Anti-Hcp1 Monoclonal Antibody Is Protective against Burkholderia pseudomallei Infection via Recognizing Amino Acids at Asp95-Leu114
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Bacterial Strains
2.2. Construction of Plasmids and B. pseudomallei Mutant Strains
2.3. Preparation of rHcp1 and Production of mAbs
2.4. Rate of Association/Dissociation
2.5. Quantitative RT-PCR (qRT-PCR) and Western Blotting
2.6. Mouse Survival and Organ Burden
2.7. Cell Infection and Experiment on Bacterial Intracellular Survival
2.8. Giemsa Stain
2.9. Immunofluorescence Assay
2.10. Statistical Analysis
3. Results
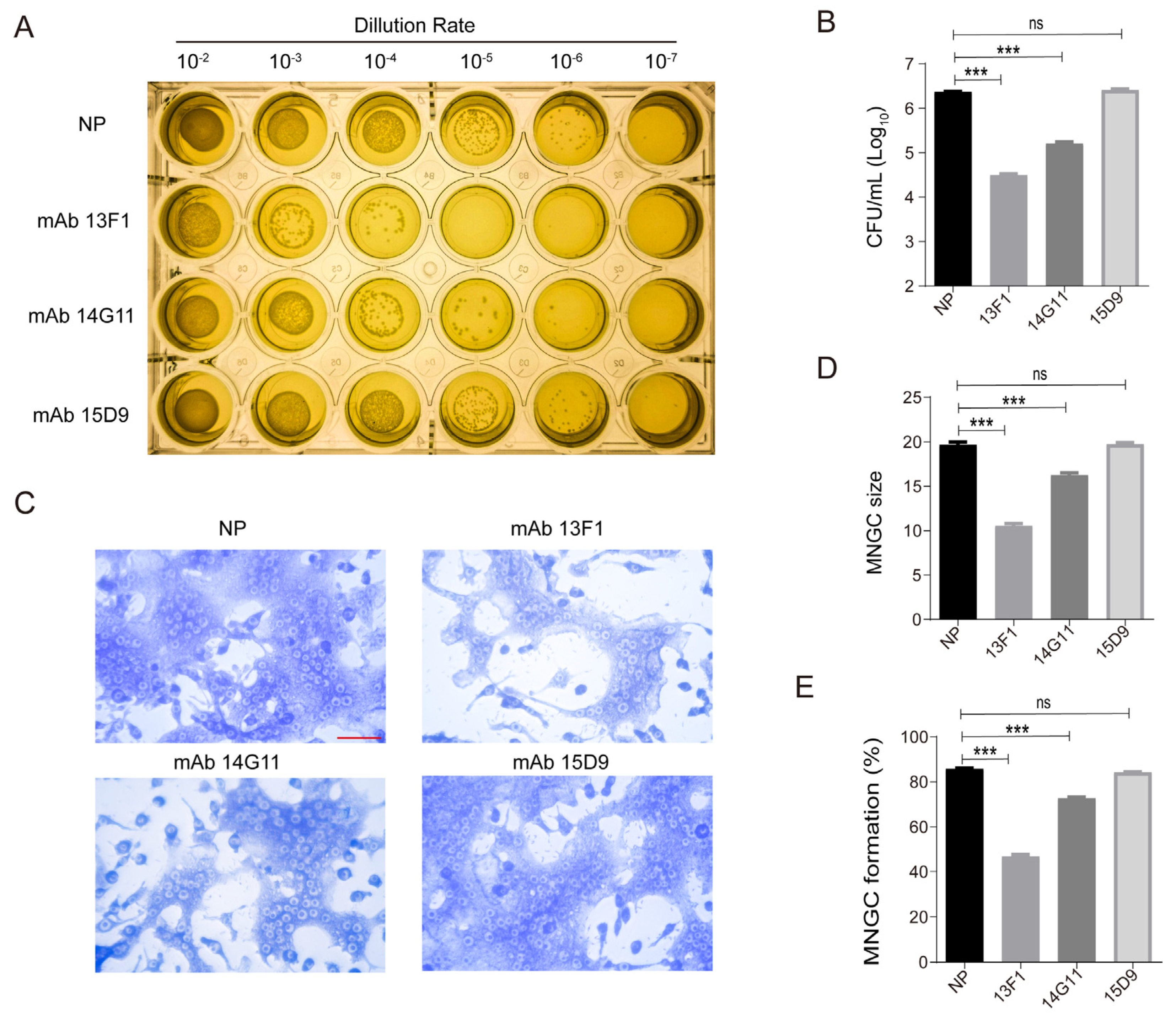
3.1. Anti-rHcp1 mAbs Reduce the Intracellular Survival of B. pseudomallei and Exhibit Protective Capacity In Vivo
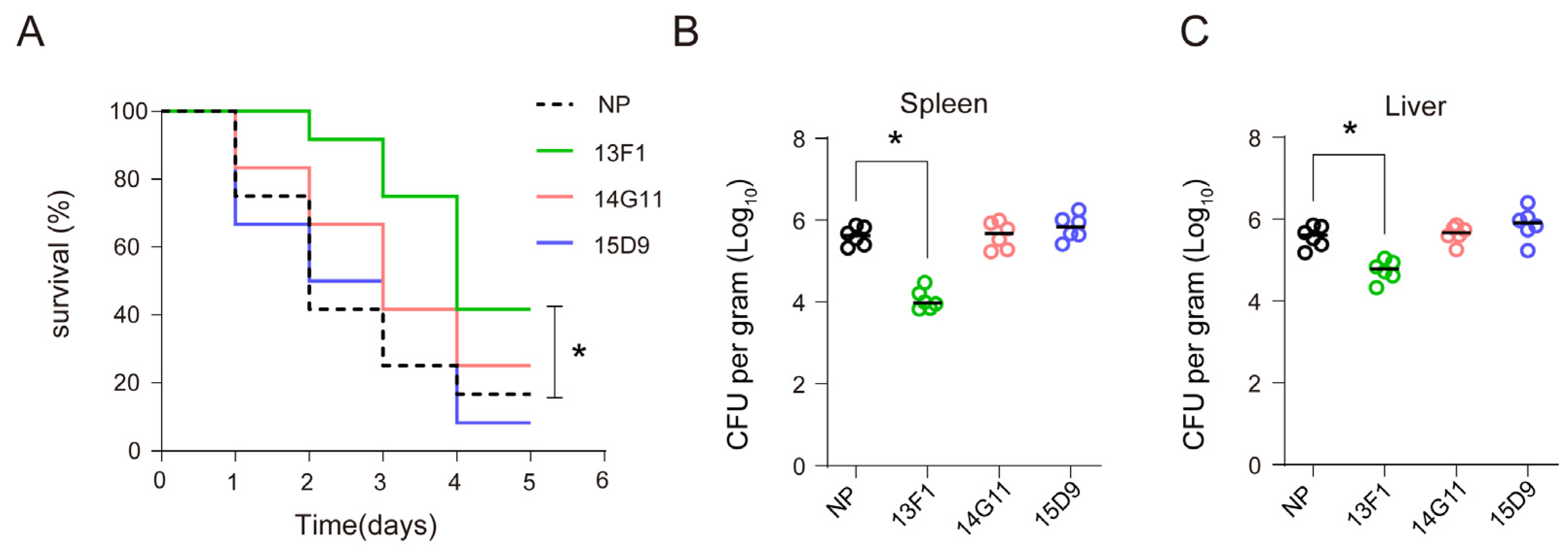
3.2. Anti–rHcp1 mAbs Exhibit Protective Capacity In Vivo
3.3. The Epitope Recognized by 13F1 Is Specifically Located Hcp1 Asp95-Leu114
3.4. 13F1 Plays Its Protective Role by Binding Hcp1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Speiser, L.J.; Graf, E.H.; Seville, M.T.; Singbartl, K.; Dalton, M.L.; Harrington, D.; Kretschmer, M.; Kuljanin, M.; Zabel, K.; Sunenshine, R.; et al. Burkholderia pseudomallei Laboratory Exposure, Arizona, USA. Emerg. Infect. Dis. 2023, 29, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Meumann, E.M.; Kaestli, M. The Expanding Global Footprint of Burkholderia pseudomallei and Melioidosis. Am. J. Trop. Med. Hyg. 2023, 108, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Gassiep, I.; Armstrong, M.; Norton, R. Human Melioidosis. Clin. Microbiol. Rev. 2020, 33, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Karunanayake, P. Melioidosis: Clinical aspects. Clin. Med. 2022, 22, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.K.; DeShazer, D.; Brett, P.J.; Burtnick, M.N. Melioidosis: Molecular aspects of pathogenesis. Expert. Rev. Anti Infect. Ther. 2014, 12, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Bzdyl, N.M.; Moran, C.L.; Bendo, J.; Sarkar–Tyson, M. Pathogenicity and virulence of Burkholderia pseudomallei. Virulence 2022, 13, 1945–1965. [Google Scholar] [CrossRef] [PubMed]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef]
- Tsao, L.C.; Force, J.; Hartman, Z.C. Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res. 2021, 81, 4641–4651. [Google Scholar] [CrossRef]
- Pelfrene, E.; Mura, M.; Cavaleiro Sanches, A.; Cavaleri, M. Monoclonal antibodies as anti–infective products: A promising future? Clin. Microbiol. Infect. 2019, 25, 60–64. [Google Scholar] [CrossRef]
- Wang–Lin, S.X.; Balthasar, J.P. Pharmacokinetic and Pharmacodynamic Considerations for the Use of Monoclonal Antibodies in the Treatment of Bacterial Infections. Antibodies 2018, 7, 5. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, Y.; Kwon, K.; Park, J.S.; Park, K.U.; Moon, S.M.; Song, K.H.; Kim, E.S.; Park, W.B.; Kim, H.B. Anti–Alpha–Toxin Antibody Responses and Clinical Outcomes of Staphylococcus aureus Bacteremia. J. Korean Med. Sci. 2023, 38, e129. [Google Scholar] [CrossRef] [PubMed]
- Proctor, L.L.; Ward, W.L.; Roggy, C.S.; Koontz, A.G.; Clark, K.M.; Quinn, A.P.; Schroeder, M.; Brooks, A.E.; Small, J.M.; Towne, F.D.; et al. Potential Therapeutic Targets for Combination Antibody Therapy against Pseudomonas aeruginosa Infections. Antibiotics 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, C.; Hamilton, M.M.; Sadowska, A.; Shi, Y.; Chang, C.S.; Chowdhury, P.; Buonapane, R.; Xiao, X.; Warrener, P.; Mediavilla, J.; et al. Targeting Alpha Toxin and ClfA with a Multimechanistic Monoclonal–Antibody–Based Approach for Prophylaxis of Serious Staphylococcus aureus Disease. mBio 2016, 7, e00528-16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.T.; Yue, Y.; Zheng, F.; Liang, X.D.; Cao, Q.X.; Wang, Y.W.; Zhu, J. Monoclonal antibody against l-lectin module of SraP blocks adhesion and protects mice against Staphylococcus aureus challenge. J. Microbiol. Immunol. Infect. 2021, 54, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qian, M.; Yi, S.; Liu, S.; Li, B.; Yu, R.; Guo, Q.; Zhang, X.; Yu, C.; Li, J.; et al. Monoclonal Antibody Targeting Staphylococcus aureus Surface Protein A (SasA) Protect Against Staphylococcus aureus Sepsis and Peritonitis in Mice. PLoS ONE 2016, 11, e0149460. [Google Scholar] [CrossRef]
- Akiyama, M.; Oishi, K.; Tao, M.; Matsumoto, K.; Pollack, M. Antibacterial properties of Pseudomonas aeruginosa immunotype 1 lipopolysaccharide–specific monoclonal antibody (MAb) in a murine thigh infection model: Combined effects of MAb and ceftazidime. Microbiol. Immunol. 2000, 44, 629–635. [Google Scholar] [CrossRef]
- Chaichana, P.; Kronsteiner, B.; Rongkard, P.; Teparrukkul, P.; Limmathurotsakul, D.; Chantratita, N.; Day, N.P.J.; Fletcher, H.A.; Dunachie, S.J. Serum from melioidosis survivors diminished intracellular Burkholderia pseudomallei growth in macrophages: A brief research report. Front. Cell. Infect. Microbiol. 2020, 10, 442. [Google Scholar] [CrossRef]
- Chaichana, P.; Jenjaroen, K.; Chumseng, S.; Sumonwiriya, M.; Rongkard, P.; Kronsteiner, B.; Teparrukkul, P.; Limmathurotsakul, D.; Day, N.; Chantratita, N.P.J.; et al. Role of Burkholderia pseudomallei–Specific IgG2 in Adults with Acute Melioidosis, Thailand. Emerg. Infect. Dis. 2021, 27, 463–470. [Google Scholar] [CrossRef]
- Taylor, A.; Jenner, D.; Rowland, C.; Laws, T.; Norville, I.; Prior, J. Monoclonal Antibodies Opsonize Burkholderia spp. and Reduce Intracellular Actin Tail Formation in a Macrophage Infection Assay. J. Bacteriol. 2021, 203, e0024421. [Google Scholar] [CrossRef]
- Mougous, J.D.; Cuff, M.E.; Raunser, S.; Shen, A.; Zhou, M.; Gifford, C.A.; Goodman, A.L.; Joachimiak, G.; Ordoñez, C.L.; Lory, S.; et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 2006, 312, 1526–1530. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Subedi, D.; Gong, Q.; Huang, H.; Li, J.; Wang, Y.; Ren, J. Hcp Proteins of the Type VI Secretion System Promote Avian Pathogenic, E. coli DE205B (O2:K1) to Induce Meningitis in Rats. Life 2022, 12, 1353. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, C.L.; Mott, T.M.; Muruato, L.A.; Sbrana, E.; Torres, A.G. Burkholderia mallei CLH001 Attenuated Vaccine Strain Is Immunogenic and Protects against Acute Respiratory Glanders. Infect. Immun. 2016, 84, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Burtnick, M.N.; Shaffer, T.L.; Ross, B.N.; Muruato, L.A.; Sbrana, E.; DeShazer, D.; Torres, A.G.; Brett, P.J. Development of subunit vaccines that provide high-level protection and sterilizing immunity against acute inhalational melioidosis. Infect. Immun. 2018, 86, e00724-17. [Google Scholar] [CrossRef] [PubMed]
- Solodushko, V.; Bitko, V.; Barrington, R.; Fouty, B. A DNA vaccine in which the rsv–f ectodomain is covalently linked to the antigens tssm and hcp1 augments the humoral and cytotoxic response in mice. Front. Immunol. 2019, 10, 2411. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Li, G.; Li, J.; Zheng, M.; Peng, X.; Rao, Y.; Li, M.; Zhou, R.; Rao, X. Hcp1-loaded staphylococcal membrane vesicle vaccine protects against acute melioidosis. Front. Immunol. 2022, 13, 1089225. [Google Scholar] [CrossRef] [PubMed]
- Pumpuang, A.; Phunpang, R.; Ekchariyawat, P.; Dulsuk, A.; Loupha, S.; Kwawong, K.; Charoensawat, Y.; Thiansukhon, E.; Day, N.P.J.; Burtnick, M.N.; et al. Distinct classes and subclasses of antibodies to hemolysin co-regulated protein 1 and O-polysaccharide and correlation with clinical characteristics of melioidosis patients. Sci. Rep. 2019, 9, 13972. [Google Scholar] [CrossRef] [PubMed]
- Burtnick, M.N.; Brett, P.J.; Harding, S.V.; Ngugi, S.A.; Ribot, W.J.; Chantratita, N.; Scorpio, A.; Milne, T.S.; Dean, R.E.; Fritz, D.L.; et al. The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect. Immun. 2011, 79, 1512–1525. [Google Scholar] [CrossRef] [PubMed]
- Phokrai, P.; Karoonboonyanan, W.; Thanapattarapairoj, N.; Promkong, C.; Dulsuk, A.; Koosakulnirand, S.; Canovali, S.; Indrawattana, N.; Jutrakul, Y.; Wuthiekanun, V.; et al. A Rapid Immunochromatography Test Based on Hcp1 Is a Potential Point-of-Care Test for Serological Diagnosis of Melioidosis. J. Clin. Microbiol. 2018, 56, 7–18. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, Y.; Li, Q.; Chen, H.; Yao, Z.; Pan, J.; Gu, J.; Tang, B.; Wang, H.-G.; Yu, B.; et al. First genome sequence of a Burkholderia pseudomallei Isolate in China, strain BPC006, obtained from a melioidosis patient in Hainan. J. Bacteriol. 2012, 194, 6604–6605. [Google Scholar] [CrossRef]
- Lefebre, M.D.; Valvano, M.A. Construction and evaluation of plasmid vectors optimized for constitutive and regulated gene expression in Burkholderia cepacia complex isolates. Appl. Environ. Microbiol. 2002, 68, 5956–5964. [Google Scholar] [CrossRef]
- Wong, J.; Chen, Y.; Gan, Y.H. Host Cytosolic Glutathione Sensing by a Membrane Histidine Kinase Activates the Type VI Secretion System in an Intracellular Bacterium. Cell Host Microbe 2015, 18, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Hu, Z.; Rao, C.; Chen, J.; Yuan, S.; Zhang, J.; Mao, C.; Yan, J.; Xia, Y.; Zhang, M.; et al. Burkholderia pseudomallei interferes with host lipid metabolism via NR1D2-mediated PNPLA2/ATGL suppression to block autophagy-dependent inhibition of infection. Autophagy 2021, 17, 1918–1933. [Google Scholar] [CrossRef] [PubMed]
- Coulthurst, S. The Type VI secretion system: A versatile bacterial weapon. Microbiology 2019, 165, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.T.; Jobichen, C.; Wong, J.; Limmathurotsakul, D.; Li, S.; Chen, Y.; Raida, M.; Srinivasan, N.; MacAry, P.A.; Sivaraman, J.; et al. Extended loop region of Hcp1 is critical for the assembly and function of type VI secretion system in Burkholderia pseudomallei. Sci. Rep. 2015, 5, 8235. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Senft, J.L.; Lockett, S.J.; Brett, P.J.; Burtnick, M.N.; DeShazer, D.; Friedlander, A.M. pH Alkalinization by Chloroquine Suppresses Pathogenic Burkholderia Type 6 Secretion System 1 and Multinucleated Giant Cells. Infect. Immun. 2017, 85, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.A.; Furniss, R.C.D.; Bonini, D.; Amin, H.; Paracuellos, P.; Zlotkin, D.; Costa, T.R.D.; Levy, A.; Mavridou, D.A.I.; Filloux, A. The Breadth and Molecular Basis of Hcp-Driven Type VI Secretion System Effector Delivery. mBio 2021, 12, e0026221. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ren, S.; Shen, D.; Yang, N.; Wang, B.; Han, S.; Shen, X.; Chou, S.-H.; Qian, G. An intrinsic mechanism for coordinated production of the contact-dependent and contact-independent weapon systems in a soil bacterium. PLoS Pathog. 2020, 16, e1008967. [Google Scholar] [CrossRef]
- Silverman, J.M.; Agnello, D.M.; Zheng, H.; Andrews, B.T.; Li, M.; Catalano, C.E.; Gonen, T.; Mougous, J.D. Haemolysin coregulated protein is an exported receptor and chaperone of type VI secretion substrates. Mol. Cell 2013, 51, 584–593. [Google Scholar] [CrossRef]
- Wang, H.; Lv, L.B.; Chen, L.P.; Xiao, J.L.; Shen, J.; Gao, B.; Zhao, J.-G.; Han, D.-M.; Chen, B.-X.; Wang, S.; et al. Hemolysin Co-Regulatory Protein 1 Enhances the Virulence of Clinically Isolated in KM Mice by Increasing Inflammation and Inducing Pyroptosis. Toxins 2023, 15, 171. [Google Scholar] [CrossRef]
- Tekedar, H.C.; Abdelhamed, H.; Kumru, S.; Blom, J.; Karsi, A.; Lawrence, M.L. Comparative Genomics of Secretion Systems and Mutational Analysis of and Genes from T6SS. Front. Microbiol. 2019, 9, 3216–3229. [Google Scholar] [CrossRef]
- Noreen, Z.; Jobichen, C.; Abbasi, R.; Seetharaman, J.; Sivaraman, J.; Bokhari, H. Structural basis for the pathogenesis of Campylobacter jejuni Hcp1, a structural and effector protein of the Type VI Secretion System. FEBS J. 2018, 285, 4060–4070. [Google Scholar] [CrossRef] [PubMed]
- Fong, R.; Kajihara, K.; Chen, M.; Hotzel, I.; Mariathasan, S.; Hazenbos, W.L.W.; Lupardus, P.J. Structural investigation of human S. aureus–targeting antibodies that bind wall teichoic acid. MAbs 2018, 10, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.L.; Hiroshi, M.J.; et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature 2015, 527, 323–328. [Google Scholar]
- Frank, D.W.; Vallis, A.; Wiener-Kronish, J.P.; Roy-Burman, A.; Spack, E.G.; Mullaney, B.P.; Megdoud, M.; Marks, J.D.; Fritz, R.; Sawa, T. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J. Infect. Dis. 2002, 186, 64–73. [Google Scholar] [CrossRef]
- Quenee, L.E.; Berube, B.J.; Segal, J.; Elli, D.; Ciletti, N.A.; Anderson, D.; Schneewind, O. Amino acid residues 196–225 of LcrV represent a plague protective epitope. Vaccine 2010, 28, 1870–1876. [Google Scholar] [CrossRef]
- Stockton, J.L.; Torres, A.G. Multinucleated Giant Cell Formation as a Portal to Chronic Bacterial Infections. Microorganisms 2020, 8, 1637. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hsiao, Y.S.; Lin, H.H.; Yen, C.M.; Chen, S.C.; Chen, Y.L. Immunogenicity and anti–burkholderia pseudomallei activity in balb/c mice immunized with plasmid DNA encoding flagellin. Vaccine 2006, 24, 750–758. [Google Scholar] [CrossRef]
- Laws, T.R.; Clark, G.C.; D’Elia, R.V. Immune profiling of the progression of a balb/c mouse aerosol infection by and the therapeutic implications of targeting hmgb1. Int. J. Infect. Dis. 2015, 40, 1–8. [Google Scholar] [CrossRef]
| Strain or Plasmid | Relevant Characteristic | Source of Reference |
|---|---|---|
| Plasmids | ||
| pMLS7 | Broad-host-range vector; Tpr; S7 ribosomal protein promoter from Burkholderia sp. strain LB400 | Lefebre, M.D. et al., 2002 [30] |
| pMLS7-hcp1 | pMLS7 containing a 510-bp full-length of B. pseudomallei hcp1 gene | This study |
| pET-28a-hcp1 | pET-28a containing a510-bp full-length of B. pseudomallei hcp1 gene | This study |
| pET-28a-hcp1283-342-null | pET-28a containing a 450-bp length of B. pseudomallei hcp1 gene | This study |
| pK18mobsacB | Conjugative, suicide vector | Wong, J. et al., 2015 [31] |
| pK18mobsacB-Δhcp1 | pK18mobsacB containing 802-bp fragment upstream and 550-bp fragment downstream of ORF BPC006_RS26143 | This study |
| Strains | ||
| E. coli | ||
| DH5α | Cloning host | Sangon Biotech |
| DH5αBL21 | Cloning host | Sangon Biotech |
| DH5αS17-λpir | Donor strain for conjugation | Wong, J. et al., 2015 [31] |
| B. pseudomallei | ||
| BPC006 | B. pseudomallei wild-type strain | Fang, Y. et al., 2012 [29] |
| Δhcp1 | BPC006 Δhcp1, codon 1-510 of BPC006_RS26143 ORF were deleted | This study |
| Δhcp1:hcp1 | Δhcp1 with plasmid pMLS7-hcp1 | This study |
| Primer Name | Sequence (5′–3′) a |
|---|---|
| pET-28a-hcp1-F | GGATCCATGCTGGCCGGAATATATCTCAA |
| pET-28a-hcp1-R | AAGCTTTCAGCCATTCGTCCAGTTTGCGGC |
| pMLS7-hcp1-F | GCTCTAGAATGCTGGCCGGAATATATCTCAAGGTCAAAGG |
| pMLS7-hcp1-R | CCAAGCTTTCAGCCATTCGTCCAGTTTGCGGC |
| hcp1-up-F | TGTAAAACGACGGCCAAGTGCCAAGCTTGTGACCGATCTGCCGCTCTAC |
| hcp1-up-R | CCCGCGACGATTCGCGATCAGCCATTCTGAGATATATTCCGGCCAGCATG |
| hcp1-down-F | GCGCCATGCTGGCCGGAATATATCTCAGAATGGCTGATCGCGAATCGTCG |
| hcp1-down-R | AAACAGCTATGACATGATTACGAATTCAGGCGAACGAGCTCGTCCTCG |
| pGEX-6P-hcp1-F1 | CGGGATCCATGCTGGCCGGAATATATCTCAAG |
| pGEX-6P-hcp1-F2 | CGGGATCCCGCGGCACGATCACGTTGA |
| pGEX-6P-hcp1-F3 | CGGGATCCGCGCTCGGCAAGCGCGAG |
| pGEX-6P-hcp1-R1 | CGGAATTCTTATCAGCCATTCGTCCAGTTTGCG |
| pGEX-6P-hcp1-R2 | CGGAATTCCAGCACTTTTTCGAACTTGTACGTGAACAG |
| pGEX-6P-hcp1-R3 | GGTCTAGATTACGTCTTCGGACGGTGGATCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Rao, C.; Liu, W.; Zhang, Z.; Nan, D.; Chen, J.; Wang, M.; Wen, Y.; Yan, J.; Yue, J.; et al. Anti-Hcp1 Monoclonal Antibody Is Protective against Burkholderia pseudomallei Infection via Recognizing Amino Acids at Asp95-Leu114. Pathogens 2024, 13, 43. https://doi.org/10.3390/pathogens13010043
Wu P, Rao C, Liu W, Zhang Z, Nan D, Chen J, Wang M, Wen Y, Yan J, Yue J, et al. Anti-Hcp1 Monoclonal Antibody Is Protective against Burkholderia pseudomallei Infection via Recognizing Amino Acids at Asp95-Leu114. Pathogens. 2024; 13(1):43. https://doi.org/10.3390/pathogens13010043
Chicago/Turabian StyleWu, Pan, Chenglong Rao, Wenzheng Liu, Ziyuan Zhang, Dongqi Nan, Jiangao Chen, Minyang Wang, Yuan Wen, Jingmin Yan, Juanjuan Yue, and et al. 2024. "Anti-Hcp1 Monoclonal Antibody Is Protective against Burkholderia pseudomallei Infection via Recognizing Amino Acids at Asp95-Leu114" Pathogens 13, no. 1: 43. https://doi.org/10.3390/pathogens13010043
APA StyleWu, P., Rao, C., Liu, W., Zhang, Z., Nan, D., Chen, J., Wang, M., Wen, Y., Yan, J., Yue, J., Mao, X., & Li, Q. (2024). Anti-Hcp1 Monoclonal Antibody Is Protective against Burkholderia pseudomallei Infection via Recognizing Amino Acids at Asp95-Leu114. Pathogens, 13(1), 43. https://doi.org/10.3390/pathogens13010043







