” by Loop-mediated Isothermal Amplification (LAMP) of the ompA gene. J. Microbiol. Meth. 2022, 202, 106601. [Google Scholar] [CrossRef] [PubMed]
- Igolkina, Y.P.; Rar, V.A.; Yakimenko, V.V.; Malkova, M.G.; Tancev, A.K.; Tikunov, A.Y.; Epikhina, T.I.; Tikunova, N.V. Genetic variability of Rickettsia spp. in Ixodes persulcatus/Ixodes trianguliceps sympatric areas from Western Siberia, Russia: Identification of a new Candidatus Rickettsia species. Infect. Genet. Evol. 2015, 34, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Q.; Zhang, X.; Li, Z.; Wang, Z.; Song, M.; Wei, F.; Wang, S.; Liu, Q. Characterization of rickettsiae in ticks in northeastern China. Parasites Vectors 2016, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K.; Kim, H.-C.; Choi, Y.-J.; Kim, J.; Park, H.-J.; Song, D.; Park, K.-H.; Jang, W.-J. Detection of Candidatus rickettsia tarasevichiae from a tick collected from a human patient in South Korea. Syst. Appl. Acarol. 2019, 24, 193–197. [Google Scholar]
- Rar, V.; Golovljova, I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 2011, 11, 1842–1861. [Google Scholar] [CrossRef]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, C.; Yan, Y.; Song, J.; Pei, Z.; Gong, P.; Wang, R.; Zhang, L.; Jian, F.; Ning, C. The novel zoonotic pathogen, Anaplasma capra, infects human erythrocytes, HL-60, and TF-1 cells in vitro. Pathogens 2021, 10, 600. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, M.; Wang, Z.; Wang, J.; Peng, Y.; Li, Y.; Guan, G.; Luo, J.; Yin, H. Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl. Environ. Microbiol. 2012, 78, 464–470. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, N.-R.; Kim, C.-M.; Park, S.; Yun, N.R.; Kim, D.-M.; Jung, D.S. First identification of Anaplasma phagocytophilum in both a biting tick Ixodes nipponensis and a patient in Korea: A case report. BMC Infect. Dis. 2020, 20, 826. [Google Scholar] [CrossRef]
- Seo, M.-G.; Kwon, O.-D.; Kwak, D. Genotypic analysis of piroplasms and associated pathogens from ticks infesting cattle in Korea. Microorganisms 2020, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.-S.; Yu, D.-H.; Shringi, S.; Klein, T.A.; Kim, H.-C.; Chong, S.-T.; Lee, I.-Y.; Foley, J. Microbial pathogens in ticks, rodents and a shrew in northern Gyeonggi-do near the DMZ, Korea. J. Vet. Sci. 2008, 9, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-I.; Na, K.-J. Ehrlichia ewingii infection in a dog from South Korea-A case report. Korean J. Vet. Serv. 2018, 41, 277–280. [Google Scholar]
- Sun, J.; Liu, Q.; Lu, L.; Ding, G.; Guo, J.; Fu, G.; Zhang, J.; Meng, F.; Wu, H.; Song, X.; et al. Coinfection with four genera of bacteria (Borrelia, Bartonella, Anaplasma, and Ehrlichia) in Haemaphysalis longicornis and Ixodes sinensis ticks from China. Vector-Borne Zoonotic Dis. 2008, 8, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Reye, A.L.; Stegniy, V.; Mishaeva, N.P.; Velhin, S.; Hübschen, J.M.; Ignatyev, G.; Muller, C.P. Prevalence of tick-borne pathogens in Ixodes ricinus and Dermacentor reticulatus ticks from different geographical locations in Belarus. PLoS ONE 2013, 8, e54476. [Google Scholar] [CrossRef]
- Karbowiak, G.; Biernat, B.; Szewczyk, T.; Sytykiewicz, H. The role of particular tick developmental stages in the circulation of tick-borne pathogens affecting humans in Central Europe. 1. The general pattern. Ann. Parasitol. 2015, 61, 221–228. [Google Scholar]
- Estrada-Peña, A.; Jongejan, F. Ticks feeding on humans: A review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acarol. 1999, 23, 685–715. [Google Scholar] [CrossRef]

| Pathogens | Target Gene | Sequence 5′ to 3′ | Amplicon Size (bp) | Reference | |
|---|---|---|---|---|---|
| Anaplasma spp. | 16S rRNA | 1st | 5′-TCCTGGCTCAGAACGAACGCTGGCGGC-3′ | 1433 | [38] |
| 5′-AGTCACTGACCCAACCTTAAATGGCTG-3′ | |||||
| 2nd | 5′-GTCGAACGGATTATTCTTTATAGCTTGC-3′ | 926 | |||
| 5′-CCCTTCCGTTAAGAAGGATCTAATCTCC-3′ | |||||
| Anaplasma phagocytophilum | ankA | 1st | 5′-GAAGAAATTACAACTCCTGAAG-3′ | 705 | [39] |
| 5′-CAGCCAGATGCAGTAACGTG-3′ | |||||
| 2nd | 5′-TTGACCGCTGAAGCACTAAC-3′ | 664 | |||
| 5′-ACCATTTGCTTCTTGAGGAG-3′ | |||||
| msp4 | 1st | 5′-ATGAATTACAGAGAATTGCTTGTAGG-3′ | 849 | [40] | |
| 5′-TTAATTGAAAGCAAATCTTGCTCCTATG-3′ | |||||
| 2nd | 5′-CTATTGGYGGNGCYAGAGT-3′ | 381 | |||
| 5′-GTTCATCGAAAATTCCGTGGTA-3′ | |||||
| Anaplasma bovis | groEL | 1st | 5′-GTTCGCAGTATTTTGCCAGT-3′ | 845 | [41] |
| 5′-CTGCRTTCAGAGTCATAAATAC-3′ | |||||
| 2nd | 5′-ATCTGGAAGRCCACTATTGAT-3′ | ||||
| 5′-CTGCRTTCAGAGTCATAAATAC-3′ | |||||
| Ehrlichia spp. | 16S rRNA | 1st | 5′-AAGCTTAACACATGCAAGTCGAA-3′ | 1406 | [42] |
| 5′-AGTCACTGACCCAACCTTAAATG-3′ | |||||
| 2nd | 5′-CAATTGCTTATAACCTTTTGGTTATAAAT-3′ | 390 | [43] | ||
| 5′-TATAGGTACCGTCATTATCTTCCCTAT-3′ | |||||
| groEL | 1st | 5′-GAAGATGCWGTWGGWTGTACKGC-3′ | 664 | [44] | |
| 5′-AGMGCTTCWCCTTCWACRTCYTC-3′ | |||||
| 2nd | 5′-ATTACTCAGAGTGCTTCTCARTG-3′ | 315 | |||
| 5′-TGCATACCRTCAGTYTTTTCAAC-3′ | |||||
| gltA | 1st | 5′-GGRRTRTTAACTTATGATCCAGG-3′ | 575 | [45] | |
| 5′-GCATTYTGYTCATGATCAGCATG-3′ | |||||
| 2nd | 5′-TTATGTCTACTGCTGCTTGTGA-3′ | 478 | |||
| 5′-TARGAAGAAAYRTCAAACATCATATG-3′ | |||||
| Rickettsia spp. | 17 kDa | 1st | 5′-TTTACAAAATTCTAAAAACCAT-3′ | 539 | [35] |
| 5′-TCAATTCACAACTTGCCATT-3′ | |||||
| 2nd | 5′-GCTCTTGCAACTTCTATGTT-3′ | 450 | |||
| 5′-TCAATTCACAACTTGCCATT-3′ | |||||
| ompA | 1st | 5′-ATGGCGAATATTTCTCCAAAAA-3′ | 634 | ||
| 5′-GTTCCGTTAATGGCAGCATCT-3′ | |||||
| 2nd | 5′-ATGGCGAATATTTCTCCAAAAA-3′ | 535 | |||
| 5′-AGTGCAGCATTCGCTCCCCCT-3′ | |||||
| gltA | 1st | 5′-GACCATGAGCAGAATGCTTCT-3′ | 479 | [46] | |
| 5′-ATTGCAAAAAGTACAGTGAACA-3′ | |||||
| 2nd | 5′-GGGGGCCTGCTCACGGCGG-3′ | 382 | |||
| 5′-ATTGCAAAAAGTACAGTGAACA-3′ | |||||
| Borrelia spp. | flagellin B | 1st | 5′-GATCARGCWCAAYATAACCAWATGCA-3′ | 459 | [47] |
| 5′-AGATTCAAGTCTGTTTTGGAAAGC-3′ | |||||
| 2nd | 5′-GCTGAAGAGCTTGGAATGCAACC-3′ | 351 | |||
| 5′-TGATCAGTTATCATTCTAATAGCA-3′ | |||||
| Species | Life Stage | No. of Collected Ticks | Sub Total (%) | Number of Bacterial Pathogens (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anaplasma phagocyto-philum | A. bovis | A. capra | Ehrlichia sp. | R. monacensis | R. tamurae | Candidatus R. Jingxinensis | Candidatus R. Tarasevichiae | ||||
| Haemaphysalis longicornis | Female | 36 | 66 (56.4) | 0 | 1 (2.8) | 1 (2.8) | 0 | 0 | 0 | 7 (19.4) | 0 |
| Male | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100.0) | 0 | ||
| Nymph | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (7.1) | 0 | ||
| Larva | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Haemaphysalis flava | Female | 4 | 6 (5.1) | 0 | 0 | 0 | 1 (25.0) | 0 | 0 | 0 | 0 |
| Nymph | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Haemaphysalis spp. | Nymph | 1 | 1 (0.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Amblyomma testudinarium | Female | 4 | 31 (26.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Male | 8 | 0 | 0 | 0 | 0 | 0 | 1 (12.5) | 0 | 0 | ||
| Nymph | 19 | 0 | 0 | 0 | 0 | 0 | 4 (21.0) | 0 | 0 | ||
| Ixodes nipponensis | Female | 10 | 10 (8.5) | 1 (10.0) | 0 | 0 | 0 | 3 (30.0) | 0 | 1 (10.0) | 0 |
| Ixodes persulcatus | Female | 1 | 1 (0.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100.0) |
| Ixodes spp. | * n.d. | 2 | 2 (1.7) | 0 | 0 | 0 | 0 | 1 (50.0) | 0 | 0 | 0 |
| Total (%) | Female | 55 (47.0) | 1 (1.8) | 1 (1.8) | 1 (1.8) | 1 (1.8) | 3 (5.4) | 0 | 8 (14.5) | 1 (1.8) | |
| Male | 9 (7.7) | 0 | 0 | 0 | 0 | 0 | 1 (11.1) | 1 (11.1) | 0 | ||
| Nymph | 50 (42.7) | 0 | 0 | 0 | 0 | 0 | 4 (8.0) | 2 (4.0) | 0 | ||
| Larva | 1 (0.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| * n.d. | 2 (1.7) | 0 | 0 | 0 | 0 | 1 (50.0) | 0 | 0 | 0 | ||
| Total | 117 | 1 (0.9) (CI 0.0–4.8) | 1 (0.9) (CI 0.0–4.8) | 1 (0.9) (CI 0.0–4.8) | 1 (0.9) (CI 0.0–4.8) | 4 (3.4) (CI 0.9–8.7) | 5 (4.2) (CI 1.4–10.0) | 11 (9.4) (CI 4.7–16.8) | 1 (0.9) (CI 0.0–4.8) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.-Y.; Kim, Y.-J.; Kim, S.-Y.; Lee, H.-I. Molecular Detection of Anaplasma, Ehrlichia and Rickettsia Pathogens in Ticks Collected from Humans in the Republic of Korea, 2021. Pathogens 2023, 12, 802. https://doi.org/10.3390/pathogens12060802
Seo J-Y, Kim Y-J, Kim S-Y, Lee H-I. Molecular Detection of Anaplasma, Ehrlichia and Rickettsia Pathogens in Ticks Collected from Humans in the Republic of Korea, 2021. Pathogens. 2023; 12(6):802. https://doi.org/10.3390/pathogens12060802
Chicago/Turabian StyleSeo, Ji-Ye, Yu-Jung Kim, Seong-Yoon Kim, and Hee-Il Lee. 2023. "Molecular Detection of Anaplasma, Ehrlichia and Rickettsia Pathogens in Ticks Collected from Humans in the Republic of Korea, 2021" Pathogens 12, no. 6: 802. https://doi.org/10.3390/pathogens12060802
APA StyleSeo, J.-Y., Kim, Y.-J., Kim, S.-Y., & Lee, H.-I. (2023). Molecular Detection of Anaplasma, Ehrlichia and Rickettsia Pathogens in Ticks Collected from Humans in the Republic of Korea, 2021. Pathogens, 12(6), 802. https://doi.org/10.3390/pathogens12060802






