Babesia divergens Shows Equal Predilection for Human ABO Blood Types in an In Vitro Erythrocyte Preference Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Babesia Divergens Culture
2.2. Parasite Multiplication Rates in Different Blood Types
2.3. Erythrocyte Labelling and Preference Assay
2.4. Statistical Analyses
3. Results
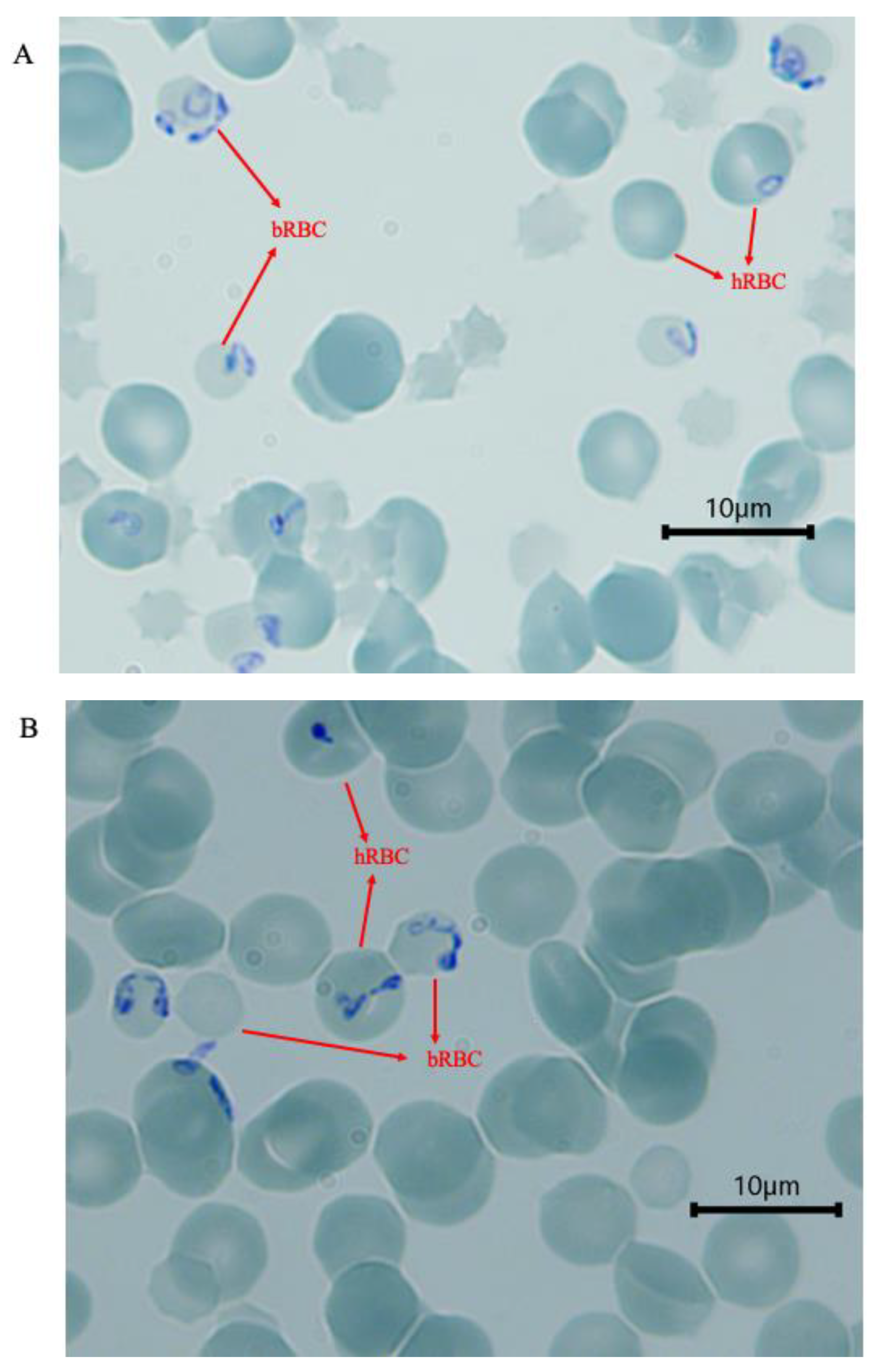
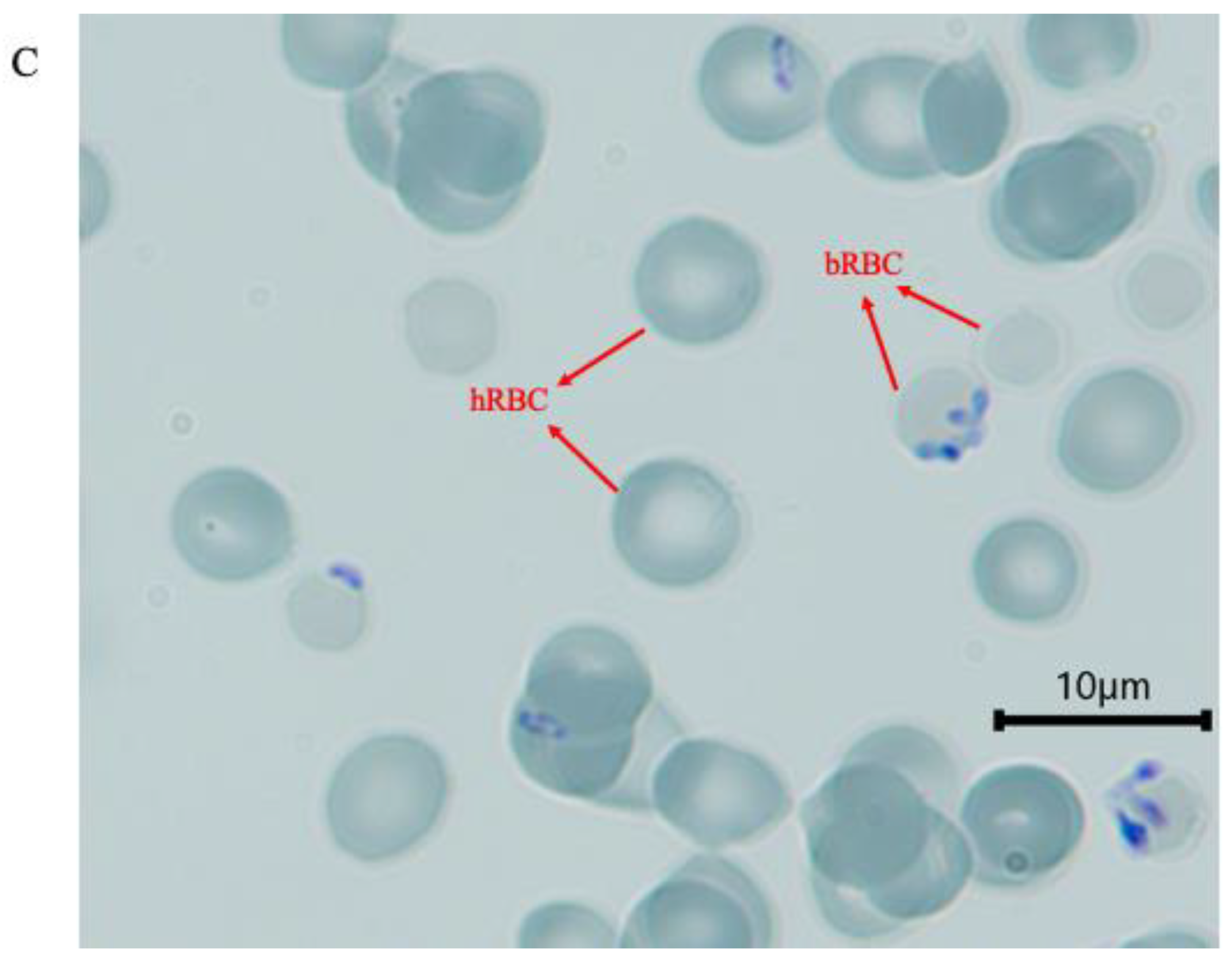
3.1. Invasion of Human Erythrocytes at 48 h
3.2. Parasite Multiplication Rate in Erythrocytes of Different ABO Blood Groups
3.3. Parasite ABO Blood Group Preference
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobo, C.A.; Singh, M.; Rodriguez, M. Human babesiosis: Recent advances and future challenges. Curr. Opin. Hematol. 2020, 27, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Burgess, M.J.; Rosenbaum, E.R.; Pritt, B.S.; Haselow, D.T.; Ferren, K.M.; Alzghoul, B.N.; Rico, J.C.; Sloan, L.M.; Ramanan, P.; Purushothaman, R.; et al. Possible Transfusion-Transmitted Babesia divergens-like/MO-1 Infection in an Arkansas Patient. Clin. Infect. Dis. 2017, 64, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Geilenkeuser, W.J.; Sireis, W.; Seifried, E.; Hourfar, K. Emerging Pathogens—How Safe is Blood? Transfus. Med. Hemother. 2014, 41, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bläckberg, J.; Lazarevic, V.L.; Hunfeld, K.P.; Persson, K.E.M. Low-virulent Babesia venatorum infection masquerading as hemophagocytic syndrome. Ann. Hematol. 2018, 97, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.E.; Andersson, M.O. Babesia species in questing Ixodes ricinus, Sweden. Ticks Tick. Borne Dis. 2016, 7, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.S. Preferential Feeding of Anopheles-Gambiae Mosquitos on Human Subjects of Blood Group-O—Relationship between Abo Polymorphism and Malaria Vectors. Hum. Biol. 1974, 46, 385–404. [Google Scholar]
- Theron, M.; Cross, N.; Cawkill, P.; Bustamante, L.Y.; Rayner, J.C. An in vitro erythrocyte preference assay reveals that Plasmodium falciparum parasites prefer Type O over Type A erythrocytes. Sci. Rep. 2018, 8, 8133. [Google Scholar] [CrossRef]
- Udomsangpetch, R.; Todd, J.; Carlson, J.; Greenwood, B.M. The effects of hemoglobin genotype and ABO blood group on the formation of rosettes by Plasmodium falciparum-infected red blood cells. Am. J. Trop. Med. Hyg. 1993, 48, 149–153. [Google Scholar] [CrossRef]
- Anjomruz, M.; Oshaghi, M.A.; Pourfatollah, A.A.; Sedaghat, M.M.; Raeisi, A.; Vatandoost, H.; Khamesipour, A.; Abai, M.R.; Mohtarami, F.; Akbarzadeh, K.; et al. Preferential feeding success of laboratory reared Anopheles stephensi mosquitoes according to ABO blood group status. Acta Trop. 2014, 140, 118–123. [Google Scholar] [CrossRef]
- Zakovska, A.; Janecek, J.; Nejezchlebova, H.; Kucerova, H.L. Pilot study of Ixodes ricinus ticks preference for human ABO blood groups using a simple in vitro method. Ann. Agric. Environ. Med. 2018, 25, 326–328. [Google Scholar] [CrossRef]
- Hatfield, P.R. Anti-mosquito antibodies and their effects on feeding, fecundity and mortality of Aedes aegypti. Med. Vet. Entomol. 1988, 2, 331–338. [Google Scholar] [CrossRef]
- Narasimhan, S.; Kurokawa, C.; Diktas, H.; Strank, N.O.; Cerny, J.; Murfin, K.; Cao, Y.; Lynn, G.; Trentleman, J.; Wu, M.J.; et al. Ixodes scapularis saliva components that elicit responses associated with acquired tick-resistance. Ticks Tick. Borne Dis. 2020, 11, 101369. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.S.; Ramasamy, R.; Kay, B.H.; Kidson, C. Anti-mosquito antibodies decrease the reproductive capacity of Aedes aegypti. Med. Vet. Entomol. 1988, 2, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R. Mosquito vector proteins homologous to alpha1-3 galactosyl transferases of tick vectors in the context of protective immunity against malaria and hypersensitivity to vector bites. Parasit. Vectors 2021, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Muthana, S.M.; Gildersleeve, J.C. Factors Affecting Anti-Glycan IgG and IgM Repertoires in Human Serum. Sci. Rep. 2016, 6, 19509. [Google Scholar] [CrossRef]
- Rispens, T.; Derksen, N.I.; Commins, S.P.; Platts-Mills, T.A.; Aalberse, R.C. IgE production to alpha-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS ONE 2013, 8, e55566. [Google Scholar] [CrossRef]
- Jajosky, R.P.; O’Bryan, J.; Spichler-Moffarah, A.; Jajosky, P.G.; Krause, P.J.; Tonnetti, L. The impact of ABO and RhD blood types on Babesia microti infection. PLoS Negl. Trop. Dis. 2023, 17, e0011060. [Google Scholar] [CrossRef]
- Mortazavi, S.E.; Lugaajju, A.; Kaddumukasa, M.; Tijani, M.K.; Kironde, F.; Persson, K.E.M. Osteopontin and malaria: No direct effect on parasite growth, but correlation with P. falciparum-specific B cells and BAFF in a malaria endemic area. BMC Microbiol. 2021, 21, 307. [Google Scholar] [CrossRef]
- Tijani, M.K.; Babalola, O.A.; Odaibo, A.B.; Anumudu, C.I.; Asinobi, A.O.; Morenikeji, O.A.; Asuzu, M.C.; Langer, C.; Reiling, L.; Beeson, J.G.; et al. Acquisition, maintenance and adaptation of invasion inhibitory antibodies against Plasmodium falciparum invasion ligands involved in immune evasion. PLoS ONE 2017, 12, e0182187. [Google Scholar] [CrossRef]
- Bajer, A.; Beck, A.; Beck, R.; Behnke, J.M.; Dwuznik-Szarek, D.; Eichenberger, R.M.; Farkas, R.; Fuehrer, H.P.; Heddergott, M.; Jokelainen, P.; et al. Babesiosis in Southeastern, Central and Northeastern Europe: An Emerging and Re-Emerging Tick-Borne Disease of Humans and Animals. Microorganisms 2022, 10, 945. [Google Scholar] [CrossRef]
- Kumar, A.; O’Bryan, J.; Krause, P.J. The Global Emergence of Human Babesiosis. Pathogens 2021, 10, 1447. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Hunfeld, K.P.; Persson, K.E.M. High seroprevalence of Babesia antibodies among Borrelia burgdorferi-infected humans in Sweden. Ticks Tick. Borne Dis. 2019, 10, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Christie, J.; Koster, L.; Du, A.; Yao, C. Emerging Human Babesiosis with “Ground Zero” in North America. Microorganisms 2021, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, T.; Clements, M.; Zhao, J.; Olsson, M.L.; Edgren, G. An agnostic study of associations between ABO and RhD blood group and phenome-wide disease risk. eLife 2021, 10, e65658. [Google Scholar] [CrossRef]
- Franchini, M.; Bonfanti, C. Evolutionary aspects of ABO blood group in humans. Clin. Chim. Acta 2015, 444, 66–71. [Google Scholar] [CrossRef]
- Rowe, J.A.; Handel, I.G.; Thera, M.A.; Deans, A.M.; Lyke, K.E.; Kone, A.; Diallo, D.A.; Raza, A.; Kai, O.; Marsh, K.; et al. Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proc. Natl. Acad. Sci. USA 2007, 104, 17471–17476. [Google Scholar] [CrossRef]
- Beri, D.; Singh, M.; Rodriguez, M.; Barbu-Stevanovic, M.; Rasquinha, G.; Mendelson, A.; An, X.; Manwani, D.; Yazdanbakhsh, K.; Lobo, C.A. Elucidating parasite and host-cell factors enabling Babesia infection in sickle red cells under hypoxic/hyperoxic conditions. Blood Adv. 2023, 7, 649–663. [Google Scholar] [CrossRef]
- Karkoska, K.; Louie, J.; Appiah-Kubi, A.O.; Wolfe, L.; Rubin, L.; Rajan, S.; Aygun, B. Transfusion-transmitted babesiosis leading to severe hemolysis in two patients with sickle cell anemia. Pediatr. Blood Cancer 2018, 65, e26734. [Google Scholar] [CrossRef]
- Krause, P.J.; Gewurz, B.E.; Hill, D.; Marty, F.M.; Vannier, E.; Foppa, I.M.; Furman, R.R.; Neuhaus, E.; Skowron, G.; Gupta, S.; et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin. Infect. Dis. 2008, 46, 370–376. [Google Scholar] [CrossRef]
- Craine, N.G.; Randolph, S.E.; Nuttall, P.A. Seasonal variation in the role of grey squirrels as hosts of Ixodes ricinus, the tick vector of the Lyme disease spirochaete, in a British woodland. Folia Parasitol. 1995, 42, 73–80. [Google Scholar]
- Humair, P.F.; Turrian, N.; Aeschilimann, A.; Gern, L. Borrelia burgdorferi in a focus of Lyme borreliosis: Epizootiologic contribution of small mammals. Folia Parasitol. 1993, 40, 65–70. [Google Scholar]
- Humair, P.F.; Turrian, N.; Aeschlimann, A.; Gern, L. Ixodes ricinus immatures on birds in a focus of Lyme borreliosis. Folia Parasitol. 1993, 40, 237–242. [Google Scholar]
- Talleklint, L.; Jaenson, T.G. Transmission of Borrelia burgdorferi s.l. from mammal reservoirs to the primary vector of Lyme borreliosis, Ixodes ricinus (Acari: Ixodidae), in Sweden. J. Med. Entomol. 1994, 31, 880–886. [Google Scholar] [CrossRef]
- Haldane, J.B. The blood-group frequencies of European peoples, and racial origins. 1940. Hum. Biol. 1989, 61, 667–702. [Google Scholar] [PubMed]
- Zintl, A.; Westbrook, C.; Mulcahy, G.; Skerrett, H.E.; Gray, J.S. Invasion, and short- and long-term survival of Babesia divergens (Phylum Apicomplexa) cultures in non-bovine sera and erythrocytes. Parasitology 2002, 124, 583–588. [Google Scholar] [CrossRef]
- Cursino-Santos, J.R.; Singh, M.; Pham, P.; Rodriguez, M.; Lobo, C.A. Babesia divergens builds a complex population structure composed of specific ratios of infected cells to ensure a prompt response to changing environmental conditions. Cell. Microbiol. 2016, 18, 859–874. [Google Scholar] [CrossRef]
- Montero, E.; Gonzalez, L.M.; Rodriguez, M.; Oksov, Y.; Blackman, M.J.; Lobo, C.A. A conserved subtilisin protease identified in Babesia divergens merozoites. J. Biol. Chem. 2006, 281, 35717–35726. [Google Scholar] [CrossRef]
- Sevilla, E.; Gonzalez, L.M.; Luque, D.; Gray, J.; Montero, E. Kinetics of the invasion and egress processes of Babesia divergens, observed by time-lapse video microscopy. Sci. Rep. 2018, 8, 14116. [Google Scholar] [CrossRef]
- Anstee, D.J. The relationship between blood groups and disease. Blood 2010, 115, 4635–4643. [Google Scholar] [CrossRef]
- Moulds, J.M.; Moulds, J.J. Blood group associations with parasites, bacteria, and viruses. Transfus. Med. Rev. 2000, 14, 302–311. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tijani, M.K.; Danielsson, L.; Storry, J.R.; Olsson, M.L.; Persson, K.E.M. Babesia divergens Shows Equal Predilection for Human ABO Blood Types in an In Vitro Erythrocyte Preference Assay. Pathogens 2023, 12, 803. https://doi.org/10.3390/pathogens12060803
Tijani MK, Danielsson L, Storry JR, Olsson ML, Persson KEM. Babesia divergens Shows Equal Predilection for Human ABO Blood Types in an In Vitro Erythrocyte Preference Assay. Pathogens. 2023; 12(6):803. https://doi.org/10.3390/pathogens12060803
Chicago/Turabian StyleTijani, Muyideen K., Lena Danielsson, Jill R. Storry, Martin L. Olsson, and Kristina E. M. Persson. 2023. "Babesia divergens Shows Equal Predilection for Human ABO Blood Types in an In Vitro Erythrocyte Preference Assay" Pathogens 12, no. 6: 803. https://doi.org/10.3390/pathogens12060803
APA StyleTijani, M. K., Danielsson, L., Storry, J. R., Olsson, M. L., & Persson, K. E. M. (2023). Babesia divergens Shows Equal Predilection for Human ABO Blood Types in an In Vitro Erythrocyte Preference Assay. Pathogens, 12(6), 803. https://doi.org/10.3390/pathogens12060803





