Abstract
Epidemiological studies have spotlighted the intricate relationship between individual oral bacteria and tumor occurrence. Porphyromonas gingivalis and Fusobacteria nucleatum, which are known periodontal pathogens, have emerged as extensively studied participants with potential pathogenic abilities in carcinogenesis. However, the complex dynamics arising from interactions between these two pathogens were less addressed. This narrative review aims to summarize the current knowledge on the prevalence and mechanism implications of P. gingivalis and F. nucleatum in the carcinogenesis of oral squamous cell carcinoma (OSCC), colorectal cancer (CRC), and pancreatic ductal adenocarcinoma (PDAC). In particular, it explores the clinical and experimental evidence on the interplay between P. gingivalis and F. nucleatum in affecting oral and gastrointestinal carcinogenesis. P. gingivalis and F. nucleatum, which are recognized as keystone or bridging bacteria, were identified in multiple clinical studies simultaneously. The prevalence of both bacteria species correlated with cancer development progression, emphasizing the potential impact of the collaboration. Regrettably, there was insufficient experimental evidence to demonstrate the synergistic function. We further propose a hypothesis to elucidate the underlying mechanisms, offering a promising avenue for future research in this dynamic and evolving field.
1. Introduction
The human microbiome consists of a diverse range of bacteria that play a vital role in maintaining the equilibrium between health and diseases [1,2]. Among them, the oral microbiome stands as the second most diverse and intricate ecosystem [3] residing in the oral cavity, which is the primary site of entrance into both the digestive and respiratory systems. The enlarged Human Oral Microbiome Database reports the presence of over 700 bacterial species involved in dynamic and intricate microbial interactions [4]. The oral microbiome has garnered increasing attention in recent years due to its potential implication for various health/disease conditions, not only inside the oral cavity but also at distant body sites [5,6].
The significant advancements in next-generation sequencing technology and bioinformatic tools have facilitated the exploration of the harmonious equilibrium among the microbiome, the host, and the environment. It has been revealed that the microbial community as a whole, rather than a few single microbes, maintains this equilibrium. In the healthy state, the microbiome and host establish a symbiotic relationship; whereas in the disease state, a dysbiosis environment promotes the prevalence of pathogenic species in a microbiome, ultimately contributing to the development of illnesses [7]. It was shown that the dysbiotic oral microbiome not only leads to oral infectious diseases, such as caries and periodontitis, but also plays an important role in the development of multiple systemic diseases, including cardiovascular disease, rheumatoid arthritis, Alzheimer’s disease, pulmonary disease, and cancer [8,9,10,11,12].
Cancer, which is characterized by uncontrolled cell growth and potential metastasis, remains a leading cause of mortality globally [13]. Traditionally, the etiological factors of cancer have been attributed to intrinsic factors, like genetic, environmental, and lifestyle components [14]. Recent discoveries in cancer research revealed that tumor growth might be affected by the dynamic interactions between all constituents inside the tumor microenvironment (TME) as well [15,16]. Within the TME, there are not only intricate signaling contacts between cellular and non-cellular factors but also reciprocal interactions involving microbial components [16,17]. It was believed that the microbiome could be operated as a powerful regulator inside the TME, thereby influencing the host (immune) responses [7,18].
Oral squamous cell carcinoma (OSCC) and gastrointestinal cancers, including colorectal cancer (CRC) and pancreatic ductal adenocarcinoma (PDAC), are among the malignancies that have been linked to the oral microbiome in an intriguing way [19]. It was hypothesized that oral microbes reach distant body sites via the circulatory system after processes such as mastication and routine oral hygiene practices, like teeth brushing and flossing [20]. The concept of the “oral-gut axis” was proposed to illustrate this connection [21]. For example, Fusobacterium nucleatum, which is a well-known Gram-negative pathogen for periodontal infections, has been implicated in the development of systemic disorders, including premature birth, inflammatory bowel disease, and CRC. Another periodontal pathogen, namely, Porphyromonas gingivalis, was found to be associated with all three types of cancers: OSCC, CRC, and PDAC [22,23]. Until now, ample studies have explored the role of oral microbes in the onset and development of cancers [6,19,24]. However, it is noteworthy that most studies focused on the role of a single bacterial species. It has been acknowledged that tumor tissues do not harbor a single bacterial species, but a multi-species microbial community that accommodates active bacterial interactions [25,26]. These bacterial interactions could change the formation of tumors. For example, Pustelny et al. demonstrated in a murine tumor model where mice were coinfected with the cystic fibrosis pathogen Pseudomonas aeruginosa and a strictly anaerobic bacterium Veillonella parvula had worse survival rates due to higher P. aeruginosa loads in tumor tissues compared with those coinfected with either bacterial species alone [27]. Lertpirlyapong et al. showed that gastric colonization with multi-species microbiota and the carcinogenic pathogen Helicobacter pylori in male mice leads to more invasive gastrointestinal intraepithelial neoplasia than colonization with H. pylori alone [28]. Although both studies were conducted in mice, the experimental evidence hints at the potential importance of microbial interaction in cancer development. Thus, the existing knowledge on the contribution of a single bacterial species to cancer progression is insufficient without considering the complex dynamics arising from interactions within the microbial community.
The oral pathogen F. nucleatum is known as a bridging bacterium that is able to coaggregate with various bacterial species, such as P. gingivalis, Treponema denticola, and Prevotella intermedia [29,30]. Similarly, the fimbriae of P. gingivalis can mediate the coaggregation with Streptococcus gordonii, Veillonella sp., and Aggregatibacter actinomycetemcomitans [31,32]. It was shown that F. nucleatum can enhance the invasion of human gingival epithelial cells by P. gingivalis, which might increase the transmission of P. gingivalis to other body sites in periodontitis patients [33]. In line with the potential role of microbial interaction in cancer development, we raise the following questions: Does the interaction between F. nucleatum and P. gingivalis have a synergistic influence on the development of cancer? Do these two bacterial species compete for resources and space inside a tumor site?
The aim of this review is to summarize the current knowledge on the association of oral pathogens P. gingivalis and F. nucleatum with local tumor OSCC and distant tumor CRC and PDAC and the underlying mechanisms. In particular, we aimed to explore clinical and experimental evidence on the interactions between these two pathogens that influence oral and gastrointestinal carcinogenesis. To this end, a literature search was conducted to classify previous studies that discovered the relationship between oral bacteria and cancer, notably, OSCC, CRC and PDAC. A comprehensive search strategy was developed, including the following terms: “oral microbiome”, “oral bacteria”, “Porphyromonas gingivalis”, “Fusobacteria nucleatum”, “cancer”, “oral squamous cell carcinoma”, “colorectal cancer”, “pancreatic ductal adenocarcinoma”, and “interaction”. The search was conducted in several databases, such as PubMed, ScienceDirect, and Google Scholar. Various levels of evidence were collected, including studies with in vitro and animal experiments, as well as clinical observational studies.
In the following sections, three distinct types of cancers, namely, OSCC, CRC, and PDAC, are initially introduced. This is followed by a summary of the association of oral pathogens P. gingivalis and F. nucleatum with these cancer types and the underlying mechanisms. Finally, clinical evidence and experimental evidence are provided to explore the interplay between P. gingivalis and F. nucleatum in these three cancer types and hypotheses regarding the underlying mechanisms are proposed.
2. OSCC, CRC, and PDAC
In this narrative review, we focused on the association between P. gingivalis and F. nucleatum with OSCC, CRC, and PDAC because these two bacterial species were frequently identified in these tumor sites. OSCC represents a local tumor environment where P. gingivalis and F. nucleatum normally reside, whereas CRC and PDAC represent the distant tumor sites where oral microbes might reach via circulation.
CRC is a malignancy that affects the colon and rectum, both of which are integral components of the gastrointestinal tract. CRC has significant heterogeneity, manifesting in diverse clinical outcomes, therapeutic responses, and morphological characteristics. Reprogrammed metabolism is a hallmark of CRC, and CRC cells are geared toward rapid proliferation, requiring nutrients and the removal of cellular waste in nutrient-poor environments [34]. CRC is a prevalent disease that ranks among the most frequently occurring cancers globally, along with an elevated mortality rate. Based on the GLOBOCAN—Global Cancer Statistics 2020 study, it was the third most often diagnosed cancer worldwide, accounting for 10% of cases. Additionally, it was shown to be the second leading cause of cancer mortality, responsible for 9.4% of deaths. It was projected that there will be an increase in the number of new CRC cases by 2040, reaching roughly 3.2 million cases. This anticipated rise in cases is expected to significantly affect the global healthcare system [35,36].
PDAC is the most common malignancy of the pancreas. The most often seen symptoms in individuals with PDAC include weight loss, abdominal discomfort, and jaundice [37,38,39]. It is an aggressive and harmful ailment, with only 26% of patients living one year after being diagnosed and the disease continues to exhibit a discouraging average 5-year survival rate of 12% [40,41]. Based on the GLOBOCAN—Global Cancer Statistics 2020 study, the global incidence of PDAC in the year 2020 reached a total of 495,773 newly diagnosed cases, while the number of fatalities attributed to this disease amounted to 466,003 [42]. As of 2023, PDAC is projected to become the second leading cause of cancer-related mortality worldwide, surpassing CRC and breast cancer [43]. The management of PDAC remains notably challenging, necessitating a concerted effort to advance our understanding of biomarkers and explore interdisciplinary strategies.
3. The Prevalence of P. gingivalis in OSCC, CRC, and PDAC
P. gingivalis is a well-known periodontal pathogen. It is a Gram-negative anaerobic bacterium associated with the onset and progression of periodontitis [44]. Previous studies showed that it can colonize malignant tissues in an oral cavity, such as OSCC, ESCC, and gingival carcinoma [45]. Sayehmiri et al. conducted a meta-analysis that revealed the presence of P. gingivalis is associated with a risk increase of more than 1.36-fold in the development of OSCC [46]. An excessive amount of P. gingivalis was identified as a potential risk factor for OSCC [47,48,49].
In addition to oral cancers, P. gingivalis was frequently related to cancers at other body sites, including esophageal cancer, lung cancer, CRC, and PDAC [50,51,52,53]. Ample clinical studies found high abundances of P. gingivalis in both tumor tissue and fecal samples of CRC patients, which were correlated with the onset of CRC and poor prognosis in patients. For example, in a cross-sectional study, Kerdreux et al. examined 247 CRC patients and 89 controls (stages I–IV). They found a significant increase in P. gingivalis levels in fecal samples of CRC patients compared with the healthy controls. P. gingivalis could be identified in the fecal samples of 2.6–5.3% of CRC patients [22]. In another cohort study, P. gingivalis was detectable in 10 out of 31 CRC tissue samples using quantitative polymerase chain reaction (qPCR). A higher prevalence of P. gingivalis was found in individuals with the latter phases of colonic carcinogenesis [35].
Similarly, a high prevalence of P. gingivalis in PDAC patients has been reported. A recent prospective cohort study of 361 individuals diagnosed with PDAC found that the presence of P. gingivalis was associated with a 59% rise in PDAC development. Results also show an imbalance in oral microbial composition occurred before the onset of the cancer [54]. Another prospective cohort study examined 405 individuals diagnosed with pancreatic cancer, together with 410 control subjects, and found that the individuals with high levels of antibodies against P. gingivalis had a greater than twofold increased risk of developing PDAC [55].
Overall, there is clear clinical evidence that a high level of P. gingivalis, either in an oral cavity, fecal samples, or tumor tissues, is associated with the development of all three types of cancers.
4. Mechanisms of P. gingivalis in Cancer Development and Chemoresistance
As a known periodontal pathogen, P. gingivalis employs many strategies to compromise tissue integrity and impair the host immune response. These strategies include the prevention of cell apoptosis, stimulation of cell proliferation, initiation of chronic inflammation, and generation of oncometabolites [56].
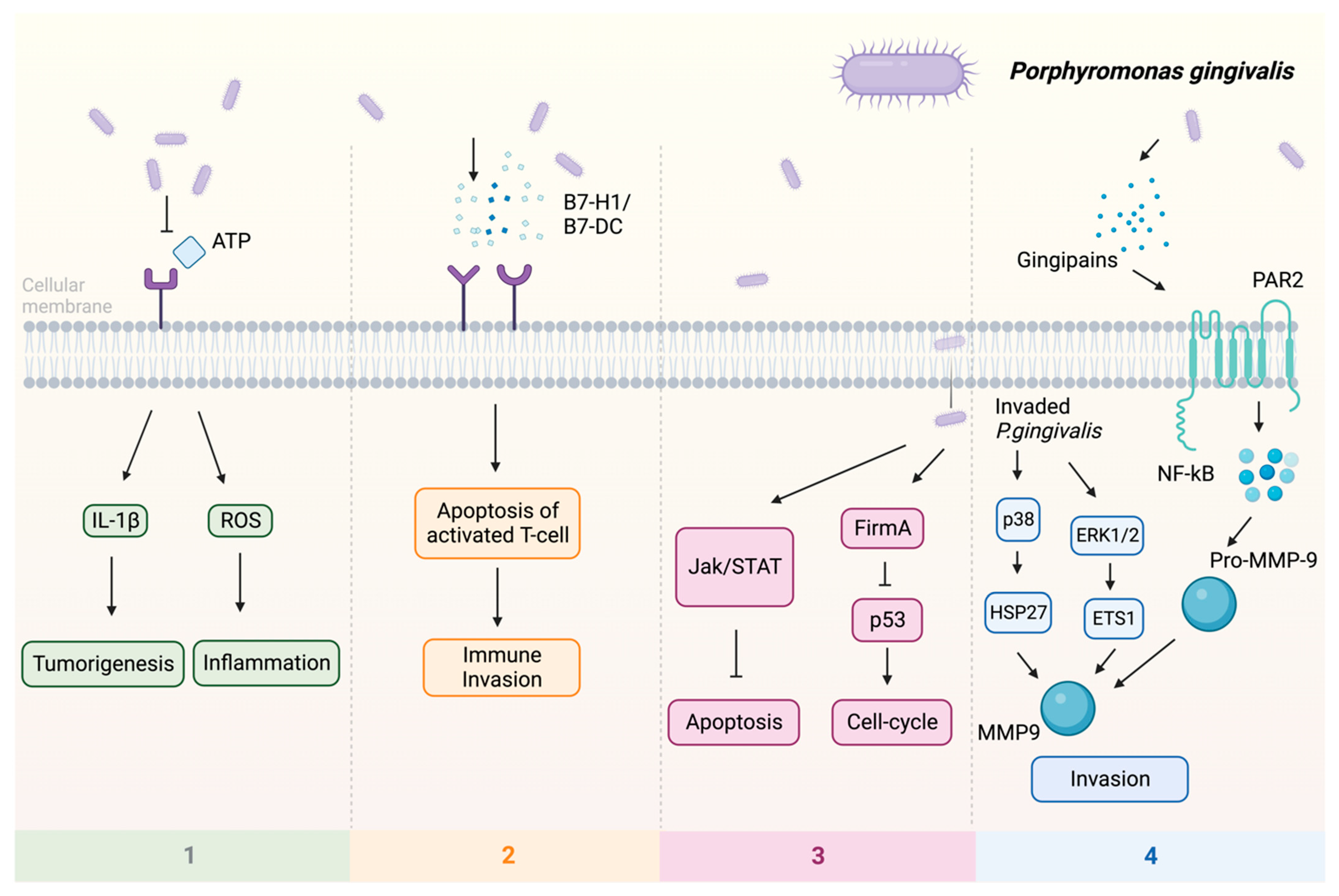
Previous studies indicate that P. gingivalis can promote tumorigenesis by influencing various signaling pathways (Figure 1): (1) Upon infection of the host by P. gingivalis, the B7-H1 receptor can be activated, facilitating the apoptosis of activated T cells. The increased expression of B7-H1 receptors in host cells may impact the persistence of inflammatory illnesses [57]. (2) Nucleoside Diphosphate Kinase (NDK), which is the effector protein produced by intracellular P. gingivalis, can block the signaling of extracellular adenosine triphosphate (ATP)/purinergic receptor (P2X7) on macrophages by consuming ATP. This prevents inflammasome activation and the secretion of interleukin-1β (IL-1β), consequently facilitating the process of tumorigenesis [58]. The NDK enzyme is also known to phosphorylate heat shock protein 27 (HSP27), and it is capable of triggering antiapoptotic processes upon phosphorylation [59]. (3) P. gingivalis activates antiapoptotic pathways, such as Janus kinase 1 (JAK1)/signal transducer and activator of transcription 3 (STAT3) and phosphoinositide 3-kinase PI3K/protein kinase B (Akt) in oral epithelial cells, thus promoting OSCC [60]. Besides inhibiting the intrinsic apoptosis of the invaded epithelial cells, P. gingivalis can also enhance the progression of the S phase of the cell cycle by inhibiting the p53 tumor suppressor gene (TSG) through the FimA adhesin [61]. Expression of the aforementioned B7-H1 receptor can inhibit the effector T cells by inducing regulatory T cells, which is beneficial for the invaded cell survival [57,62]. Due to the induction of regulatory T cells by the B7-H1 receptor, the immune system is (partly) evaded. (4) The activation of extracellular signal-regulated kinase 1/2 (ERK1/2)-protein ETS1, p38/HSP27, and PAR2 (protease-activated receptor 2)/nuclear factor kappa B (NF-κB) pathways was observed in response to P gingivalis infection, leading to the induction of pro-matrix metalloproteinase-9 (pro-MMP-9) expression, hence the increasing levels of MMP-9 and enhanced cellular invasion [63,64,65].
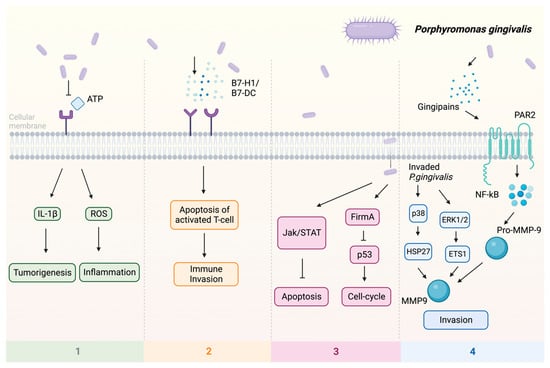
Figure 1.
Multiple pathways employed by P. gingivalis in tumor induction: (1) P2X7 activation via ATP is blocked, leading to the stimulation of IL-1β, which promotes tumorigenesis, and the induction of ROS, which fosters a pro-inflammatory microenvironment. (2) Facilitation of immune evasion occurs through the activation of B7-H1 and B7-DC receptors, contributing to a (partial) circumvention of the immune system. Immune evasion is facilitated through the activation of B7-H1 and B7-DC receptors, contributing to a (partial) evasion of the immune system. (3) Activation of FimA results in the downregulation of p53, enhancing the host cell’s cell cycle while simultaneously suppressing apoptosis. The JAK/STAT axis is also implicated in the downregulation of apoptosis. (4) Additionally, P. gingivalis stimulates invasion through PAR2 activation via gingipains, activating NF-κB signaling, which leads to the formation of MMP-9, thereby enhancing P. gingivalis invasion. Upon invasion, pro-MMP-9 undergoes upregulation facilitated by ERK1/2 and ETS1, along with activation of p38 and HSP27. Abbreviations: ATP—adenosine triphosphate; ERK1/2—extracellular signal-regulated kinase 1/2; ETS1—protein; FimA—protein; HSP27—heat shock protein 27; IL-1β—interleukin-1β; JAK—Janus kinase 1; MMP-9—matrix metalloproteinase-9; NF-κB—nuclear factor kappa B; P2X7—purinergic receptor; pro-MMP-9—pro-matrix metalloproteinase-9; p38, p53—protein; ROS—reaction oxygen species; STAT—signal transducer and activator of transcription.
5. The Prevalence of F. nucleatum in OSCC, CRC, and PDAC
As mentioned above, F. nucleatum is another Gram-negative anaerobic bacterium often found in the resident oral microbiome and periodontal disease sites. It is known for its adhesive properties, which facilitate its attachment to other bacterial species and host cells [66]. Beyond its oral habitat, F. nucleatum was found in various cancer types, including CRC and PDAC [67,68,69]. Furthermore, its presence has been linked to worse survival rates in patients diagnosed with CRC and PDAC [70,71].
Extensive clinical investigations have reported a high prevalence of F. nucleatum in OSCC. A clinical cross-sectional study examined 80 paired OSCC tumors and adjacent normal tissues; F. nucleatum was detected in a striking 75.7% of OSCC tissue samples, contrasting with approximately 33.6% prevalence in normal samples, which is a reduction by 2.25-fold [68]. This significant difference highlights the potential diagnostic value of F. nucleatum in OSCC. The abundance of F. nucleatum in oral rinse samples has also been associated with the progress of OSCC. Yang et al. scrutinized the microbiota composition of oral rinses collected from a cohort of 51 healthy individuals and 197 patients diagnosed with OSCC at varying stages [72]. They found a notable increase in F. nucleatum abundance as oral cancer progressed. Its abundance increased from 2.98% in healthy controls to 4.35% in OSCC stage 1 and 7.92% in stage 4. These compelling findings underscore the potential relevance of F. nucleatum in both the onset and progression of OSCC.
Notably, F. nucleatum is a key member of CRC-associated bacteria. Multiple narrative review and systematic review articles summarized clinical evidence on the enrichment of F. nucleatum in CRC patients [70,73]. Generally, the abundance of F. nucleatum in CRC tumor tissues was found to be higher than in neighboring normal tissues [74]. Its abundance was also positively associated with CRC progression [75,76]. However, differential prevalence rates of F. nucleatum in CRC tissues have been reported. In particular, there was a notable disparity in the prevalence of F. nucleatum inside tumor tissues compared with normal tissues across different geographical cohorts, including the United States, Japan, and Europe. The occurrence of F. nucleatum in CRC tissues could vary from 13% to 75% [76]. Nevertheless, F. nucleatum has been considered a diagnostic and prognostic determinant in CRC patients [75].
The correlation between F. nucleatum prevalence and PDAC was not conclusive. Although Mitsuhashi et al. reported a detection rate of 8.8% for Fusobacterium species in 302 PDAC tissue specimens, with 283 positive and 25 negative detections of Fusobacterium species, Yamamura et al. did not detect any F. nucleatum in pancreatic cancer tissue [77,78]. Given these disparate findings, further in-depth analysis is warranted to elucidate the relationships between F. nucleatum and PDAC.
Similar to P. gingivalis, the high level of F. nucleatum is associated with the onset and progression of OSCC and CRC. But more clinical evidence is needed to clarify the association between F. nucleatum prevalence and PDAC.
6. Mechanisms of F. nucleatum in Cancer Development
Two major virulence factors of F. nucleatum are believed to be the most important factors in cancer progression [79,80]. The first factor is Fusobacterium adhesin A (FadA), which is also an important kinase in OSCC, induces oncogenic gene expression and promotes the growth of CRC cells; the other factor, namely, Fap2, which is derived from F. nucleatum, potentiates the progression of CRC by its inhibiting potential for immune cell activity through interacting with T-cell immunoreceptors with Ig and ITIM domains (TIGIT) [81,82]. Fap2 is unique to CRC but inactive in OSCC [83].
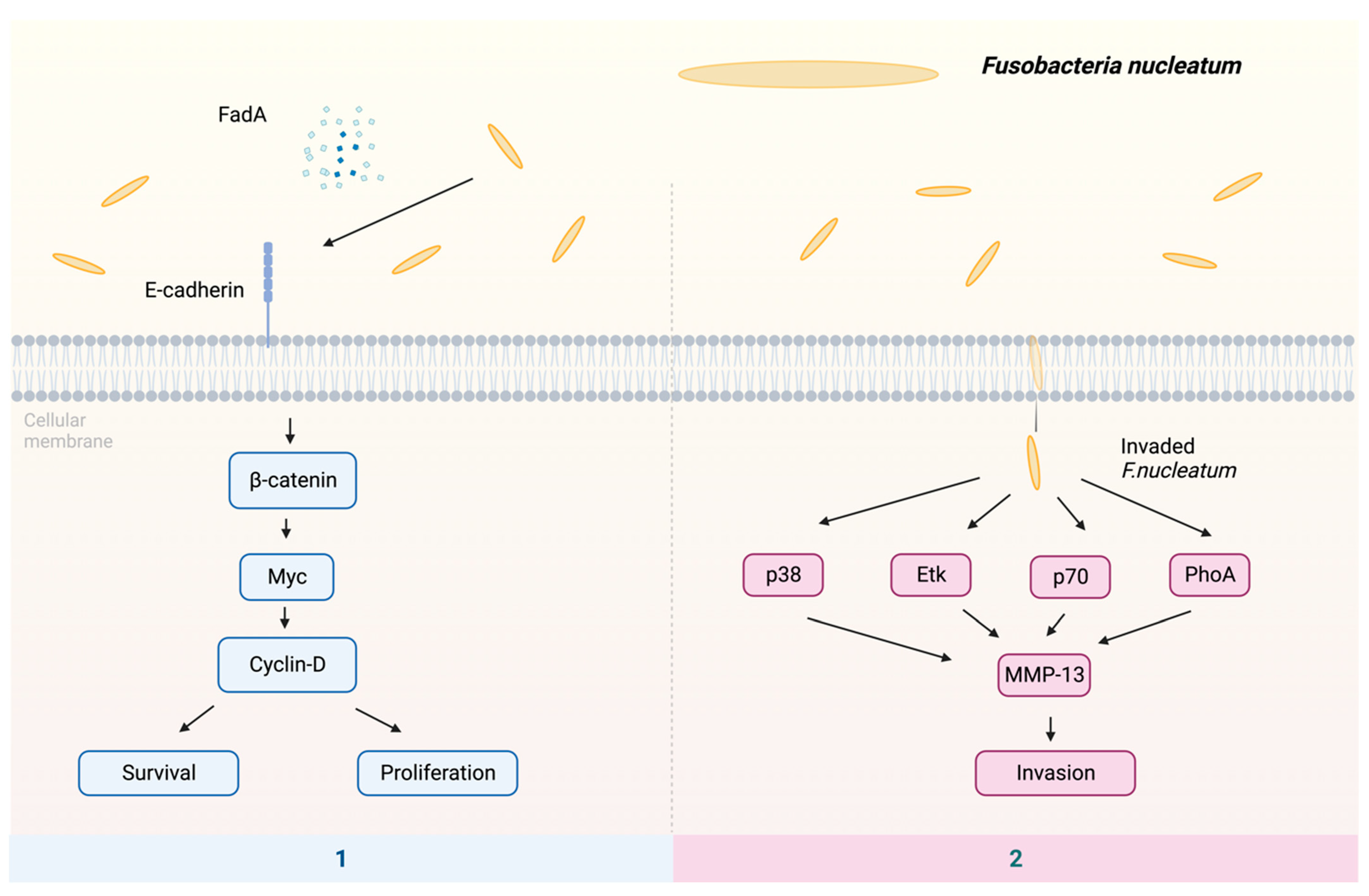
The putative processes by which F. nucleatum may contribute to the development of malignancies include the following pathways (Figure 2): (1) The proliferation of oral epithelial cells is induced by F. nucleatum FadA [84,85]. Factor FadA binds to E-cadherin, which activates β-catenin [86]. The translocation of activated β-catenin from the cytoplasm to the cell nucleus leads to the production of oncogenes, such as Myc and Cyclin D, hence inducing cellular proliferation and upregulating the expression of oncogenic and inflammatory genes [83,87,88]. (2) The F. nucleatum infection can lead to an elevation in the synthesis of MMP-13, which is also known as collagenase 3, facilitating the movement of cells by activating Etk/BMX, S6 kinase p70, and RhoA kinase. In addition, F. nucleatum is able to activate mitogen-activated protein kinase p38, subsequently triggering the activation of heat shock protein-27 (HSP-27), leading to the production of MMP-9 and MMP-13 [89,90]. Remarkably, these enzymes are imperative in promoting tumor invasion and metastasis.
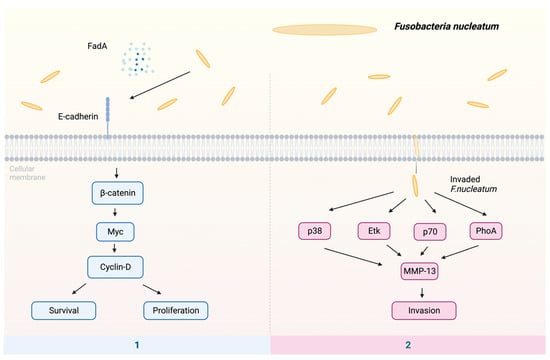
Figure 2.
The potential mechanisms of F. nucleatum in cancer: (1) The virulence factor FadA binds to E-cadherin, subsequently activating β-catenin. This activation, in turn, triggers the transcription factor Myc, leading to the activation of cyclin-D. The activation of cyclin-D stimulates host cell survival and proliferation. (2) F. nucleatum enhances invasion by activating p38, Etk, p70, and RhoA, resulting in the upregulation of MMP-13. Abbreviations: Etk—tyrosine kinase; FadA—Fusobacterium adhesin A; MMP-13—matrix metalloproteinase-13; p38—protein kinase; p70—S6 kinase; RhoA—kinase.
7. Coexistence of P. gingivalis and F. nucleatum in OSCC, CRC, and PDAC
As abovementioned, the association between P. gingivalis/F. nucleatum and cancer, including OSCC, CRC, and PDAC, and the underlying mechanisms have been extensively reviewed [19,81,84,86,91,92]. Many of these reviews summarized the role of F. nucleatum in the development of OSCC and CRC [81,84,86,91], whereas Saikia et al. and Irfan et al. discussed the involvement of both P. gingivalis and F. nucleatum in carcinogenesis [19,92]. We noticed that most reviews discussed the role of individual bacterial species in carcinogenesis. Although the interaction between P. gingivalis and F. nucleatum was mentioned in several reviews [19,84,86], the exact role and potential influence of this interaction on carcinogenesis were not extensively discussed. This current review attempted to summarize the clinical evidence on the co-existence of P. gingivalis and F. nucleatum in three cancer types. Furthermore, we highlight the intricate web of microorganisms that may influence the landscape of cancer. Table 1 summarizes the clinical studies that have reported the co-existence of P. gingivalis and F. nucleatum in patients with OSCC, CRC, and PDAC.
In oral infection diseases, such as periodontitis, the two pathogens P. gingivalis and F. nucleatum were often detected simultaneously [93,94]. Similarly, in OSCC patients, these two bacterial species were often detected simultaneously. In a cross-sectional study, Zhang et al. found that OSCC tissues were enriched with the Fusobacterium and Porphyromonas genera [95]. Torralba et al. reported significantly elevated levels of both P. gingivalis and F. nucleatum in OSCC tissue samples compared with those obtained from healthy subjects [96]. Furthermore, microbiota composition analysis demonstrated a marked increase in P. gingivalis and F. nucleatum abundance in tumor sites when compared with control groups. Chang et al. examined 61 cancer tissues, pericancerous tissues, subgingival plaque samples, and 30 normal tissues using qPCR. They revealed that both P. gingivalis and F. nucleatum existed at higher levels in cancer tissues than in normal tissues [45]. In addition, the relative number of these two bacterial species in cancer tissues positively correlated with that in subgingival plaque, indicating a link between periodontitis and OSCC. Compared with F. nucleatum, it seems that P. gingivalis infection was more positively associated with late clinical staging, low differentiation, and lymph node metastasis in OSCC patients. This phenomenon was observed in a cohort study by Park et al., where a higher serum level of P. gingivalis IgG was associated with a worse prognosis, even though the IgGs of both P. gingivalis and F. nucleatum were detected in OSCC patients [97].
The coexistence of P. gingivalis and F. nucleatum has also been reported for CRC patients. Several clinical studies found significant enrichment of both P. gingivalis and F. nucleatum in the tumor tissue, saliva, or fecal samples compared with healthy controls [49,98,99]. In an observational study by Purcell et al., the abundance of P. gingivalis and F. nucleatum were correlated with the consensus molecular subtypes (CMS) of CRC [49]. It was found that both bacterial species were strongly associated with CMS subtype 1 (CMS1), which is characterized by its inflammatory signatures, high mutation rate, and hypermethylation of CpG [100]. CMS1 tumors had a favorable prognosis when detected before metastasis but a poor prognosis after relapse. The enrichment of P. gingivalis and F. nucleatum in CMS1 might imply a potential microbial synergy within the tumor microenvironment. The results presented by Guven et al. confirmed the presence of P. gingivalis and F. nucleatum in saliva samples from CRC patients [98]. Gao et al. also found that the relative abundance of P. gingivalis and F. nucleatum was associated with CRC. Further microbial network analysis revealed that both genera Porphyromonas and Fusobacterium played central roles in inter-microbial interactions by being associated with other bacterial species in the microbial network [49]. P. gingivalis has been considered a keystone pathogen because it was able to alter oral microbiome composition and function, despite a low abundance [101,102]. It is likely that P. gingivalis and F. nucleatum act as key modulators for CRC tumorigenesis.
In PDAC patients, the evidence of the coexistence of P. gingivalis and F. nucleatum was inconclusive. In a case–control study, Fan et al. examined the microbial compositions of oral rinse samples collected from 361 PDAC patients and 371 healthy controls and found that the prevalence of P. gingivalis was associated with a higher risk of pancreatic cancer, but that of phylum Fusobacteria was associated with a decreased pancreatic cancer risk [54]. This observation was in line with the finding of a prospective cohort study where blood samples were taken from 405 pancreatic cancer patients and 416 matched controls. P. gingvialis was found to be twofold higher in the samples of cancer patients than those in the control. But there was no difference in the level of F. nucleatum between the cancer and control samples [55]. Conversely, Kartal et al. found elevated levels of F. nucleatum in the fecal samples of PDAC patients when compared with those of the controls [103]. However, P. gingivalis was not detected.
Generally, the clinical evidence showed that P. gingivalis and F. nucleatum co-existed in both OSCC and CRC. Higher levels of both bacterial species were associated with a worse prognosis. When going through the clinical evidence, we found that other bacterial genera or species than P. gingivalis and F. nucleatum were identified in tumor tissues or patients with tumors. This information is summarized in Section 8 below in order to give a glimpse of the complexity of the cancer-associated microbiome.

Table 1.
Coexistence of P. gingivalis and F. nucleatum in OSCC, CRC, and PDAC samples.
Table 1.
Coexistence of P. gingivalis and F. nucleatum in OSCC, CRC, and PDAC samples.
| Type of Cancer | Sample Types | Bacterial Detection Method | Pg, Fn a | Major Bacterial Genera b | References |
|---|---|---|---|---|---|
| OSCC | Tumor tissue | 16S rRNA | Pg ↑, Fn ↑ | Streptococcus, Prevotella, Fusobacterium | [95] |
| OSCC | Tumor tissue | 16S rRNA | Pg ↑, Fn ↑ | Fusobacterium, Prevotella, Actinomyces | [96] |
| OSCC | Tumor tissue | 16S rRNA | Pg ↑, Fn ↑ | Capnocytophaga, Fusobacterium, Haemophilus | [45] |
| OSCC | Serum | ELISA | Pg ↑, Fn ↑ | Porphyromonas, Fusobacterium | [97] |
| CRC | Tumor tissue | 16s rRNA | Pg ↑, Fn ↑ | Porphyromonas, Parvimonas, Peptostreptococcus | [49] |
| CRC | Saliva | Real-time PCR | Pg -, Fn - | Fusobacterium, Porphyromonas, Streptococcus | [98] |
| CRC | Stool | Real-time PCR | Pg ↑, Fn ↑ | Porphyromonas, Fusobacterium, Bacteroides | [99] |
| PDAC | Oral wash | 16S rRNA | Pg ↑, Fn ↓ | Porphyromonas, Aggregatibacter | [54] |
| PDAC | Fecal | 16S rRNA | Pg: n. m, Fn ↑ | Faecalibacterium, Romboutsia, Bacteroides | [103] |
a: bacteria coexisting in specific type of samples; b: top two to three bacterial genera with increased abundance within tumor tissues or patients; ↑: increased abundance in tumor tissues or patients; ↓: decreased abundance in tumor tissues or patients; -: no significant change; 16S rRNA: 16S ribosomal RNA gene amplicon sequencing; ELISA: enzyme-linked immunosorbent assay; real-time PCR: real-time polymerase chain reaction; Fn: F. nucleatum; Pg: P. gingivalis.
8. Complex Microbiome Related to OSCC, CRC, and PDAC
Table 1 also includes information on the top 2–3 major bacterial genera identified in clinical studies. Although it is not the goal of this review to provide a comprehensive overview of the compositions of the cancer-associated microbiome, the information in Table 1 highlights key aspects of the microbial interactions, emphasizing the presence of a diverse, multi-species community in the three tumor types. The findings from the listed clinical studies showed that multiple bacterial species, other than P. gingivalis and F. nucleatum, can be associated with cancer progression. There is no consensus on the tumor-specific bacterial genera. Up to 14 different bacterial genera are reported, which are enriched in either tumor tissue or cancer patients (Table 1). This evidence underlies the complex nature of the cancer-associated microbiome.
9. Interaction between P. gingivalis and F. nucleatum in Cancer Development
Although multiple clinical studies revealed the co-existence of P. gingivalis and F. nucleatum in cancer patients, the functions of this co-existence have not been well studied. We searched for various types of evidence in order to answer the following key questions: Do these two pathogens impose a synergistic effect on cancer development? Are there growth or niche competitions between these two bacterial species within a tumor site?
Using in vitro cell culture or murine models, researchers showed that the dual species of P. gingivalis and F. nucleatum can promote OSCC tumorigenesis via Toll-like receptors on the oral epithelial cells, leading to increased pro-inflammatory cytokine production, the epithelial–mesenchymal transition (EMT), and cell apoptosis inhibition [104,105,106,107,108]. Unfortunately, most studies did not include a P. gingivalis- or F. nucleatum-alone group as a control. It was impossible to identify whether there was a synergy between the two pathogens. So far, only two studies have compared the function of dual species with the corresponding single species alone [105,106]. Sztukowska et al. found that P. gingivalis alone induced the expression of transcription factor ZEB1 and promoted the migration of epithelial cells TGK1 in vitro [105]. Combining F. nucleatum or Streptococcus gordonii with P. gingivalis did not improve or inhibit the ZEB1 induction of P. gingivalis. Therefore, no synergy or competition between P. gingivalis and F. nucleatum was found in this experimental setting. Lee et al. confirmed that P. gingivalis alone can increase the expression of key EMT-promoting transcription factors, including Zeb1. But they found that the combination of P. gingivalis and F. nucleatum slightly enhances cell migration compared with each bacterial species alone [106]. Overall, there was insufficient experimental evidence to demonstrate any synergy between P. gingivalis and F. nucleatum in contributing to carcinogenesis. However, the coinfection of P. gingivalis and F. nucleatum has been reported in the field of periodontology [109]. Polak et al., using a rat model, revealed that the simultaneous presence of P. gingivalis and F. nucleatum resulted in a synergistic effect, leading to increased bone loss and intensified inflammatory reactions in the periodontal tissues compared with each pathogen alone [109]. Maekawa observed that the survival of intracellular P. gingivalis facilitated the intracellular survival of F. nucleatum in a coinfection subcutaneous chamber model [110]. Inversely, F. nucleatum can enhance the invasion of P. gingivalis into gingival epithelial cells [93]. This evidence from the field of periodontology indicates a possible collaboration between these two pathogens within a microbial community. Given the polymicrobial nature of the cancer-associated microbiome, it is critical to understand the microbe-induced tumorigenesis using dual- or multi-species coinfection models in the future.
10. Potential Mechanisms of P. gingivalis–F. nucleatum Co-Infection in Cancer Development
As mentioned above, there has been a lack of mechanistic studies that investigated the co-infection of P. gingivalis and F. nucleatum in in vitro or animal models. Hence, the potential roles of P. gingivalis or F. nucleatum in carcinogenesis have mainly been summarized based on single-species studies. Here, based on the existing knowledge, we propose our hypothesis on the mechanisms of the coinfections in cancer development.
It was suggested that the divergence in nutrient utilization could lead to the coexistence of P. gingivalis and F. nucleatum. For instance, P. gingivalis primarily degrades dipeptides, while Prevotella and F. nucleatum prefer smaller amino acids. This metabolic synergy is further underscored by P. gingivalis’ proteolytic nature, enabling it to provide amino acids to F. nucleatum, which lacks proteolytic capabilities. This mutualistic interaction fosters the colonization of additional P. gingivalis, thus creating a positive feedback loop, where more amino acids are supplied to other cohabiting bacteria [32]. The roles of single P. gingivalis and F. nucleatum in cancer development summarized in Figure 1 and Figure 2 indicate that these two bacterial species can utilize different pathways to function. P. gingivalis causes cancers via immune evasion, apoptosis inhibition, EMT, and establishing a chronic inflammatory state, while F. nucleatum promotes the occurrence and development of cancer through localization, proliferation, immune suppression, and metastasis [56,89]. Hence, it is possible that the co-infection of P. gingivalis and F. nucleatum can enhance tumorigenesis compared with single-species infection. The diverse mechanisms employed by both P. gingivalis and F. nucleatum pose challenges to the development of targeting therapeutics since multiple targets should be taken into account.
11. Potential Mechanisms of Microbiome in Cancer Development
Increasing evidence has pointed out that cancer development does not depend on the abundance of individual or several bacterial species, but is modulated by the function of an entire microbial community, which consists of hundreds of bacterial species. How a microbiome causes the onset and progression of cancer has not been systematically studied. Several hypotheses were proposed, including the driver–passenger model and cancer–microbiome–immune axis theory [111,112]. Although the effect of the cancer-associated microbiome was not the focus of this review, we highlight two hypotheses in order to increase awareness of the microbiome in mechanistic research.
The “driver-passenger” hypothesis was initiated in 2012 by Tjalsma et al. while aiming to explain the striking differences in CRC-associated microbiome compositions reported by various studies [111]. It proposes that pathogenic bacteria like Bacteriodes spp. can initiate CRC development and function as a driver. The driver-induced changes in the tumor microenvironment and cellular metabolism provide a competitive advantage to the passenger bacteria, which are opportunistic pathogens, such as Fusobacterium or Streptococcus spp. Eventually, the passengers can replace the drivers and subsequently either suppress or promote CRC progression. This hypothesis acknowledges the dynamic changes of microbiomes and suggests that the microbiome “snapshots” obtained from numerous clinical cross-sectional studies may not fully capture the comprehensive dynamics of the microbiome throughout the development of CRC.
According to Tjalsma, the driver–passenger hypothesis is unique to CRC and cannot be generalized as a microbial etiology [111]. Recently, Al-Hebshi et al. adapted the “driver-passenger” hypothesis and proposed a “passenger-tuning-driver” model to illustrate the role of microbiomes in OSCC [113]. Different from the “driver-passenger” model, it was believed that the oral microbiome was not involved in OSCC initiation. The tumor microenvironment selected or enriched the initial “passenger”. As it matures, the intra-tumor microbiome can turn into a functional “driver” by expressing pro-inflammatory components and virulence factors, consequently, enhance the OSCC progression.
The two models above emphasize the dynamic interaction between the microbiome and the host. The cancer–microbiome–immune axis concept proposed by Jain et al. includes the aspect of immunity, which explains the interplay between the microbiome, immunity, and cancer [112]. The microbiome can affect the tumor cells directly by serving as antigens, or indirectly by adjuvant signals, which lead to immunomodulation. The adjuvant signals can be sent in the form of various microbial-secreted products, such as metabolites, toxins, and vesicles, or cytokines secreted through the manipulation of host cells. The understanding of the cancer–microbiome–immune axis brings up new ideas for cancer therapy through microbial modulation. Modulation of the microbiome can be harnessed to potentiate the efficacy of immunotherapies and decrease their toxicity. So far, antibiotics, probiotics, and prebiotics have been developed for microbiome modulation [114,115,116]. But the actual clinical efficacy is yet to be improved.
It is important to acknowledge certain limitations of this narrative review. The clinical studies cited for demonstrating the co-existence of P. gingivalis and F. nucleatum in cancer patients were mostly cohort studies, which are relatively low in the hierarchy of evidence. To confirm the association between the co-existence of these two bacterial species and cancer development, a systematic review based on randomized control trials is necessary. Furthermore, this review focused only on the interaction between P. gingivalis and F. nucleatum. As shown in Table 1, other bacterial genera might be involved in the onset and progression of cancer. Within the complex microbial community, various microbial interactions might modulate the structure and function of the community, and hence, influence the process of carcinogenesis. Future research on the collective impact of bacterial consortia on cancer-related processes is poised to reveal novel insights into the complex relationship between the oral microbiome and tumors developed in both the oral cavity and at distant/internal body sites.
12. Conclusions
Within the aforementioned limitations, this review demonstrates the association of P. gingivalis and F. nucleatum, alone or together, in the initiation and development of OSCC, CRC, and PDAC. Based on the results of clinical studies, the prevalence of both bacteria species correlated with cancer development progression, emphasizing the potential impact of the collaboration. Regrettably, there was insufficient experimental evidence to demonstrate the synergistic function. Since the existing reported underlying mechanisms were based on single-species P. gingivalis or F. nucleatum studies, we propose that the P. gingivalis–F. nucleatum interaction might provide colonization advantages for both bacterial species. The diverse pathways employed by both bacterial species might enhance their pathogenicity and complicate the therapeutic targets. A deeper understanding of the microbial interplay may hold the potential to unlock innovative strategies for cancer management, underscoring the feasibility of targeted modulation of the microbiome to alter cancer trajectories.
Author Contributions
Writing—original draft preparation, B.W., J.D. and D.D.; writing—editing, B.W., J.D., D.D. and E.G.; literature research, B.W., V.D., N.M., A.E.F. and D.D.; supervision, A.E.F., E.G. and D.D. All authors read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
This research was partly supported by the China Scholarship Council and by the KWF-Dutch Cancer Society. The funders had no role in study design, data collection, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Relman, D.A. The human microbiome and the future practice of medicine. JAMA 2015, 314, 1127–1128. [Google Scholar] [CrossRef] [PubMed]
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Escapa, I.F.; Chen, T.; Huang, Y.; Gajare, P.; Dewhirst, F.E.; Lemon, K.P. New Insights into Human Nostril Microbiome from the Expanded Human Oral Microbiome Database (eHOMD): A Resource for the Microbiome of the Human Aerodigestive Tract. mSystems 2018, 3, e00187-18. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.G.; Sarkar, S.; Umar, S.; Lee, S.T.M.; Thomas, S.M. The Contribution of the Human Oral Microbiome to Oral Disease: A Review. Microorganisms 2023, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral. Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Zhang, L. Role of the microbiome in oral cancer occurrence, progression and therapy. Microb. Pathog. 2022, 169, 105638. [Google Scholar] [CrossRef]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Cao, Y.; Xue, J.; Zhou, X. The oral microbiome diversity and its relation to human diseases. Folia Microbiol. 2015, 60, 69–80. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, Y.; Li, S.; Yang, L.; Wang, H.; Wang, T.; Bin, S.; Gai, Z.; Heng, X.; Zhang, C.; et al. Variations in oral microbiome profiles in rheumatoid arthritis and osteoarthritis with potential biomarkers for arthritis screening. Sci. Rep. 2018, 8, 17126. [Google Scholar] [CrossRef]
- Shoemark, D.K.; Allen, S.J. The microbiome and disease: Reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Varadhachary, G.R.; Tamm, E.P.; Abbruzzese, J.L.; Xiong, H.Q.; Crane, C.H.; Wang, H.; Lee, J.E.; Pisters, P.W.; Evans, D.B.; Wolff, R.A. Borderline resectable pancreatic cancer: Definitions, management, and role of preoperative therapy. Ann. Surg. Oncol. 2006, 13, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Sevcikova, A.; Izoldova, N.; Stevurkova, V.; Kasperova, B.; Chovanec, M.; Ciernikova, S.; Mego, M. The Impact of the Microbiome on Resistance to Cancer Treatment with Chemotherapeutic Agents and Immunotherapy. Int. J. Mol. Sci. 2022, 23, 488. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Ciernikova, S.; Sevcikova, A.; Stevurkova, V.; Mego, M. Tumor microbiome—An integral part of the tumor microenvironment. Front. Oncol. 2022, 12, 1063100. [Google Scholar] [CrossRef]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef]
- Choy, A.T.F.; Carnevale, I.; Coppola, S.; Meijer, L.L.; Kazemier, G.; Zaura, E.; Deng, D.; Giovannetti, E. The microbiome of pancreatic cancer: From molecular diagnostics to new therapeutic approaches to overcome chemoresistance caused by metabolic inactivation of gemcitabine. Expert. Rev. Mol. Diagn. 2018, 18, 1005–1009. [Google Scholar] [CrossRef]
- Lam, G.A.; Albarrak, H.; McColl, C.J.; Pizarro, A.; Sanaka, H.; Gomez-Nguyen, A.; Cominelli, F.; Paes Batista da Silva, A. The Oral-Gut Axis: Periodontal Diseases and Gastrointestinal Disorders. Inflamm. Bowel Dis. 2023, 29, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Kerdreux, M.; Edin, S.; Löwenmark, T.; Bronnec, V.; Löfgren-Burström, A.; Zingmark, C.; Ljuslinder, I.; Palmqvist, R.; Ling, A. Porphyromonas gingivalis in Colorectal Cancer and its Association to Patient Prognosis. J. Cancer 2023, 14, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, J.; Binder Gallimidi, A.; Saba, E.; Pandi, K.; Eli Berchoer, L.; Hermano, E.; Angabo, S.; Makkawi, H.a.; Khashan, A.; Daoud, A. Intracellular Porphyromonas gingivalis promotes the tumorigenic behavior of pancreatic carcinoma cells. Cancers 2020, 12, 2331. [Google Scholar] [CrossRef] [PubMed]
- Reitano, E.; de’Angelis, N.; Gavriilidis, P.; Gaiani, F.; Memeo, R.; Inchingolo, R.; Bianchi, G.; de’Angelis, G.L.; Carra, M.C. Oral Bacterial Microbiota in Digestive Cancer Patients: A Systematic Review. Microorganisms 2021, 9, 2585. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.J.; Crean, S.J.; Lewis, M.A.; Spratt, D.A.; Wade, W.G.; Wilson, M.J. Viable bacteria present within oral squamous cell carcinoma tissue. J. Clin. Microbiol. 2006, 44, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Ji, X.; Li, Y.; Estilo, C.; Yegnanarayana, R.; Singh, B.; Li, X.; Saxena, D. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012, 12, 144. [Google Scholar] [CrossRef]
- Pustelny, C.; Komor, U.; Pawar, V.; Lorenz, A.; Bielecka, A.; Moter, A.; Gocht, B.; Eckweiler, D.; Müsken, M.; Grothe, C. Contribution of Veillonella parvula to Pseudomonas aeruginosa-mediated pathogenicity in a murine tumor model system. Infect. Immun. 2015, 83, 417–429. [Google Scholar] [CrossRef]
- Lertpiriyapong, K.; Whary, M.T.; Muthupalani, S.; Lofgren, J.L.; Gamazon, E.R.; Feng, Y.; Ge, Z.; Wang, T.C.; Fox, J.G. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut 2014, 63, 54–63. [Google Scholar] [CrossRef]
- Coppenhagen-Glazer, S.; Sol, A.; Abed, J.; Naor, R.; Zhang, X.; Han, Y.W.; Bachrach, G. Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect. Immun. 2015, 83, 1104–1113. [Google Scholar] [CrossRef]
- Okuda, T.; Kokubu, E.; Kawana, T.; Saito, A.; Okuda, K.; Ishihara, K. Synergy in biofilm formation between Fusobacterium nucleatum and Prevotella species. Anaerobe 2012, 18, 110–116. [Google Scholar] [CrossRef]
- Rosen, G.; Sela, M.N. Coaggregation of Porphyromonas gingivalis and Fusobacterium nucleatum PK 1594 is mediated by capsular polysaccharide and lipopolysaccharide. FEMS Microbiol. Lett. 2006, 256, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Kolenbrander, P.E. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J. Bacteriol. 2009, 191, 6804–6811. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Inagaki, S.; Kimizuka, R.; Okuda, K.; Hosaka, Y.; Nakagawa, T.; Ishihara, K. Fusobacterium nucleatum enhances invasion of human gingival epithelial and aortic endothelial cells by Porphyromonas gingivalis. FEMS Immunol. Med. Microbiol. 2008, 54, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.C.; Yilmaz, Ö.H.; Roper, J. Metabolism and colorectal cancer. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, Y.; Wen, L.; Mu, W.; Wu, X.; Liu, T.; Liu, X.; Fang, J.; Luan, Y.; Chen, P.; et al. Porphyromonas gingivalis promotes colorectal carcinoma by activating the hematopoietic NLRP3 inflammasome. Cancer Res. 2021, 81, 2745–2759. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Fabregat, X.; Malats, N.; Guarner, L.; Carrato, A.; De Miguel, A.; Ruiz, L.; Jariod, M.; Costafreda, S.; Coll, S. Exocrine pancreatic cancer: Symptoms at presentation and their relation to tumour site and stage. Clin. Transl. Oncol. 2005, 7, 189–197. [Google Scholar] [CrossRef]
- Hue, J.J.; Sugumar, K.; Kyasaram, R.K.; Shanahan, J.; Lyons, J.; Ocuin, L.M.; Rothermel, L.D.; Hardacre, J.M.; Ammori, J.B.; Rao, G. Weight loss as an untapped early detection marker in pancreatic and periampullary cancer. Ann. Surg. Oncol. 2021, 28, 6283–6292. [Google Scholar] [CrossRef]
- Drewes, A.M.; Campbell, C.M.; Ceyhan, G.O.; Delhaye, M.; Garg, P.K.; van Goor, H.; Laquente, B.; Morlion, B.; Olesen, S.S.; Singh, V.K. Pain in pancreatic ductal adenocarcinoma: A multidisciplinary, International guideline for optimized management. Pancreatology 2018, 18, 446–457. [Google Scholar] [CrossRef]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic cancer: Advances and challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, E.; van der Borden, C.L.; Frampton, A.E.; Ali, A.; Firuzi, O.; Peters, G.J. Never let it go: Stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin. Cancer Biol. 2017, 44, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Geng, F.; Shi, X.; Li, Y.; Zhang, X.; Zhao, X.; Pan, Y. The prevalence rate of periodontal pathogens and its association with oral squamous cell carcinoma. Appl. Microbiol. Biotechnol. 2019, 103, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Sayehmiri, F.; Sayehmiri, K.; Asadollahi, K.; Soroush, S.; Bogdanovic, L.; Jalilian, F.A.; Emaneini, M.; Taherikalani, M. The prevalence rate of Porphyromonas gingivalis and its association with cancer: A systematic review and meta-analysis. Int. J. Immunopathol. Pharmacol. 2015, 28, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Yilmaz, Ö. Possible role of Porphyromonas gingivalis in orodigestive cancers. J. Oral. Microbiol. 2019, 11, 1563410. [Google Scholar] [CrossRef]
- Li, Q.; Hu, Y.; Zhou, X.; Liu, S.; Han, Q.; Cheng, L. Role of Oral Bacteria in the Development of Oral Squamous Cell Carcinoma. Cancers 2020, 12, 2797. [Google Scholar] [CrossRef]
- Purcell, R.V.; Visnovska, M.; Biggs, P.J.; Schmeier, S.; Frizelle, F.A. Distinct gut microbiome patterns associate with consensus molecular subtypes of colorectal cancer. Sci. Rep. 2017, 7, 11590. [Google Scholar] [CrossRef]
- Bellotti, R.; Speth, C.; Adolph, T.E.; Lass-Flörl, C.; Effenberger, M.; Öfner, D.; Maglione, M. Micro- and Mycobiota Dysbiosis in Pancreatic Ductal Adenocarcinoma Development. Cancers 2021, 13, 3431. [Google Scholar] [CrossRef]
- Malinowski, B.; Węsierska, A.; Zalewska, K.; Sokołowska, M.M.; Bursiewicz, W.; Socha, M.; Ozorowski, M.; Pawlak-Osińska, K.; Wiciński, M. The role of Tannerella forsythia and Porphyromonas gingivalis in pathogenesis of esophageal cancer. Infect. Agent. Cancer 2019, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, X.; Chen, K.; Zhou, F.; Yang, H.; Yang, H.; Qi, Y.; Kong, J.; Sun, W.; Gao, S. Clinical significance and prognostic value of Porphyromonas gingivalis infection in lung cancer. Transl. Oncol. 2021, 14, 100972. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yuan, X.; Wang, J.; Liu, Y.; Sun, W.; Gu, B.; Lan, Z.; Gao, S. Frequencies of Porphyromonas gingivalis detection in oral-digestive tract tumors. Pathol. Oncol. Res. 2021, 27, 628942. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.H.; Grote, V.A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, K.; Amiri Moghaddam, M.; Etajuri, E.A.; Badkoobeh, A.; Tavakol, O.; Rafinejad, M.; Forutan Mirhosseini, A.; Fathi, A. Periodontitis and progression of gastrointestinal cancer: Current knowledge and future perspective. Clin. Transl. Oncol. 2023, 25, 2801–2811. [Google Scholar] [CrossRef]
- Groeger, S.; Domann, E.; Gonzales, J.R.; Chakraborty, T.; Meyle, J. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology 2011, 216, 1302–1310. [Google Scholar] [CrossRef]
- Yilmaz, Ö. The chronicles of Porphyromonas gingivalis: The microbium, the human oral epithelium and their interplay. Microbiology 2008, 154, 2897–2903. [Google Scholar] [CrossRef]
- Garrido, C.; Brunet, M.; Didelot, C.; Zermati, Y.; Schmitt, E.; Kroemer, G. Heat shock proteins 27 and 70: Anti-apoptotic proteins with tumorigenic properties. Cell Cycle 2006, 5, 2592–2601. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Kuboniwa, M.; Hasegawa, Y.; Mao, S.; Shizukuishi, S.; Amano, A.; Lamont, R.J.; Yilmaz, O.P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect. 2008, 10, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Luo, G.H. Porphyromonas gingivalis and digestive system cancers. World J. Clin. Cases 2019, 7, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.J.; Tu, H.P.; Chin, Y.T.; Lu, S.H.; Chiang, C.Y.; Chen, R.Y.; Fu, E. Cyclosporine-A inhibits MMP-2 and -9 activities in the presence of Porphyromonas gingivalis lipopolysaccharide: An experiment in human gingival fibroblast and U937 macrophage co-culture. J. Periodontal Res. 2012, 47, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014, 16, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.H.; Park, D.G.; Woo, B.H.; Kim, D.J.; Choi, J.I.; Park, B.S.; Kim, Y.D.; Lee, J.H.; Park, H.R. Porphyromonas gingivalis increases the invasiveness of oral cancer cells by upregulating IL-8 and MMPs. Cytokine 2016, 86, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Jo, Y.H.; Luke Yeo, I.S.; Yoon, H.I.; Lee, J.H.; Han, J.S. The effect of surface material, roughness and wettability on the adhesion and proliferation of Streptococcus gordonii, Fusobacterium nucleatum and Porphyromonas gingivalis. J. Dent. Sci. 2023, 18, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Zheng, W.; Chen, G.; Yin, Y.; Huang, X.; Wo, K.; Lei, H.; et al. F. nucleatum facilitates oral squamous cell carcinoma progression via GLUT1-driven lactate production. EBioMedicine 2023, 88, 104444. [Google Scholar] [CrossRef]
- Gaiser, R.A.; Halimi, A.; Alkharaan, H.; Lu, L.; Davanian, H.; Healy, K.; Hugerth, L.W.; Ateeb, Z.; Valente, R.; Fernández Moro, C.; et al. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut 2019, 68, 2186–2194. [Google Scholar] [CrossRef]
- Gethings-Behncke, C.; Coleman, H.G.; Jordao, H.W.T.; Longley, D.B.; Crawford, N.; Murray, L.J.; Kunzmann, A.T. Fusobacterium nucleatum in the Colorectum and Its Association with Cancer Risk and Survival: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 539–548. [Google Scholar] [CrossRef]
- Mima, K.; Cao, Y.; Chan, A.T.; Qian, Z.R.; Nowak, J.A.; Masugi, Y.; Shi, Y.; Song, M.; da Silva, A.; Gu, M.; et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location. Clin. Transl. Gastroenterol. 2016, 7, e200. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Yeh, Y.M.; Yu, H.Y.; Chin, C.Y.; Hsu, C.W.; Liu, H.; Huang, P.J.; Hu, S.N.; Liao, C.T.; Chang, K.P.; et al. Oral Microbiota Community Dynamics Associated With Oral Squamous Cell Carcinoma Staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Janati, A.I.; Karp, I.; Laprise, C.; Sabri, H.; Emami, E. Detection of Fusobaterium nucleatum in feces and colorectal mucosa as a risk factor for colorectal cancer: A systematic review and meta-analysis. Syst. Rev. 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Wang, H.; Tao, Y.; Luo, K.; Ye, J.; Ran, S.; Guan, Z.; Wang, Y.; Hu, H.; Huang, R. Fusobacterium nucleatum and colorectal cancer: From phenomenon to mechanism. Front. Cell Infect. Microbiol. 2022, 12, 1020583. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.; Schmid, J.; Ebert, M.; Soucek, P.; Kunicka, T.; Liska, V.; Bruha, J.; Neary, P.; Dezeeuw, N.; Tommasino, M.; et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Zorron Cheng Tao Pu, L.; Yamamoto, K.; Honda, T.; Nakamura, M.; Yamamura, T.; Hattori, S.; Burt, A.D.; Singh, R.; Hirooka, Y.; Fujishiro, M. Microbiota profile is different for early and invasive colorectal cancer and is consistent throughout the colon. J. Gastroenterol. Hepatol. 2020, 35, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, K.; Nosho, K.; Sukawa, Y.; Matsunaga, Y.; Ito, M.; Kurihara, H.; Kanno, S.; Igarashi, H.; Naito, T.; Adachi, Y.; et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 2015, 6, 7209–7220. [Google Scholar] [CrossRef]
- Yamamura, K.; Baba, Y.; Miyake, K.; Nakamura, K.; Shigaki, H.; Mima, K.; Kurashige, J.; Ishimoto, T.; Iwatsuki, M.; Sakamoto, Y.; et al. Fusobacterium nucleatum in gastroenterological cancer: Evaluation of measurement methods using quantitative polymerase chain reaction and a literature review. Oncol. Lett. 2017, 14, 6373–6378. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Heidarzadeh, S.; Jahangiri, S.; Farhood, B.; Mortezaee, K.; Khanlarkhani, N.; Negahdari, B. Fusobacterium nucleatum and colorectal cancer: A mechanistic overview. J. Cell Physiol. 2019, 234, 2337–2344. [Google Scholar] [CrossRef]
- Ranjbar, M.; Salehi, R.; Haghjooy Javanmard, S.; Rafiee, L.; Faraji, H.; Jafarpor, S.; Ferns, G.A.; Ghayour-Mobarhan, M.; Manian, M.; Nedaeinia, R. The dysbiosis signature of Fusobacterium nucleatum in colorectal cancer-cause or consequences? A systematic review. Cancer Cell Int. 2021, 21, 194. [Google Scholar] [CrossRef]
- Sun, C.-H.; Li, B.-B.; Wang, B.; Zhao, J.; Zhang, X.-Y.; Li, T.-T.; Li, W.-B.; Tang, D.; Qiu, M.-J.; Wang, X.-C. The role of Fusobacterium nucleatum in colorectal cancer: From carcinogenesis to clinical management. Chronic Dis. Transl. Med. 2019, 5, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- McIlvanna, E.; Linden, G.J.; Craig, S.G.; Lundy, F.T.; James, J.A. Fusobacterium nucleatum and oral cancer: A critical review. BMC Cancer 2021, 21, 1212. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yu, Y.; Yin, Y.; Wang, L.; Yang, H.; Luo, S.; Zheng, Q.; Pan, Y.; Zhang, D. Potential role of epithelial-mesenchymal transition induced by periodontal pathogens in oral cancer. J. Cell Mol. Med. 2023. early view. [Google Scholar] [CrossRef]
- Pignatelli, P.; Nuccio, F.; Piattelli, A.; Curia, M.C. The Role of Fusobacterium nucleatum in Oral and Colorectal Carcinogenesis. Microorganisms 2023, 11, 2358. [Google Scholar] [CrossRef]
- Van der Merwe, M.; Van Niekerk, G.; Botha, A.; Engelbrecht, A.M. The onco-immunological implications of Fusobacterium nucleatum in breast cancer. Immunol. Lett. 2021, 232, 60–66. [Google Scholar] [CrossRef]
- Yu, T.C.; Zhou, Y.L.; Fang, J.Y. Oral pathogen in the pathogenesis of colorectal cancer. J. Gastroenterol. Hepatol. 2022, 37, 273–279. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. Inflammatory Bacteriome and Oral Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 725–732. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Eslami, H.; Kafil, H.S. Carcinogenesis mechanisms of Fusobacterium nucleatum. Biomed. Pharmacother. 2017, 89, 918–925. [Google Scholar] [CrossRef]
- Alon-Maimon, T.; Mandelboim, O.; Bachrach, G. Fusobacterium nucleatum and cancer. Periodontology 2000 2022, 89, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Saikia, P.J.; Pathak, L.; Mitra, S.; Das, B. The emerging role of oral microbiota in oral cancer initiation, progression and stemness. Front. Immunol. 2023, 14, 1198269. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Kokubu, E.; Inagaki, S.; Imamura, K.; Kita, D.; Lamont, R.J.; Ishihara, K. Porphyromonas gingivalis entry into gingival epithelial cells modulated by Fusobacterium nucleatum is dependent on lipid rafts. Microb. Pathog. 2012, 53, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.Y.; Zhang, Q.; Li, J.L.; Yang, S.H.; Shi, Q. Progression of periodontal inflammation in adolescents is associated with increased number of Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythensis, and Fusobacterium nucleatum. Int. J. Paediatr. Dent. 2014, 24, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell Infect. Microbiol. 2019, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Torralba, M.G.; Aleti, G.; Li, W.; Moncera, K.J.; Lin, Y.H.; Yu, Y.; Masternak, M.M.; Golusinski, W.; Golusinski, P.; Lamperska, K.; et al. Oral Microbial Species and Virulence Factors Associated with Oral Squamous Cell Carcinoma. Microb. Ecol. 2021, 82, 1030–1046. [Google Scholar] [CrossRef] [PubMed]
- Park, D.G.; Woo, B.H.; Lee, B.J.; Yoon, S.; Cho, Y.; Kim, Y.D.; Park, H.R.; Song, J.M. Serum Levels of Interleukin-6 and Titers of Antibodies Against Porphyromonas gingivalis Could Be Potential Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2749. [Google Scholar] [CrossRef]
- Guven, D.C.; Dizdar, O.; Alp, A.; Akdoğan Kittana, F.N.; Karakoc, D.; Hamaloglu, E.; Lacin, S.; Karakas, Y.; Kilickap, S.; Hayran, M.; et al. Analysis of Fusobacterium nucleatum and Streptococcus gallolyticus in saliva of colorectal cancer patients. Biomark. Med. 2019, 13, 725–735. [Google Scholar] [CrossRef]
- Gao, R.; Zhu, Y.; Kong, C.; Xia, K.; Li, H.; Zhu, Y.; Zhang, X.; Liu, Y.; Zhong, H.; Yang, R.; et al. Alterations, Interactions, and Diagnostic Potential of Gut Bacteria and Viruses in Colorectal Cancer. Front. Cell Infect. Microbiol. 2021, 11, 657867. [Google Scholar] [CrossRef]
- Thanki, K.; Nicholls, M.E.; Gajjar, A.; Senagore, A.J.; Qiu, S.; Szabo, C.; Hellmich, M.R.; Chao, C. Consensus Molecular Subtypes of Colorectal Cancer and their Clinical Implications. Int. Biol. Biomed. J. 2017, 3, 105–111. [Google Scholar]
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral. Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P.; Hajishengallis, G.; Curtis, M.A. Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 2012, 91, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Kartal, E.; Schmidt, T.S.B.; Molina-Montes, E.; Rodríguez-Perales, S.; Wirbel, J.; Maistrenko, O.M.; Akanni, W.A.; Alashkar Alhamwe, B.; Alves, R.J.; Carrato, A.; et al. A faecal microbiota signature with high specificity for pancreatic cancer. Gut 2022, 71, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Binder Gallimidi, A.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef] [PubMed]
- Sztukowska, M.N.; Ojo, A.; Ahmed, S.; Carenbauer, A.L.; Wang, Q.; Shumway, B.; Jenkinson, H.F.; Wang, H.; Darling, D.S.; Lamont, R.J. Porphyromonas gingivalis initiates a mesenchymal-like transition through ZEB1 in gingival epithelial cells. Cell Microbiol. 2016, 18, 844–858. [Google Scholar] [CrossRef]
- Lee, J.; Roberts, J.S.; Atanasova, K.R.; Chowdhury, N.; Han, K.; Yilmaz, Ö. Human Primary Epithelial Cells Acquire an Epithelial-Mesenchymal-Transition Phenotype during Long-Term Infection by the Oral Opportunistic Pathogen, Porphyromonas gingivalis. Front. Cell Infect. Microbiol. 2017, 7, 493. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shen, X.; Tian, J. The effects of periodontitis associated microbiota on the development of oral squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2021, 576, 80–85. [Google Scholar] [CrossRef]
- Yao, Y.; Shen, X.; Zhou, M.; Tang, B. Periodontal Pathogens Promote Oral Squamous Cell Carcinoma by Regulating ATR and NLRP3 Inflammasome. Front. Oncol. 2021, 11, 722797. [Google Scholar] [CrossRef]
- Polak, D.; Wilensky, A.; Shapira, L.; Halabi, A.; Goldstein, D.; Weiss, E.I.; Houri-Haddad, Y. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: Bone loss and host response. J. Clin. Periodontol. 2009, 36, 406–410. [Google Scholar] [CrossRef]
- Maekawa, T.; Krauss, J.L.; Abe, T.; Jotwani, R.; Triantafilou, M.; Triantafilou, K.; Hashim, A.; Hoch, S.; Curtis, M.A.; Nussbaum, G.; et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 2014, 15, 768–778. [Google Scholar] [CrossRef]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A bacterial driver-passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Sharma, P.; Are, A.C.; Vickers, S.M.; Dudeja, V. New Insights Into the Cancer-Microbiome-Immune Axis: Decrypting a Decade of Discoveries. Front. Immunol. 2021, 12, 622064. [Google Scholar] [CrossRef] [PubMed]
- Al-Hebshi, N.N.; Borgnakke, W.S.; Johnson, N.W. The microbiome of oral squamous cell carcinomas: A functional perspective. Current Oral. Health Rep. 2019, 6, 145–160. [Google Scholar] [CrossRef]
- Gori, S.; Inno, A.; Belluomini, L.; Bocus, P.; Bisoffi, Z.; Russo, A.; Arcaro, G. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit. Rev. Oncol. Hematol. 2019, 143, 139–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).