Formulating Parasiticidal Fungi in Dried Edible Gelatins to Reduce the Risk of Infection by Trichuris sp. among Continuous Grazing Bison
Abstract
:1. Introduction
2. Materials and Methods
2.1. Edible Gelatins with Fungal Chlamydospores
2.2. Effect of Drying on the Viability of Fungal Chlamydospores
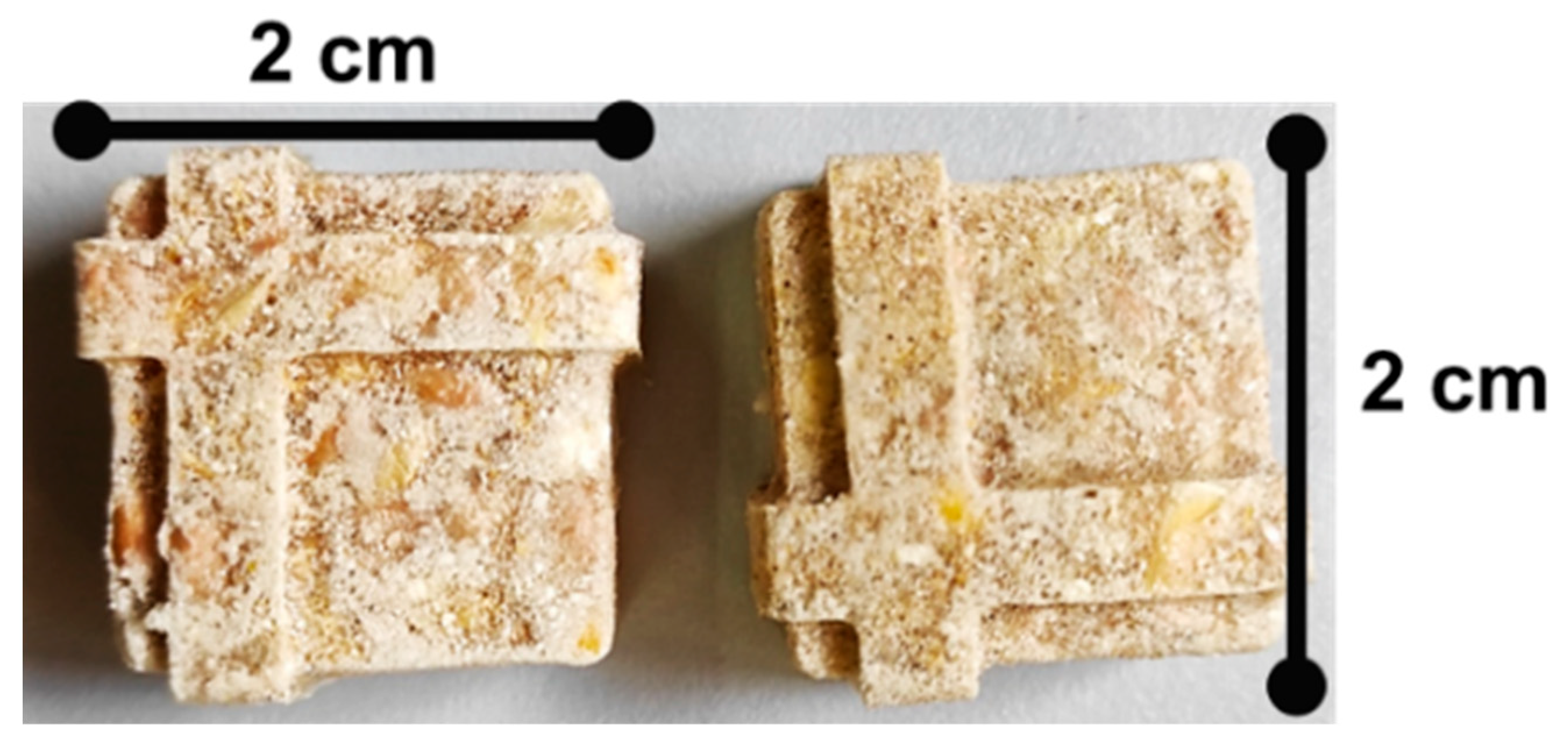
2.3. Captive Bison
2.4. Experimental Design
2.5. Evaluation of the Strategy
2.6. Statistical Analysis
3. Results
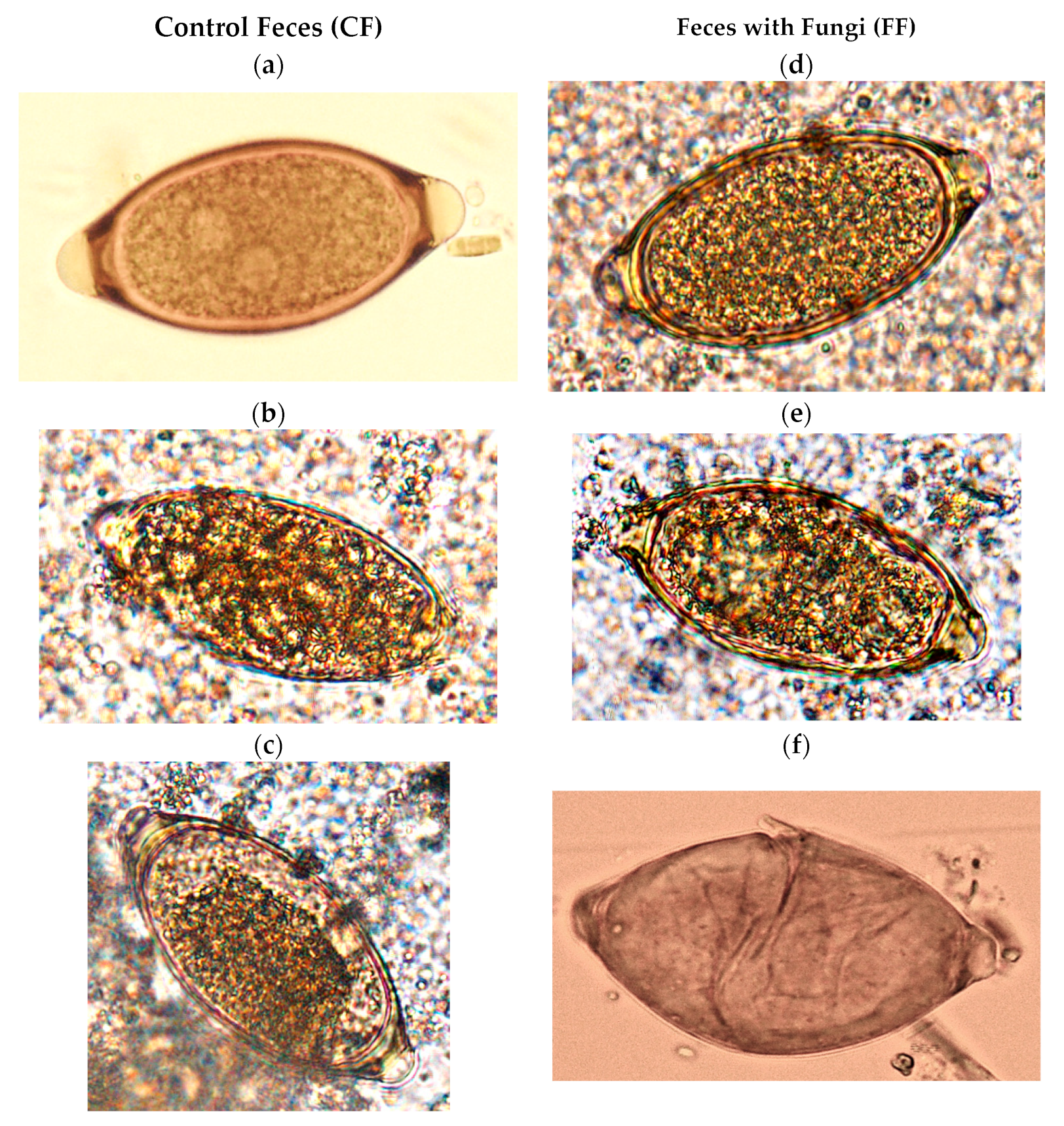
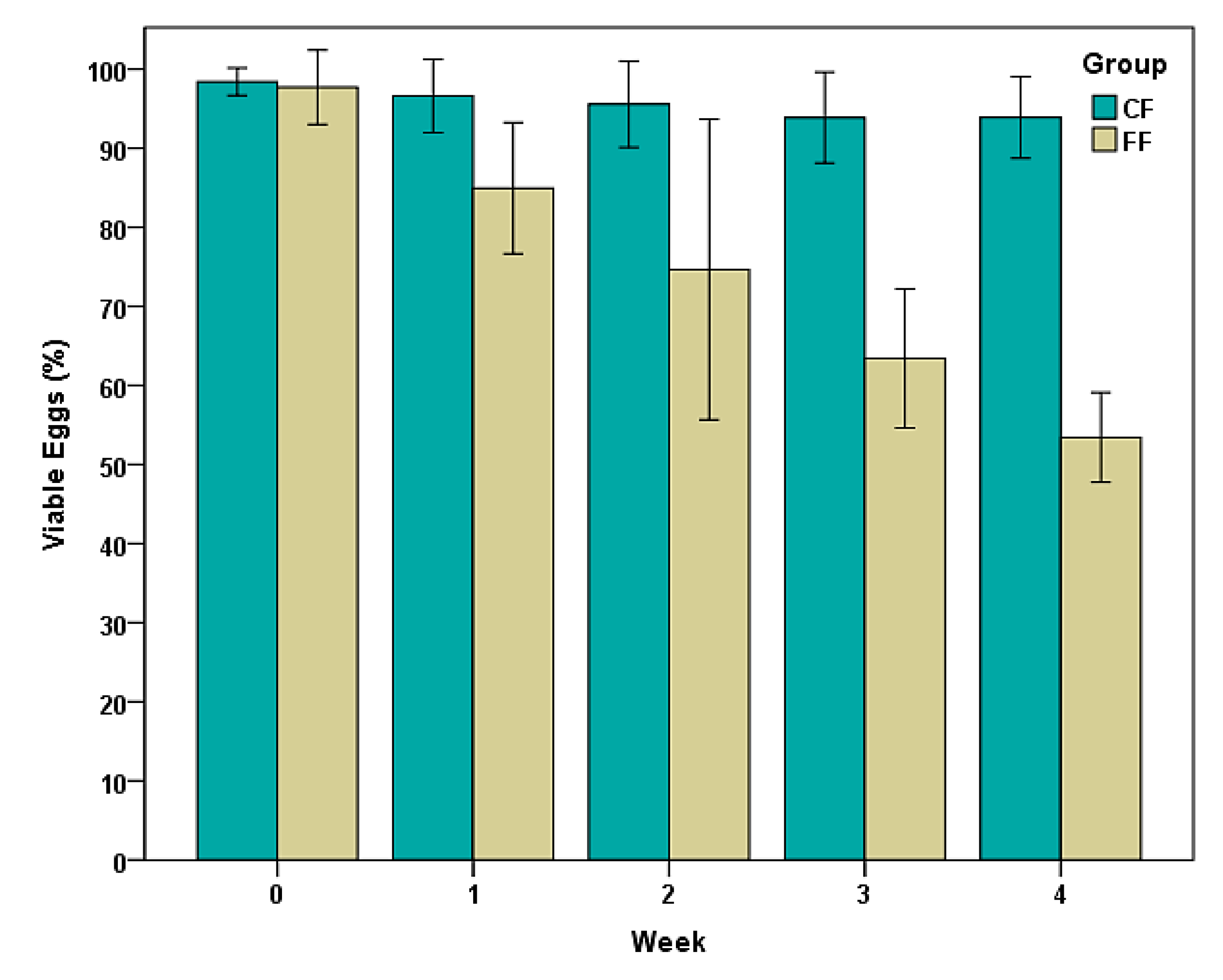
3.1. Variations on the Viability of Eggs of Trichuris sp.
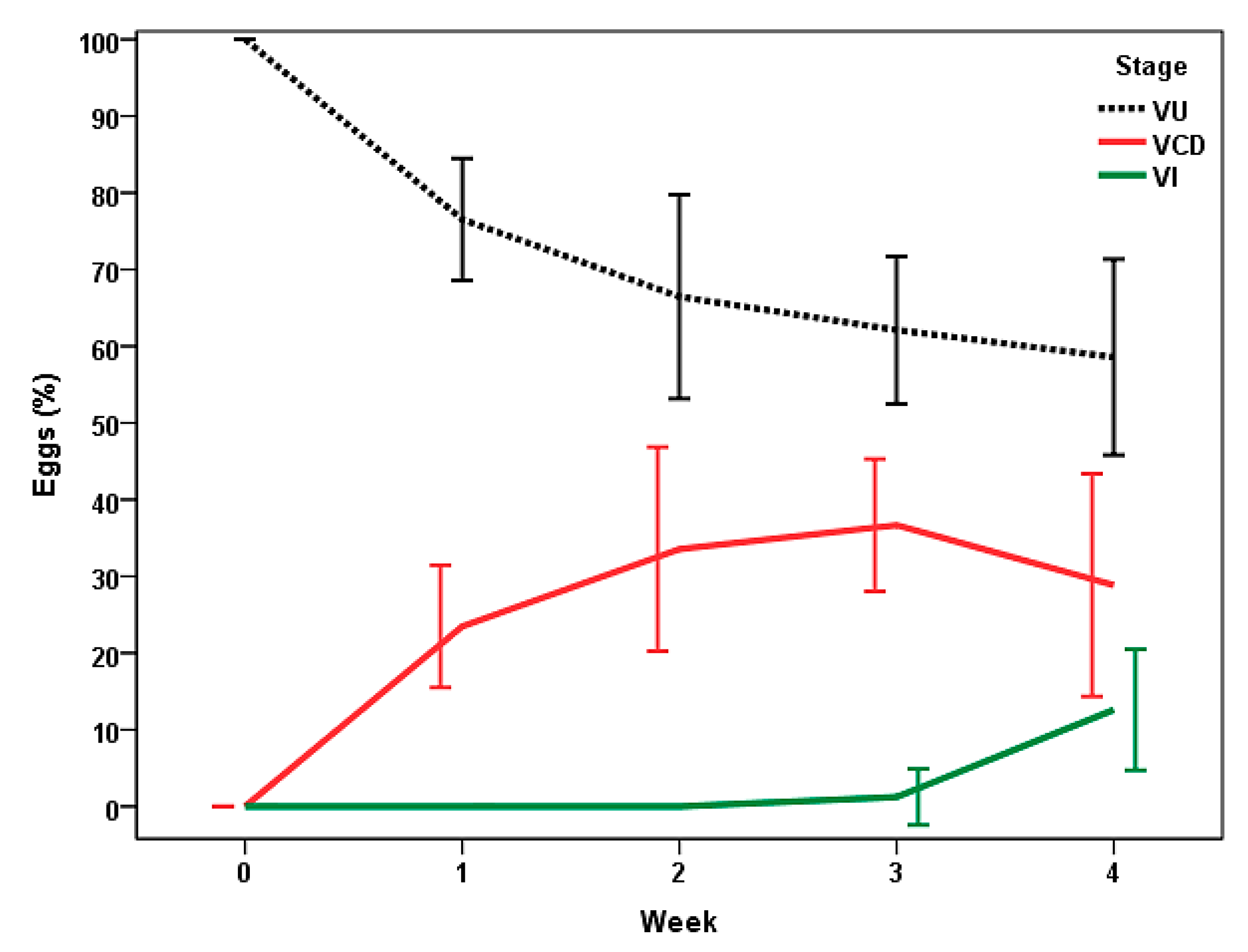
3.2. Evolution of Viable Eggs of Trichuris sp.
3.3. Effect of Ingestion of Fungal Chlamydospores on Eggs of Trichuris sp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández, J.Á.; Sánchez-Andrade, R.; Cazapal-Monteiro, C.F.; Arroyo, F.L.; Sanchís, J.M.; Paz-Silva, A.; Arias, M.S. A combined effort to avoid strongyle infection in horses in an oceanic climate region: Rotational grazing and parasiticidal fungi. Parasit. Vectors 2018, 11, 240. [Google Scholar] [CrossRef]
- McAllister, T.A.; Stanford, K.; Chaves, A.V.; Evans, P.R.; de Souza Figueiredo, E.E.; Ribeiro, G. Chapter 5—Nutrition, feeding and management of beef cattle in intensive and extensive production systems. In Animal Agriculture; Bazer, F.W., Lamb, G.C., Wu, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 75–98. [Google Scholar] [CrossRef]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Veterinary Parasitology, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Bulbul, K.; Akand, A.; Hussain, J.; Parbin, S.; Hasin, D. A brief understanding of Trichuris ovis in ruminants. Int. J. Vet. Sci. Anim. Husb. 2020, 5, 72–74. [Google Scholar]
- Eysker, M.; Bakker, N.; Kooyman, F.N.; Ploeger, H.W. The possibilities and limitations of evasive grazing as a control measure for parasitic gastroenteritis in small ruminants in temperate climates. Vet. Parasitol. 2005, 129, 95–104. [Google Scholar] [CrossRef]
- Bambou, J.C.; Ceï, W.; Arquet, R.; Calif, V.; Bocage, B.; Mandonnet, N.; Alexandre, G. Mixed Grazing and Dietary Supplementation Improve the Response to Gastrointestinal Nematode Parasitism and Production Performances of Goats. Front. Vet. Sci. 2021, 8, 628686. [Google Scholar] [CrossRef]
- Kolodziej-Sobocińska, M.; Pyziel, A.; Demiaszkiewicz, A.; Borowik, T.; Kowalczyk, R. Pattern of parasite egg shedding by European bison (Bison bonasus) in the Białowieża Primeval Forest, Poland. Mammal Res. 2016, 61, 179–186. [Google Scholar] [CrossRef]
- Wiese, J.D.; Caven, A.J.; Zarlenga, D.S.; Topliff, C.L.; Kelling, C.L.; Salter, J. Gastrointestinal parasites of a reintroduced semi-wild plains bison (Bison bison bison) herd: Examining effects of demographic variation, deworming treatments, and management strategy. Int. J. Parasitol. Parasites Wildl. 2021, 14, 216–227. [Google Scholar] [CrossRef]
- Mendoza-de-Gives, P. Soil-Borne Nematodes: Impact in Agriculture and Livestock and Sustainable Strategies of Prevention and Control with Special Reference to the Use of Nematode Natural Enemies. Pathogens 2022, 11, 640. [Google Scholar] [CrossRef]
- Waller, P.J. Sustainable nematode parasite control strategies for ruminant livestock by grazing management and biological control. Anim. Feed Sci. Technol. 2006, 1, 277–289. [Google Scholar] [CrossRef]
- Horton, J. Helminth-Nematode: Trichuris trichiura. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 111–115. [Google Scholar] [CrossRef]
- Spickler, A.R. Trichuriasis. 2019. Available online: http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php (accessed on 18 December 2023).
- Wideman, G.N. Fatal Trichuris spp. infection in a Holstein heifer persistently infected with bovine viral diarrhea virus. Can. Vet. J. 2004, 45, 511–512. [Google Scholar]
- Forman, R.; Partridge, F.A.; Sattelle, D.B.; Else, K.J. Un-‘Egg’-Plored: Characterisation of Embryonation in the Whipworm Model Organism Trichuris muris. Front. Trop. Dis. 2021, 2, 790311. [Google Scholar] [CrossRef]
- Arias, M.S.; Cazapal-Monteiro, C.F.; Suárez, J.; Miguélez, S.; Francisco, I.; Arroyo, F.L.; Suárez, J.L.; Paz-Silva, A.; Sánchez-Andrade, R.; Mendoza de Gives, P. Mixed production of filamentous fungal spores for preventing soil-transmitted helminth zoonoses: A preliminary analysis. BioMed Res. Int. 2013, 2013, 567876. [Google Scholar] [CrossRef]
- Araújo, J.V.; Braga, F.R.; Mendoza-de-Gives, P.; Paz-Silva, A.; Vilela, V.L.R. Recent Advances in the Control of Helminths of Domestic Animals by Helminthophagous Fungi. Parasitologia 2021, 1, 168–176. [Google Scholar] [CrossRef]
- Palomero, A.M.; Cazapal-Monteiro, C.F.; Valderrábano, E.; Paz-Silva, A.; Sánchez-Andrade, R.; Arias, M.S. Soil fungi enable the control of gastrointestinal nematodes in wild bovidae captive in a zoological park: A 4-year trial. Parasitology 2020, 147, 791–798. [Google Scholar] [CrossRef]
- Voinot, M.; Bonilla, R.; Sousa, S.; Sanchís, J.; Canhão-Dias, M.; Romero Delgado, J.; Lozano, J.; Sánchez-Andrade, R.; Arias, M.S.; Madeira de Carvalho, L. Control of Strongyles in First-Season Grazing Ewe Lambs by Integrating Deworming and Thrice-Weekly Administration of Parasiticidal Fungal Spores. Pathogens 2021, 10, 1338. [Google Scholar] [CrossRef] [PubMed]
- Voinot, M.; Cazapal-Monteiro, C.; Hernández, J.Á.; Palomero, A.M.; Arroyo, F.L.; Sanchís, J.; Pedreira, J.; Sánchez-Andrade, R.; Paz-Silva, A.; Arias, M.S. Integrating the control of helminths in dairy cattle: Deworming, rotational grazing and nutritional pellets with parasiticide fungi. Vet. Parasitol. 2020, 278, 109038. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.A.; Cazapal-Monteiro, C.F.; Arroyo, F.L.; Silva, M.I.; Palomero, A.M.; Paz-Silva, A.; Sánchez-Andrade, R.; Arias, M.S. Biological control of soil transmitted helminths (STHs) in a zoological park by using saprophytic fungi. Biol. Control 2018, 122, 24–30. [Google Scholar] [CrossRef]
- Viña, C.; Silva, M.I.; Palomero, A.M.; Voinot, M.; Vilá, M.; Hernández, J.Á.; Paz-Silva, A.; Sánchez-Andrade, R.; Cazapal-Monteiro, C.F.; Arias, M.S. The Control of Zoonotic Soil-Transmitted Helminthoses Using Saprophytic Fungi. Pathogens 2020, 9, 1071. [Google Scholar] [CrossRef]
- Van Metre, D.C.; Tennant, B.C.; Whitlock, R.H. Chapter 6—Infectious Diseases of the Gastrointestinal Tract. In Rebhun’s Diseases of Dairy Cattle, 2nd ed.; Divers, T.J., Peek, S.F., Eds.; W.B. Saunders: St. Louis, Missouri, USA, 2008; pp. 200–294. [Google Scholar] [CrossRef]
- Peek, S.F.; Mcguirk, S.M.; Sweeney, R.W.; Cummings, K.J. Chapter 6—Infectious Diseases of the Gastrointestinal Tract. In Rebhun’s Diseases of Dairy Cattle, 3rd ed.; Divers, T.J., Peek, S.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 249–356. [Google Scholar] [CrossRef]
- Buzatti, A.; de Paula Santos, C.; Fernandes, M.A.; Yoshitani, U.Y.; Sprenger, L.K.; dos Santos, C.D.; Molento, M.B. Duddingtonia flagrans in the control of gastrointestinal nematodes of horses. Exp. Parasitol. 2015, 159, 1–4. [Google Scholar] [CrossRef]
- Ortiz Pérez, D.O.; Sánchez Muñoz, B.; Nahed Toral, J.; Orantes Zebadúa, M.Á.; Cruz López, J.L.; Reyes García, M.E.; Mendoza de Gives, P. Using Duddingtonia flagrans in calves under an organic milk farm production system in the Mexican tropics. Exp. Parasitol. 2017, 175, 74–78. [Google Scholar] [CrossRef]
- Vieira, Í.S.; Oliveira, I.C.; Campos, A.K.; Araújo, J.V. Association and predatory capacity of fungi Pochonia chlamydosporia and Arthrobotrys cladodes in the biological control of parasitic helminths of bovines. Parasitology 2019, 146, 1347–1351. [Google Scholar] [CrossRef]
- Oliveira, I.C.; Vieira, Í.S.; Campos, A.K.; Araújo, J.V. In vitro compatibility and nematicidal activity of Monacrosporium sinense and Pochonia chlamydosporia for biological control of bovine parasitic nematodes. Parasitology 2021, 148, 956–961. [Google Scholar] [CrossRef]
- Hernández, J.Á.; Cazapal-Monteiro, C.F.; Sanchís, J.; Sánchez-Andrade, R.; Paz-Silva, A.; Arias, M.S. Potential Usefulness of Filamentous Fungi to Prevent Zoonotic Soil-Transmitted Helminths. Vector-Borne Zoonotic Dis. 2018, 18, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Paraud, C.; Hoste, H.; Lefrileux, Y.; Pommaret, A.; Paolini, V.; Pors, I.; Chartier, C. Administration of Duddingtonia flagrans chlamydospores to goats to control gastro-intestinal nematodes: Dose trials. Vet. Res. 2005, 36, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.E.; Uriostegui, M.A.; Millán-Orozco, J.; Gives, P.M.; Hernández, E.L.; Braga, F.R.; Araújo, J.V. Predatory activity of Butlerius nematodes and nematophagous fungi against Haemonchus contortus infective larvae. Rev. Bras. Parasitol. Vet. 2017, 26, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Marcelino, L.; Mendoza-de-Gives, P.; Torres-Hernández, G.; López-Arellano, M.E.; Becerril-Pérez, C.M.; Orihuela-Trujillo, A.; Torres-Acosta, J.F.J.; Olmedo-Juárez, A. Consumption of nutritional pellets with Duddingtonia flagrans fungal chlamydospores reduces infective nematode larvae of Haemonchus contortus in faeces of Saint Croix lambs. J. Helminthol. 2017, 91, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Healey, K.; Lawlor, C.; Knox, M.R.; Chambers, M.; Lamb, J.; Groves, P. Field evaluation of Duddingtonia flagrans IAH 1297 for the reduction of worm burden in grazing animals: Pasture larval studies in horses, cattle and goats. Vet. Parasitol. 2018, 258, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Fausto, G.C.; Fausto, M.C.; Vieira, Í.S.; Freitas, S.G.; Carvalho, L.M.; Oliveira, I.C.; Silva, E.N.; Campos, A.K.; Araújo, J.V. Formulation of the nematophagous fungus Duddingtonia flagrans in the control of equine gastrointestinal parasitic nematodes. Vet. Parasitol. 2021, 295, 109458. [Google Scholar] [CrossRef]
- Sanjari, G.; Bofu, Y.; Hossein, G.; Cyril, C. Effects of time-controlled and continuous grazing on total herbage mass and ground cover. J. Agric. Rural Dev. Trop. 2009, 47, 165–174. [Google Scholar] [CrossRef]
- Acharya, M.; Burke, J.M.; Miller, J.E.; Terrill, T.H.; Wood, E.L.; Muir, J.P. Quebracho tannins aid in the control of Eimeria spp. and gastrointestinal nematodes in lambs and goat kids. Vet. Parasitol. 2020, 288, 109295. [Google Scholar] [CrossRef]
- Hoste, H.; Meza-OCampos, G.; Marchand, S.; Sotiraki, S.; Sarasti, K.; Blomstrand, B.M.; Williams, A.R.; Thamsborg, S.M.; Athanasiadou, S.; Enemark, H.L.; et al. Use of agro-industrial by-products containing tannins for the integrated control of gastrointestinal nematodes in ruminants. Parasite 2022, 29, 10. [Google Scholar] [CrossRef]
- Paller, V.G.V.; Babia-Abion, S. Soil-Transmitted Helminth (STH) Eggs Contaminating Soils in Selected Organic and Conventional Farms in the Philippines. Parasit. Epidemiol. Control 2019, 7, e00119. [Google Scholar] [CrossRef] [PubMed]


| Week | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| VU | 1 | 0.9 | 0.8 | 0.8 | 0.5 |
| VCD | - | 1.4 | 1.1 | 0.9 | 1.2 |
| VI | - | - | - | 15 | 3 |
| Viable Eggs | 1 | 1.1 | 1.3 | 1.5 | 1.8 |
| Week | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| VR (%) | 12 | 22 | 33 | 44 |
| Week | 0 | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|---|
| SCI (%) | CF | 0 | 0 | 7 | 14 | 36 |
| FF | 0 | 0 | 0 | 1 | 7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmo, R.; Viña, C.; Zubiria, I.; Malagón, J.Á.H.; Sanchís, J.M.; Cazapal, C.; Arias, M.S.; Sánchez-Andrade, R.; Paz-Silva, A. Formulating Parasiticidal Fungi in Dried Edible Gelatins to Reduce the Risk of Infection by Trichuris sp. among Continuous Grazing Bison. Pathogens 2024, 13, 82. https://doi.org/10.3390/pathogens13010082
Salmo R, Viña C, Zubiria I, Malagón JÁH, Sanchís JM, Cazapal C, Arias MS, Sánchez-Andrade R, Paz-Silva A. Formulating Parasiticidal Fungi in Dried Edible Gelatins to Reduce the Risk of Infection by Trichuris sp. among Continuous Grazing Bison. Pathogens. 2024; 13(1):82. https://doi.org/10.3390/pathogens13010082
Chicago/Turabian StyleSalmo, Rami, Cándido Viña, Izaro Zubiria, José Ángel Hernández Malagón, Jaime M. Sanchís, Cristiana Cazapal, María Sol Arias, Rita Sánchez-Andrade, and Adolfo Paz-Silva. 2024. "Formulating Parasiticidal Fungi in Dried Edible Gelatins to Reduce the Risk of Infection by Trichuris sp. among Continuous Grazing Bison" Pathogens 13, no. 1: 82. https://doi.org/10.3390/pathogens13010082
APA StyleSalmo, R., Viña, C., Zubiria, I., Malagón, J. Á. H., Sanchís, J. M., Cazapal, C., Arias, M. S., Sánchez-Andrade, R., & Paz-Silva, A. (2024). Formulating Parasiticidal Fungi in Dried Edible Gelatins to Reduce the Risk of Infection by Trichuris sp. among Continuous Grazing Bison. Pathogens, 13(1), 82. https://doi.org/10.3390/pathogens13010082








