Feline Parvovirus Lethal Outbreak in a Group of Adult Cohabiting Domestic Cats
Abstract
:1. Introduction
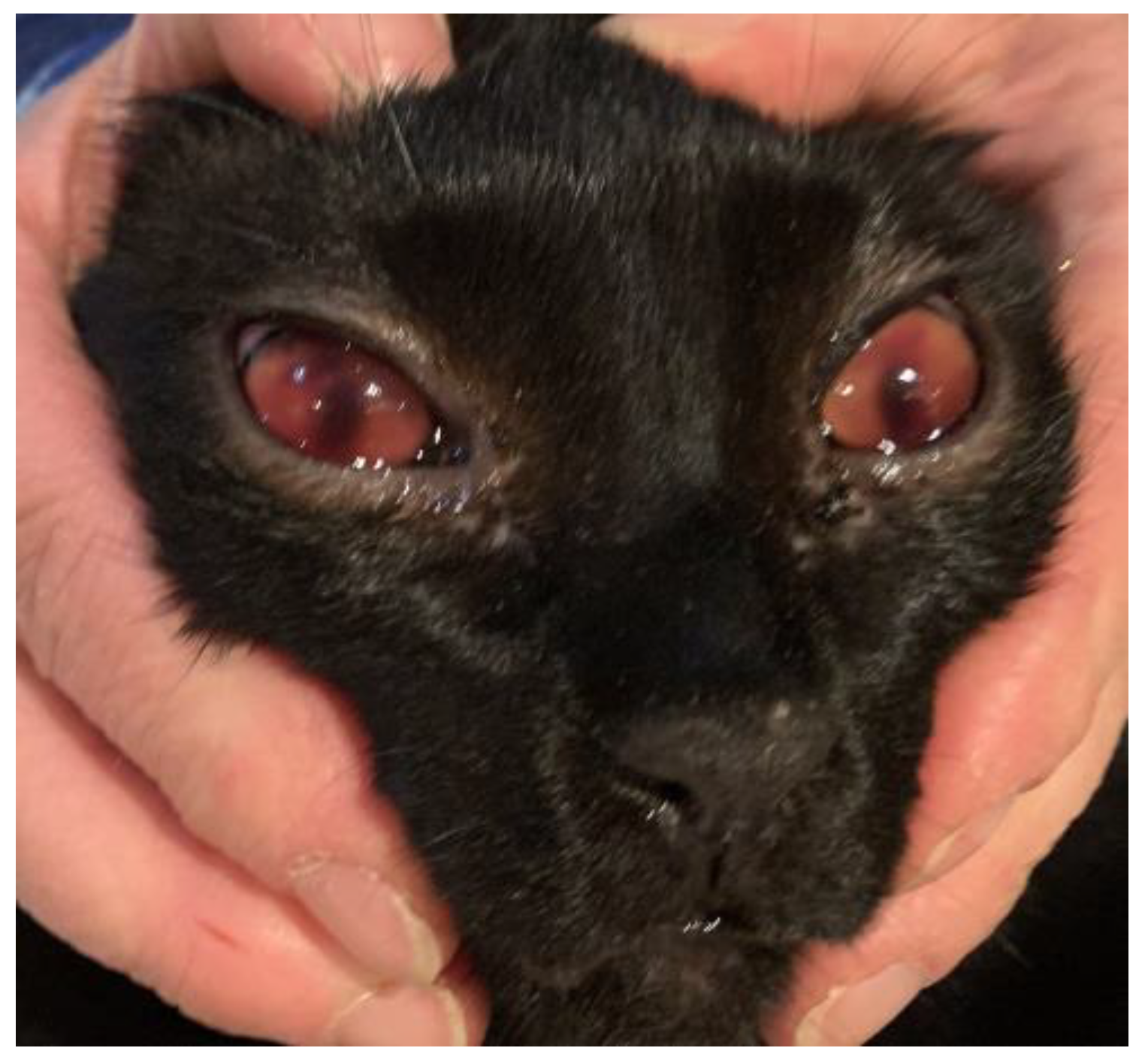
2. Case Report
2.1. Anatomopathological Examination
2.2. Bacteriology
2.3. Virology
2.3.1. Viral Isolation
2.3.2. Molecular Analyses
2.3.3. Phylogenetic Analysis
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotmore, S.F.; Agbandje-McKenna, M.; Chiorini, J.A.; Mukha, D.V.; Pintel, D.J.; Qiu, J.; Soderlund-Venermo, M.; Tattersall, P.; Tijssen, P.; Gatherer, D.; et al. The Family Parvoviridae. Arch. Virol. 2014, 159, 1239–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrs, V.R. Feline Panleukopenia: A Re-Emergent Disease. Vet. Clin. Small Anim. Pract. 2019, 49, 651–670. [Google Scholar] [CrossRef]
- Sykes, J.E. Feline Panleukopenia Virus Infection and Other Viral Enteritides. In Canine and Feline Infectious Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 187–194. ISBN 9781437707953. [Google Scholar]
- Lamm, C.G.; Rezabek, G.B. Parvovirus Infection in Domestic Companion Animals. Vet. Clin. N. Am.—Small Anim. Pract. 2008, 38, 837–850. [Google Scholar] [CrossRef]
- Tuzio, H. Feline Panleukopenia. Infect. Dis. Manag. Anim. Shelter. Second Ed. 2021, Chapter 15, 337–366. [Google Scholar] [CrossRef]
- Steinel, A.; Parrish, C.R.; Bloom, M.E.; Truyen, U. Parvovirus Infections in Wild Carnivores. J. Wildl. Dis. 2001, 37, 594–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truyen, U.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline Panleukopenia ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2009, 11, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Poole, G.M. Stability of a Modified, Live Panleucopenia Virus Stored in Liquid Phase. Appl. Microbiol. 1972, 24, 663–664. [Google Scholar] [CrossRef]
- Rehme, T.; Hartmann, K.; Truyen, U.; Zablotski, Y.; Bergmann, M. Feline Panleukopenia Outbreaks and Risk Factors in Cats in Animal Shelters. Viruses 2022, 14, 1248. [Google Scholar] [CrossRef]
- Stone, A.E.S.; Brummet, G.O.; Carozza, E.M.; Kass, P.H.; Petersen, E.P.; Sykes, J.; Westman, M.E. 2020 AAHA/AAFP Feline Vaccination Guidelines. J. Feline Med. Surg. 2020, 22, 813–830. [Google Scholar] [CrossRef]
- Scott, F.W.; Geissinger, C.M. Long-Term Immunity in Cats Vaccinated with an Inactivated Trivalent Vaccine. Am. J. Vet. Res. 1999, 60, 652–658. [Google Scholar]
- Schunck, B.; Kraft, W.; Truyen, U. A Simple Touch-down Polymerase Chain Reaction for the Detection of Canine Parvovirus and Feline Panleukopenia Virus in Feces. J. Virol. Methods 1995, 55, 427–433. [Google Scholar] [CrossRef]
- Schatzberg, S.J.; Haley, N.J.; Barr, S.C.; Parrish, C.; Steingold, S.; Summers, B.A.; Lahunta, A.; Kornegay, J.N.; Sharp, N.J.H. Polymerase Chain Reaction (PCR) Amplification of Parvoviral DNA from the Brains of Dogs and Cats with Cerebellar Hypoplasia. J. Vet. Intern. Med. 2003, 17, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Truyen, U.; Evermann, J.F.; Vieler, E.; Parrish, C.R. Evolution of Canine Parvovirus Involved Loss and Gain of Feline Host Range. Virology 1996, 215, 186–189. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Mochizuki, M.; Naito, R.; Nakamura, K.; Miyazawa, T.; Mikami, T.; Takahashi, E. Predominance of Canine Parvovirus (CPV) in Unvaccinated Cat Populations and Emergence of New Antigenic Types of CPVs in Cats. Virology 2000, 278, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nader, N.; Sonea, C.; Cretu, D.M.; Gurau, M.R.; Aniela, R.; Gheorghe, I.; Otelea, F.; Udrea, L. Panleukopenia Outbreak Diagnosed by Real Time qPCR in a Catterry in Romania. Available online: https://sciendo.com/pdf/10.2478/agr-2022-0018 (accessed on 10 May 2023).
- Demeter, Z.; Palade, E.A.; Rusvai, M. Feline Panleukopenla Virus Infection in Various Species from Hungary. Lucr. St. Med. Vet. 2010, 43, 73–81. [Google Scholar]
- Van Brussel, K.; Carrai, M.; Lin, C.; Kelman, M.; Setyo, L.; Aberdein, D.; Brailey, J.; Lawler, M.; Maher, S.; Plaganyi, I.; et al. Distinct Lineages of Feline Parvovirus Associated with Epizootic Outbreaks in Australia, New Zealand and the United Arab Emirates. Viruses 2019, 11, 1155. [Google Scholar] [CrossRef] [Green Version]
- Tucciarone, C.M.; Franzo, G.; Legnardi, M.; Lazzaro, E.; Zoia, A.; Petini, M.; Furlanello, T.; Caldin, M.; Cecchinato, M.; Drigo, M. Genetic Insights into Feline Parvovirus: Evaluation of Viral Evolutionary Patterns and Association between Phylogeny and Clinical Variables. Viruses 2021, 13, 1033. [Google Scholar] [CrossRef]
- Mazzaferro, E.; Powell, L.L. Fluid Therapy for the Emergent Small Animal Patient: Crystalloids, Colloids, and Albumin Products. Vet. Clin. N. Am.—Small Anim. Pract. 2022, 52, 781–796. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Woo, P.C.Y.; Yeung, H.C.; Teng, J.L.L.; Wu, Y.; Bai, R.; Fan, R.Y.Y.; Chan, K.H.; Yuen, K.Y. Identification and Characterization of Bocaviruses in Cats and Dogs Reveals a Novel Feline Bocavirus and a Novel Genetic Group of Canine Bocavirus. J. Gen. Virol. 2012, 93, 1573–1582. [Google Scholar] [CrossRef]
- Chu, D.K.W.; Leung, C.Y.H.; Gilbert, M.; Joyner, P.H.; Ng, E.M.; Tse, T.M.; Guan, Y.; Peiris, J.S.M.; Poon, L.L.M. Avian Coronavirus in Wild Aquatic Birds. J. Virol. 2011, 85, 12815–12820. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.K.W.; Poon, L.L.M.; Guan, Y.; Peiris, J.S.M. Novel Astroviruses in Insectivorous Bats. J. Virol. 2008, 82, 9107. [Google Scholar] [CrossRef] [Green Version]
- Flores, P.S.; Mendes, C.A.S.; Travassos, C.E.P.F.; Mariano, F.A.; Rangel, M.F.N.; Mendes, G.S.; Santos, N. RVA in Pet, Sheltered, and Stray Dogs and Cats in Brazil. Top. Companion Anim. Med. 2022, 49, 100667. [Google Scholar] [CrossRef]
- Pérez, R.; Calleros, L.; Marandino, A.; Sarute, N.; Iraola, G.; Grecco, S.; Blanc, H.; Vignuzzi, M.; Isakov, O.; Shomron, N.; et al. Phylogenetic and Genome-Wide Deep-Sequencing Analyses of Canine Parvovirus Reveal Co-Infection with Field Variants and Emergence of a Recent Recombinant Strain. PLoS ONE 2014, 9, e111779. [Google Scholar] [CrossRef] [Green Version]
- Tucciarone, C.M.; Franzo, G.; Mazzetto, E.; Legnardi, M.; Caldin, M.; Furlanello, T.; Cecchinato, M.; Drigo, M. Molecular Insight into Italian Canine Parvovirus Heterogeneity and Comparison with the Worldwide Scenario. Infect. Genet. Evol. 2018, 66, 171–179. [Google Scholar] [CrossRef]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An Important Software for Molecular Biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2018, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.R. Pathogenesis of Feline Panleukopenia Virus and Canine Parvovirus. In Parvoviruses; Taylor & Francis Ltd.: Abingdon, UK, 2005; pp. 429–434. ISBN 9781444114782. [Google Scholar]
- Litster, A.; Benjanirut, C. Case Series of Feline Panleukopenia Virus in an Animal Shelter. J. Feline Med. Surg. 2014, 16, 346–353. [Google Scholar] [CrossRef]
- Jenkins, E.; Davis, C.; Carrai, M.; Ward, M.P.; O’Keeffe, S.; van Boeijen, M.; Beveridge, L.; Desario, C.; Buonavoglia, C.; Beatty, J.A.; et al. Feline Parvovirus Seroprevalence Is High in Domestic Cats from Disease Outbreak and Non-outbreak Regions in Australia. Viruses 2020, 12, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, C.; Vieira, M.J.; Silva, E.; Carvalheira, J.; Parrish, C.R.; Thompson, G. Genetic Analysis of Feline Panleukopenia Virus Full-Length VP2 Gene in Domestic Cats Between 2006–2008 and 2012–2014, Portugal. Transbound. Emerg. Dis. 2017, 64, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Kelman, M.; Harriott, L.; Carrai, M.; Kwan, E.; Ward, M.P.; Barrs, V.R. Phylogenetic and Geospatial Evidence of Canine Parvovirus Transmission Betweenwild Dogs and Domestic Dogs at the Urban Fringe in Australia. Viruses 2020, 12, 663. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Santos, N.; Parrish, C.; Thompson, G. Genetic Characterization of Canine Parvovirus in Sympatric Free-Ranging Wild Carnivores in Portugal. J. Wildl. Dis. 2017, 53, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Canuti, M.; Mira, F.; Sorensen, R.G.; Rodrigues, B.; Bouchard, É.; Walzthoni, N.; Hopson, M.; Gilroy, C.; Whitney, H.G.; Lang, A.S. Distribution and Diversity of Dog Parvoviruses in Wild, Free-Roaming and Domestic Canids of Newfoundland and Labrador, Canada. Transbound. Emerg. Dis. 2022, 69, e2694–e2705. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.D.; Henriques, A.M.; Barros, S.C.; Fagulha, T.; Mendonça, P.; Carvalho, P.; Monteiro, M.; Fevereiro, M.; Basto, M.P.; Rosalino, L.M.; et al. Snapshot of Viral Infections in Wild Carnivores Reveals Ubiquity of Parvovirus and Susceptibility of Egyptian Mongoose to Feline Panleukopenia Virus. PLoS ONE 2013, 8, e59399. [Google Scholar] [CrossRef]
- Calatayud, O.; Esperón, F.; Velarde, R.; Oleaga, Á.; Llaneza, L.; Ribas, A.; Negre, N.; de la Torre, A.; Rodríguez, A.; Millán, J. Genetic Characterization of Carnivore Parvoviruses in Spanish Wildlife Reveals Domestic Dog and Cat-Related Sequences. Transbound. Emerg. Dis. 2020, 67, 626–634. [Google Scholar] [CrossRef]







| Cat No. | Age | Status |
|---|---|---|
| 1 | 5 | Died few hours after clinical signs |
| 2 (cat #1) | 7 | Died 24 h after clinical signs; submitted to DSV |
| 3 (cat #2) | 5 | Died 48 h after clinical signs; submitted to DSV |
| 4 | 1 | I–V |
| 5 | 1 | I–V |
| 6 | 2 | I–V |
| 7 | 4 | I–V |
| 8 | 5 | I–V |
| 9 | 8 | I–V |
| 10 | 9 | I–V |
| 11 | 11 | I–V |
| 12 | 11 | I–V |
| Viral Target | Primers Sequences | Melting T° (°C) | Amplicon Length (bps) | Reference | |
|---|---|---|---|---|---|
| Protoparvovirus carnivoran1 | Pair 1 | Fw: ACGTGGTGTAACTCAAATGG Rw: GCATTTGGTAGACAACATGGT | 55 | 215 | [13] |
| Pair 2 | Fw: GGGTGTGTTAGTAAAGTGGG Rw: CGCTGCTTATCTTCGCTCTG | 193 | |||
| Pair 3 | Fw: CAAACAAATAGAGCATTGGGC Rw: GCTGAGGTTGGTTATAGTGCACC | 184 | |||
| Bocaparvovirus | Fw: GCCAGCACNGGNAARACMAA Rw: CATNAGNCAYTCYTCCCACCA | 55 | 141 | [21] | |
| Coronavirus | Fw: GGKTGGGAYTAYCCKAARTG Rw: TGYTGTSWRCARAAYTCRTG Fw nested: GGTTGGGACTATCCTAAGTGTGA Rw nested: CCATCATCAGATAGAATCATCAT | 48 | 440 | [22] | |
| Astrovirus | Fw a: GARTTYGATTGGRCKCGKTAYGA Fw b: GARTTYGATTGGRCKAGGTAYGA Fw nested a: CGKTAYGATGGKACKATHCC Fw nested b: AGGTAYGATGGKACKATHCC Rw: GGYTTKACCCACATNCCRAA | 50 | 422 | [23] | |
| Rotavirus | Fw: GACGGVGCRACTACATGGT Rw: GTCCAATTCATNCCTGGTGG5 | 55 | 379 | [24] | |
| Primer Name | Sequence 5′-3′ | Position | Reference |
|---|---|---|---|
| NS_Fext | GACCGTTACTGACATTCGCTTC | 206–227 | [25] |
| NS_Fint | GTTGAAACCACAGTGACGACAG | 1055–1076 | |
| NS_Rext | GGAGAACCAACTAACCCTTC | 2460–2441 | |
| NS_Rint | CACCTGAAGACTGGATGATG | 1186–1167 | |
| 2161_F | TTGGCGTTACTCACAAAGACGTGC | 2161–2184 | |
| 4823_R | GTTGTTATGGTGTGGGTGGTTGGT | 4823–4800 | |
| VP1_Seq_F2 | GGATTTCTACGGGTACTTTC | 2713–2732 | [26] |
| VP1_Seq_F3 | AGGTGATGAATTTGCTACAGG | 3368–3388 | |
| VP1_Seq_F4 | GCTACCAACAGATCCAATTG | 3887–3906 | |
| VP1_Seq_R2 | CTCAGCCACCAACTAAAGTTT | 3090–3070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacini, M.I.; Forzan, M.; Franzo, G.; Tucciarone, C.M.; Fornai, M.; Bertelloni, F.; Sgorbini, M.; Cantile, C.; Mazzei, M. Feline Parvovirus Lethal Outbreak in a Group of Adult Cohabiting Domestic Cats. Pathogens 2023, 12, 822. https://doi.org/10.3390/pathogens12060822
Pacini MI, Forzan M, Franzo G, Tucciarone CM, Fornai M, Bertelloni F, Sgorbini M, Cantile C, Mazzei M. Feline Parvovirus Lethal Outbreak in a Group of Adult Cohabiting Domestic Cats. Pathogens. 2023; 12(6):822. https://doi.org/10.3390/pathogens12060822
Chicago/Turabian StylePacini, Maria Irene, Mario Forzan, Giovanni Franzo, Claudia Maria Tucciarone, Milena Fornai, Fabrizio Bertelloni, Micaela Sgorbini, Carlo Cantile, and Maurizio Mazzei. 2023. "Feline Parvovirus Lethal Outbreak in a Group of Adult Cohabiting Domestic Cats" Pathogens 12, no. 6: 822. https://doi.org/10.3390/pathogens12060822
APA StylePacini, M. I., Forzan, M., Franzo, G., Tucciarone, C. M., Fornai, M., Bertelloni, F., Sgorbini, M., Cantile, C., & Mazzei, M. (2023). Feline Parvovirus Lethal Outbreak in a Group of Adult Cohabiting Domestic Cats. Pathogens, 12(6), 822. https://doi.org/10.3390/pathogens12060822






