Predation of Cyclopoid Copepods on the Theronts of Ichthyophthirius multifiliis: Shedding Light on Biocontrol of White Spot Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ichthyophthirius multifiliis Culture and Isolation
2.2. Macro-zooplankton Collection and Identification
2.3. Fluorescent Labeling of Infective Theronts
2.4. Co-Culture and Predation Experiments
2.5. Molecular Analyses
2.6. Challenge and Infection Level Determination
2.7. Statistical Analysis
3. Results
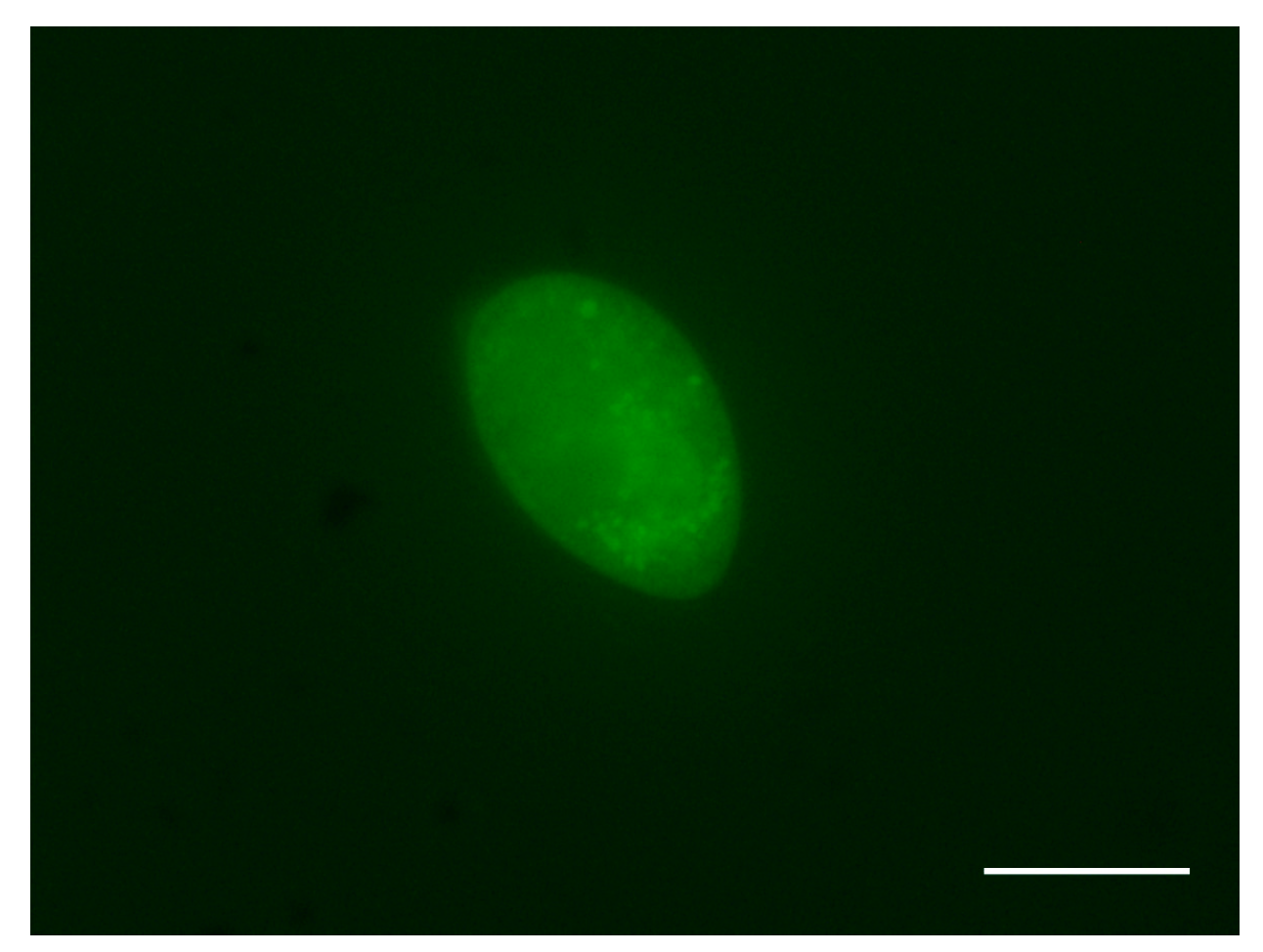
3.1. Theronts Labeled with CFDA-SE
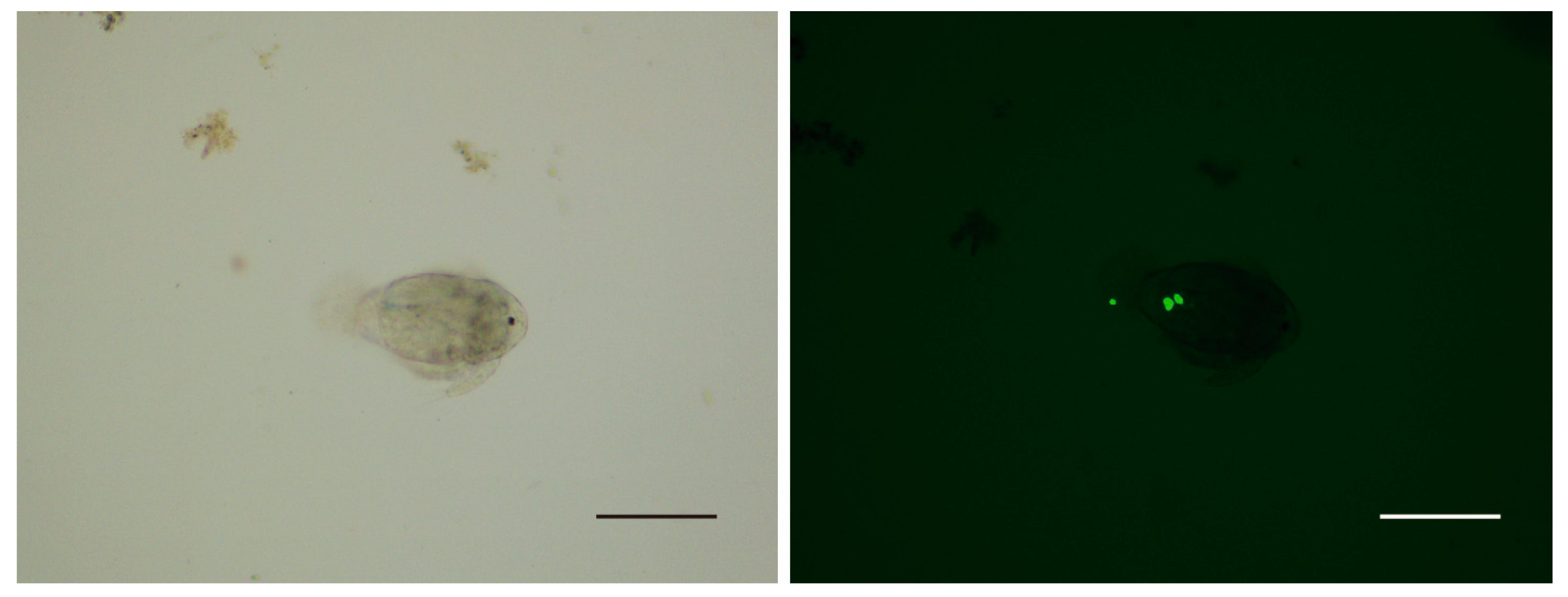
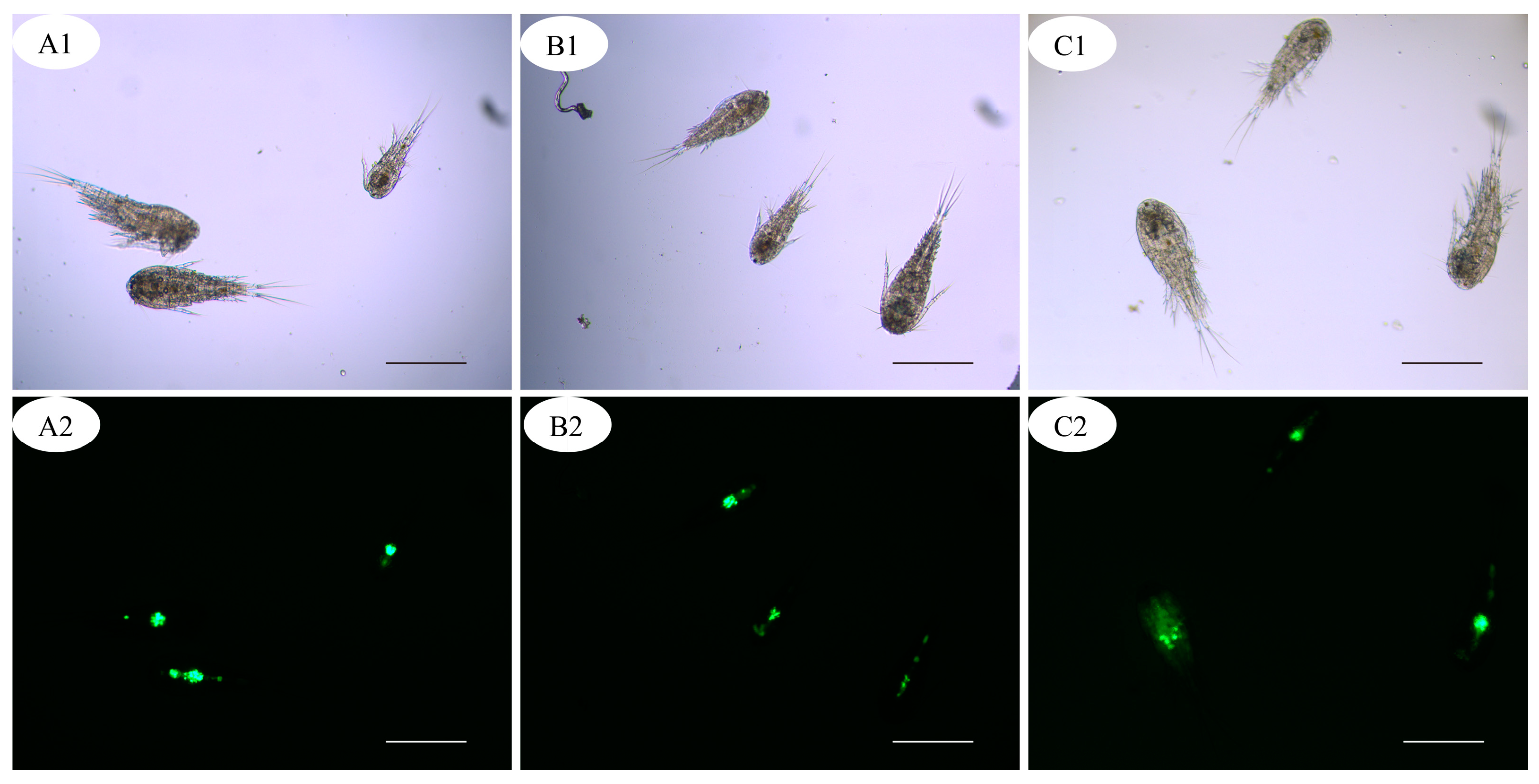
3.2. Predation of Copepods on Theronts
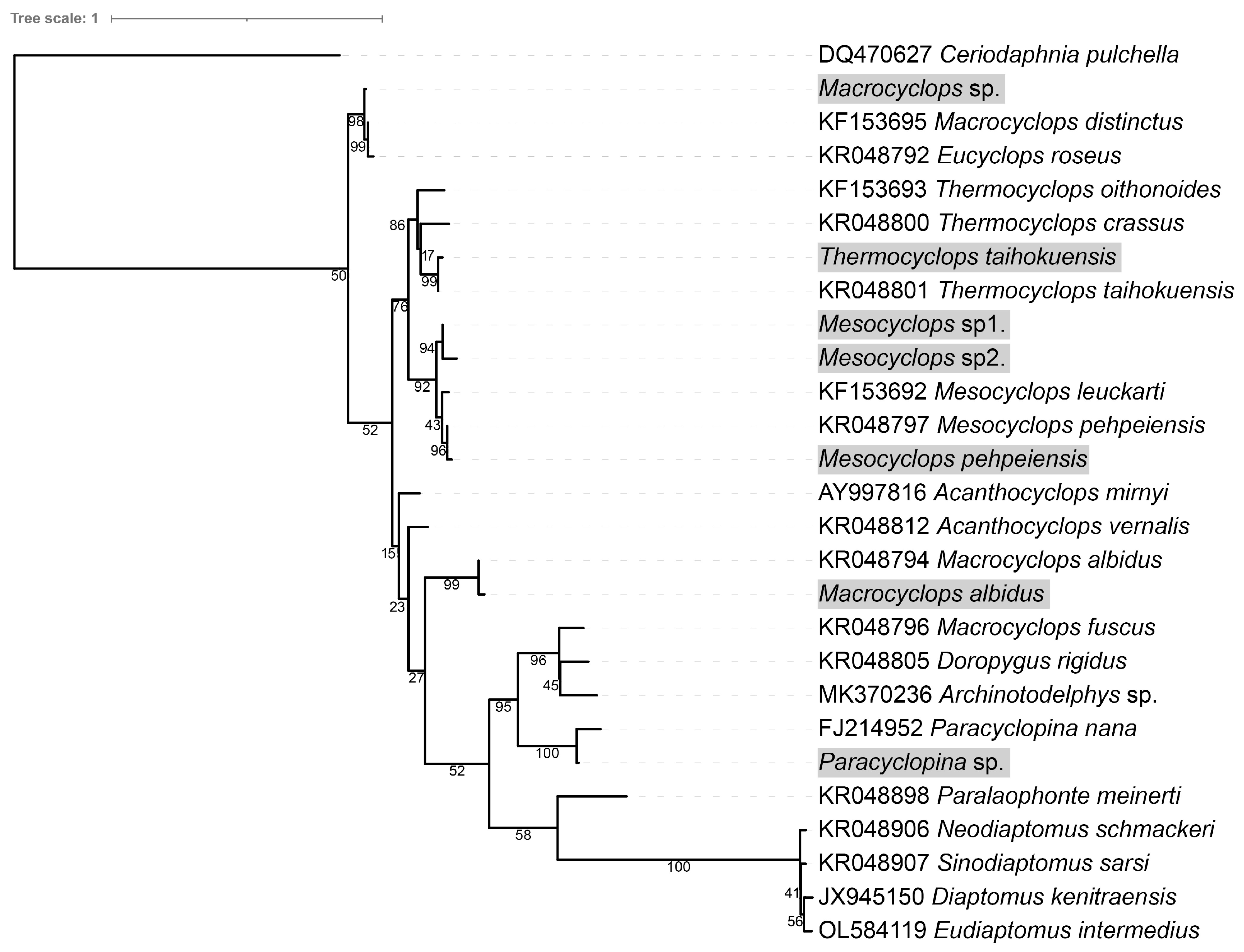
3.3. Species of Zooplankton and Predators of Theronts
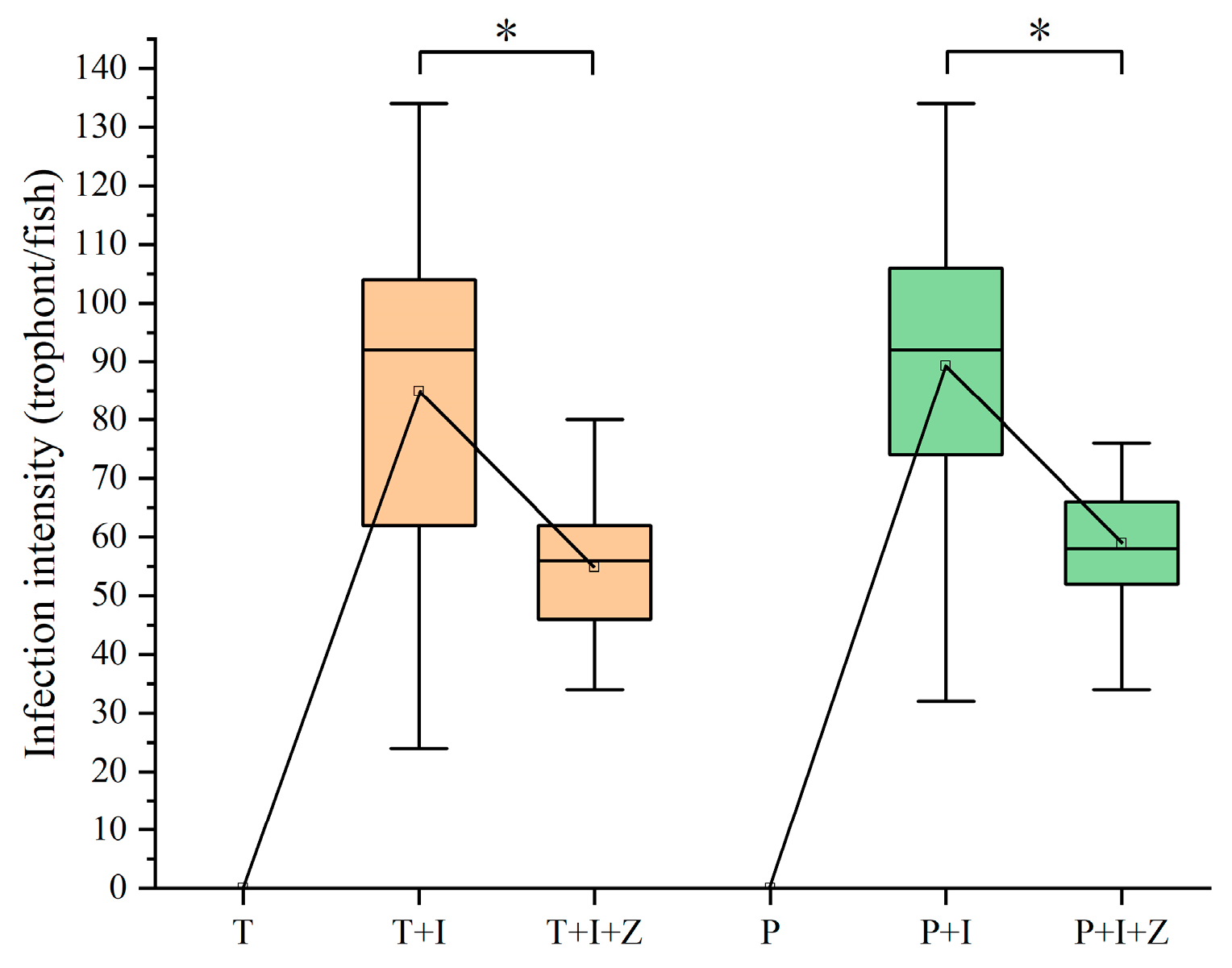
3.4. Intervention Effect of Macro-zooplankton on I. multifiliis Infection in Goldfish
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dickerson, H.W.; Dawe, D.L. Ichthyophthirius multifiliis and Cryptocaryon irritans (Phylum Ciliophora). In Fish Diseases and Disorders. Volume 1: Protozoan and Metazoan Infections; Woo, P.T.K., Ed.; CABI Publishing: Wallingford, UK, 1995; ISBN 978-0-85198-823-8. [Google Scholar]
- von Gersdorff Jørgensen, L. The Fish Parasite Ichthyophthirius multifiliis—Host Immunology, Vaccines and Novel Treatments. Fish Shellfish Immunol. 2017, 67, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.A. Ichthyophthirius multifiliis Fouquet and Ichthyophthiriosis in Freshwater Teleosts. In Advances in Parasitology; Baker, J.R., Muller, R., Rollinson, D., Eds.; Academic Press: Cambridge, MA, USA, 2005; Volume 59, pp. 159–241. [Google Scholar]
- Picón-Camacho, S.M.; Leclercq, E.; Bron, J.E.; Shinn, A.P. The Potential Utility of the Leopard Pleco (Glyptoperichthys gibbiceps) as a Biological Control of the Ciliate Protozoan Ichthyophthirius multifiliis. Pest Manag. Sci. 2012, 68, 557–563. [Google Scholar] [CrossRef]
- Tieman, D.M.; Goodwin, A.E. Treatments for Ich Infestations in Channel Catfish Evaluated under Static and Flow-Through Water Conditions. N. Am. J. Aquac. 2001, 63, 293–299. [Google Scholar] [CrossRef]
- Nie, D.; Lee, L.S. Studies on the Morphology and Life Cycle of Ichthyophthirius nultifliis and Its Control, with a Description of a New Species. Acta Hydrobiol. Sin. 1960, 2, 197–215. [Google Scholar]
- Abdel-Hafez, G.; Lahnsteiner, F.; Mansour, N.; Licek, E. Pathophysiology of Ichthyophthirius multifiliis Infection in Rainbow Trout (Oncorhynchus mykiss) and Chub (Leuciscus cephalus). J. Comp. Pathol. 2014, 151, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Brierley, A.S. Plankton. Curr. Biol. 2017, 27, R478–R483. [Google Scholar] [CrossRef]
- Kosiba, J.; Wilk-Woźniak, E.; Krztoń, W.; Strzesak, M.; Pociecha, A.; Walusiak, E.; Pudaś, K.; Szarek-Gwiazda, E. What Underpins the Trophic Networks of the Plankton in Shallow Oxbow Lakes? Microb. Ecol. 2017, 73, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Weisse, T. Functional Diversity of Aquatic Ciliates. Eur. J. Protistol. 2017, 61, 331–358. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Xie, Y.; Song, C.; Zhang, Y.; Yu, H.; Burton, G.A. Zooplankton Community Profiling in a Eutrophic Freshwater Ecosystem-Lake Tai Basin by DNA Metabarcoding. Sci. Rep. 2017, 7, 1773. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Bastos Gomes, G.; Zhao, W.; Hu, G.; Huang, K.; Yoshinaga, T.; Clark, T.G.; Li, W.; Zou, H.; Wu, S.; et al. Cultivation of Fish Ciliate Parasites: Progress and Prospects. Rev. Aquac. 2023, 15, 142–162. [Google Scholar] [CrossRef]
- Clayton, G.M.; Price, D.J. Ichthyophthirius multifiliis: Standardization of the Infection-Response Model in Ameca splendens (Miller & Fitzsimons). J. Fish Dis. 1988, 11, 371–377. [Google Scholar] [CrossRef]
- Hermosilla, C.; Stamm, I.; Taubert, A.; Lutz, K.; Zahner, H.; Menge, C. Fluorescent Eimeria bovis Sporozoites and Meront Stages In Vitro: A Helpful Tool to Study Parasite–Host Cell Interactions. Parasitol. Res. 2008, 102, 777–786. [Google Scholar] [CrossRef]
- Shen, C. Fauna Sinica (Crustacea, Freshwater Copepoda); Science Press: Beijing, China, 1979. [Google Scholar]
- Bissett, A.; Gibson, J.A.E.; Jarman, S.N.; Swadling, K.M.; Cromer, L. Isolation, Amplification, and Identification of Ancient Copepod DNA from Lake Sediments. Limnol. Oceanogr. Methods 2005, 3, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A Fast Online Phylogenetic Tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Zhou, Q.; Chen, K.; Xi, B.; Xie, J.; Pan, L.; Mao, Y. Establishment and Application of PCR and SYBR Green Real-Time PCR Assays for Detection of Ichthyophthirius multifiliis. J. Fish. China 2023, 1–9. Available online: http://kns.cnki.net/kcms/detail/31.1283.S.20230109.1250.001.html (accessed on 2 June 2023).
- Lašt’ovička, J.; Budinský, V.; Špíšek, R.; Bartůňková, J. Assessment of Lymphocyte Proliferation: CFSE Kills Dividing Cells and Modulates Expression of Activation Markers. Cell. Immunol. 2009, 256, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.J. The Effect of Daphnia Interference on a Natural Rotifer and Ciliate Community: Short-Term Bottle Experiments. Limnol. Oceanogr. 1989, 34, 606–617. [Google Scholar] [CrossRef]
- Wickham, S.A.; Gilbert, J.J. Relative Vulnerabilities of Natural Rotifer and Ciliate Communities to Cladocerans: Laboratory and Field Experiments. Freshw. Biol. 1991, 26, 77–86. [Google Scholar] [CrossRef]
- Landry, M.R.; Gifford, D.J.; Kirchman, D.L.; Wheeler, P.A.; Monger, B.C. Direct and Indirect Effects of Grazing by Neocalanus plumchrus on Plankton Community Dynamics in the Subarctic Pacific. Prog. Oceanogr. 1993, 32, 239–258. [Google Scholar] [CrossRef]
- Atkinson, A. Subantarctic Copepods in an Oceanic, Low Chlorophyll Environment: Ciliate Predation, Food Selectivity and Impact on Prey Populations. Mar. Ecol. Prog. Ser. 1996, 130, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Vincent, D.; Hartmann, H.J. Contribution of Ciliated Microprotozoans and Dinoflagellates to the Diet of Three Copepod Species in the Bay of Biscay. Hydrobiologia 2001, 443, 193–204. [Google Scholar] [CrossRef]
- Lu, X.; Weisse, T. Top-down Control of Planktonic Ciliates by Microcrustacean Predators Is Stronger in Lakes than in the Ocean. Sci. Rep. 2022, 12, 10501. [Google Scholar] [CrossRef]
- Dhanker, R.; Kumar, R.; Tseng, L.C.; Hwang, J.S. Ciliate (Euplotes sp.) Predation by Pseudodiaptomus annandalei (Copepoda: Calanoida) and the Effects of Mono-Algal and Pluri-Algal Diets. Zool. Stud. 2013, 52, 34. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.V.; De Stasio, B.T., Jr.; Huizenga, K.N.; Silow, E.A. Trophic Coupling of the Microbial and the Classical Food Web in Lake Baikal, Siberia. Freshw. Biol. 2019, 64, 138–151. [Google Scholar] [CrossRef] [Green Version]
- Böttjer, D.; Morales, C.E.; Bathmann, U. Trophic Role of Small Cyclopoid Copepod Nauplii in the Microbial Food Web: A Case Study in the Coastal Upwelling System off Central Chile. Mar. Biol. 2010, 157, 689–705. [Google Scholar] [CrossRef]
- Kim Hue, N.T.; Deruyck, B.; Decaestecker, E.; Vandamme, D.; Muylaert, K. Biological Control of Ciliate Contamination in Chlamydomonas Culture Using the Predatory Copepod Acanthocyclops robustus. Algal Res. 2019, 37, 269–276. [Google Scholar] [CrossRef]
| Sample Code | Type | Location | Health Condition of the Fish |
|---|---|---|---|
| NQ1-3 | farming ponds | Nanquan Base of FFRC, Wuxi, Jiangsu | healthy |
| YX1-3 | farming ponds | Nancheng Village, Yixing, Jiangsu | yellow-head catfish Pelteobagrus fulvidraco with serious white spot disease |
| LK1 | wild water body | The Gonghu Lake, near Ping’an Bridge, Wuxi, Jiangsu | healthy |
| LK2 | wild water body | The Taihu Lake, near Renzi Harbor Bridge, Wuxi, Jiangsu | healthy |
| LK3 | wild water body | The Lihu Lake, near Changguangxi Bridge, Wuxi, Jiangsu | healthy |
| Group Code | Water Source | Zooplankton | Theront (Cells) |
|---|---|---|---|
| T | 2 L aerated tap water | null | null |
| T+I | 2 L aerated tap water | null | 50,000 |
| T+I+Z | 2 L aerated tap water | ~1000 ind./L | 50,000 |
| P | 2 L pond water | ~1000 ind./L | null |
| P+I | 2 L pond water | null | 50,000 |
| P+I+Z | 2 L pond water | ~1000 ind./L | 50,000 |
| Species | Source | Numbers | Similar Species and Acc. No. | Sequence Similarity | |
|---|---|---|---|---|---|
| Paracyclopina sp. | LK2–3 | 2 | P. nana | FJ214952 | 90.62% |
| Thermocyclops taihokuensis | NQ1–3 | 60 | T. taihokuensis | KR048801 | 99.22% |
| LK1–3 | |||||
| Macrocyclops albidus | LK2 | 1 | M. albidus | KR048794 | 99.62% |
| Macrocyclops sp. | YX3 | 1 | M. distinctus | KF153695 | 98.02% |
| Eucyclops roseus | KR048792 | 96.03% | |||
| Mesocyclops pehpeiensis | LK2 | 2 | M. pehpeiensis | KR048797 | 99.22% |
| Mesocyclops sp1. | YX1–3 | 27 | M. leuckarti | KF153692 | 93.44% |
| M. pehpeiensis | KR048797 | 93.05% | |||
| Mesocyclops sp2. | YX1–3 | 3 | M. leuckarti | KF153692 | 89.96% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.-Y.; Xi, B.-W.; Zhou, Q.-J.; Chen, K.; Xie, J. Predation of Cyclopoid Copepods on the Theronts of Ichthyophthirius multifiliis: Shedding Light on Biocontrol of White Spot Disease. Pathogens 2023, 12, 860. https://doi.org/10.3390/pathogens12070860
Cao Z-Y, Xi B-W, Zhou Q-J, Chen K, Xie J. Predation of Cyclopoid Copepods on the Theronts of Ichthyophthirius multifiliis: Shedding Light on Biocontrol of White Spot Disease. Pathogens. 2023; 12(7):860. https://doi.org/10.3390/pathogens12070860
Chicago/Turabian StyleCao, Ze-Yi, Bing-Wen Xi, Qing-Jie Zhou, Kai Chen, and Jun Xie. 2023. "Predation of Cyclopoid Copepods on the Theronts of Ichthyophthirius multifiliis: Shedding Light on Biocontrol of White Spot Disease" Pathogens 12, no. 7: 860. https://doi.org/10.3390/pathogens12070860





