Pandemic-Proofing: Intercepting Zoonotic Spillover Events
Abstract
1. Introduction
2. Methodology
3. Historical Instances of Zoonotic Spillover Leading to Pandemics/Endemics
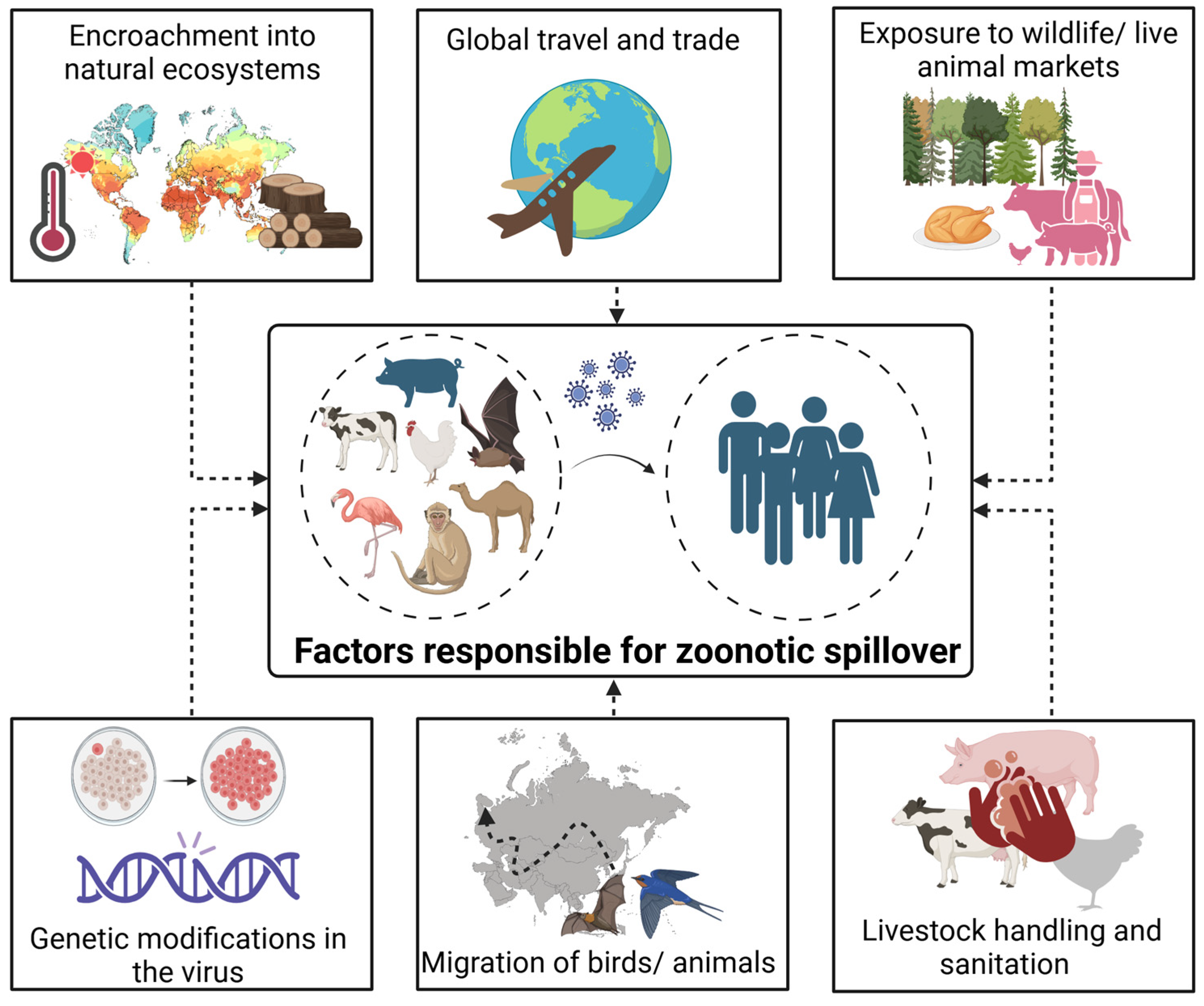
4. Factors Behind Zoonotic Spillover
4.1. Increased Human-Animal Contact
4.2. Global Travel and Trade
4.3. Lack of Monitoring and Surveillance
4.4. Ecological and Climate Changes
4.5. Sociocultural Factors
4.6. Inadequate Healthcare Systems
5. Challenges
5.1. Reservoir Diversity
5.2. Virus Adaptation and Evolution
5.3. Lack of Highly Sensitive and Robust Diagnostics
6. Future Predictions
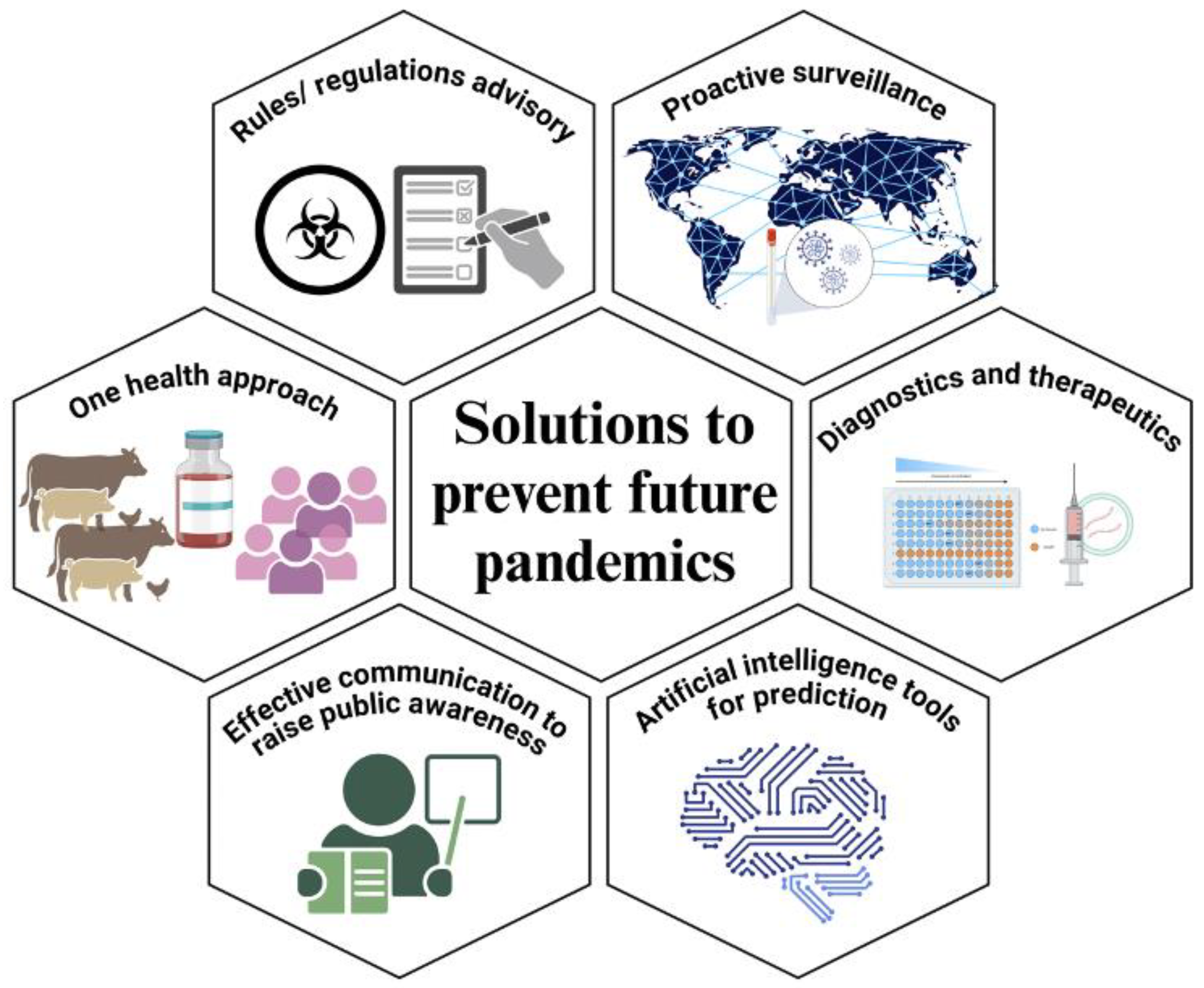
7. Solutions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rehman, H.; Ahmad, M.I. COVID-19: A wreak havoc across the globe. Arch. Physiol. Biochem. 2023, 129, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.; Baker, N.; Phillips, N. Coronapod: Our future with an ever-present coronavirus. Nature 2021. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.E. Pre-spillover prevention of emerging zoonotic diseases: What are the targets and what are the tools? Curr. Top. Microbiol. Immunol. 2007, 315, 389–443. [Google Scholar] [CrossRef] [PubMed]
- Spyrou, M.A.; Musralina, L.; Gnecchi Ruscone, G.A.; Kocher, A.; Borbone, P.G.; Khartanovich, V.I.; Buzhilova, A.; Djansugurova, L.; Bos, K.I.; Kuhnert, D.; et al. The source of the Black Death in fourteenth-century central Eurasia. Nature 2022, 606, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Lycett, S.J.; Duchatel, F.; Digard, P. A brief history of bird flu. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180257. [Google Scholar] [CrossRef]
- Hemelaar, J. The origin and diversity of the HIV-1 pandemic. Trends Mol. Med. 2012, 18, 182–192. [Google Scholar] [CrossRef]
- Richard, M.; de Graaf, M.; Herfst, S. Avian influenza A viruses: From zoonosis to pandemic. Future Virol. 2014, 9, 513–524. [Google Scholar] [CrossRef]
- Peiris, J.S.; Poon, L.L.; Guan, Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J. Clin. Virol. 2009, 45, 169–173. [Google Scholar] [CrossRef]
- Vilcek, S. SARS-CoV-2: Zoonotic origin of pandemic coronavirus. Acta Virol. 2020, 64, 281–287. [Google Scholar] [CrossRef]
- Glatter, K.A.; Finkelman, P. History of the Plague: An Ancient Pandemic for the Age of COVID-19. Am. J. Med. 2021, 134, 176–181. [Google Scholar] [CrossRef]
- Yang, R.; Atkinson, S.; Chen, Z.; Cui, Y.; Du, Z.; Han, Y.; Sebbane, F.; Slavin, P.; Song, Y.; Yan, Y.; et al. Yersinia pestis and Plague: Some knowns and unknowns. Zoonoses 2023, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Viboud, C.; Simonsen, L.; Fuentes, R.; Flores, J.; Miller, M.A.; Chowell, G. Global Mortality Impact of the 1957-1959 Influenza Pandemic. J. Infect. Dis. 2016, 213, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Boulos, C.; Memoli, M.J. The 1968 Influenza Pandemic and COVID-19 Outcomes. medRxiv 2022. [Google Scholar] [CrossRef]
- Lam, W.K.; Zhong, N.S.; Tan, W.C. Overview on SARS in Asia and the world. Respirology 2003, 8 (Suppl. S1), S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Dawood, F.S.; Iuliano, A.D.; Reed, C.; Meltzer, M.I.; Shay, D.K.; Cheng, P.Y.; Bandaranayake, D.; Breiman, R.F.; Brooks, W.A.; Buchy, P.; et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: A modelling study. Lancet Infect. Dis. 2012, 12, 687–695. [Google Scholar] [CrossRef]
- Memish, Z.A.; Perlman, S.; Van Kerkhove, M.D.; Zumla, A. Middle East respiratory syndrome. Lancet 2020, 395, 1063–1077. [Google Scholar] [CrossRef]
- Zhu, W.; Li, X.; Dong, J.; Bo, H.; Liu, J.; Yang, J.; Zhang, Y.; Wei, H.; Huang, W.; Zhao, X.; et al. Epidemiologic, Clinical, and Genetic Characteristics of Human Infections with Influenza A(H5N6) Viruses, China. Emerg. Infect. Dis. 2022, 28, 1332–1344. [Google Scholar] [CrossRef]
- Paul, L. Nipah virus in Kerala: A deadly Zoonosis. Clin. Microbiol. Infect. 2018, 24, 1113–1114. [Google Scholar] [CrossRef]
- Wong, S.S.; Yuen, K.Y. Avian influenza virus infections in humans. Chest 2006, 129, 156–168. [Google Scholar] [CrossRef]
- Ye, Y.; Ye, Z.; Yang, L.; Xiang, B.; Zheng, C. Unignorable public health risk of avian influenza virus during COVID-19 pandemic. J. Med. Virol. 2022, 94, 4058–4060. [Google Scholar] [CrossRef]
- Sivanandy, P.; Jun, P.H.; Man, L.W.; Wei, N.S.; Mun, N.F.K.; Yii, C.A.J.; Ying, C.C.X. A systematic review of Ebola virus disease outbreaks and an analysis of the efficacy and safety of newer drugs approved for the treatment of Ebola virus disease by the US Food and Drug Administration from 2016 to 2020. J. Infect. Public Health 2022, 15, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Choi, G.K.; Yip, C.C.; Cheng, V.C.; Yuen, K.Y. Zika fever and congenital Zika syndrome: An unexpected emerging arboviral disease. J. Infect. 2016, 72, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sharma, L.; Chang, D. Pathophysiology and clinical management of coronavirus disease (COVID-19): A mini-review. Front. Immunol. 2023, 14, 1116131. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Khan, S. A Global emergence of West Nile virus: Threat & preparedness in special perspective to India. Indian J. Med. Res. 2021, 154, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Lapa, D.; Pauciullo, S.; Ricci, I.; Garbuglia, A.R.; Maggi, F.; Scicluna, M.T.; Tofani, S. Rift Valley Fever Virus: An Overview of the Current Status of Diagnostics. Biomedicines 2024, 12, 540. [Google Scholar] [CrossRef]
- Pattnaik, P.; Mahal, A.; Mishra, S.; Alkhouri, A.; Mohapatra, R.K.; Kandi, V. Alarming Rise in Global Rabies Cases Calls for Urgent Attention: Current Vaccination Status and Suggested Key Countermeasures. Cureus 2023, 15, e50424. [Google Scholar] [CrossRef]
- Laurenson-Schafer, H.; Sklenovska, N.; Hoxha, A.; Kerr, S.M.; Ndumbi, P.; Fitzner, J.; Almiron, M.; de Sousa, L.A.; Briand, S.; Cenciarelli, O.; et al. Description of the first global outbreak of mpox: An analysis of global surveillance data. Lancet Glob. Health 2023, 11, e1012–e1023. [Google Scholar] [CrossRef]
- Han, H.J.; Yu, H.; Yu, X.J. Evidence for zoonotic origins of Middle East respiratory syndrome coronavirus. J. Gen. Virol. 2016, 97, 274–280. [Google Scholar] [CrossRef]
- Mari Saez, A.; Weiss, S.; Nowak, K.; Lapeyre, V.; Zimmermann, F.; Dux, A.; Kuhl, H.S.; Kaba, M.; Regnaut, S.; Merkel, K.; et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol. Med. 2015, 7, 17–23. [Google Scholar] [CrossRef]
- Song, B.H.; Yun, S.I.; Woolley, M.; Lee, Y.M. Zika virus: History, epidemiology, transmission, and clinical presentation. J. Neuroimmunol. 2017, 308, 50–64. [Google Scholar] [CrossRef]
- Epstein, J.H.; Field, H.E.; Luby, S.; Pulliam, J.R.; Daszak, P. Nipah virus: Impact, origins, and causes of emergence. Curr. Infect. Dis. Rep. 2006, 8, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.L.; Conly, J.M. West Nile virus—Where did it come from and where might it go? Can. J. Infect. Dis. 2000, 11, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J. West nile virus: An historical overview. Ochsner. J. 2003, 5, 6–10. [Google Scholar] [PubMed]
- Kwasnik, M.; Rozek, W.; Rola, J. Rift Valley Fever—A Growing Threat to Humans and Animals. J. Vet. Res. 2021, 65, 7–14. [Google Scholar] [CrossRef]
- Fisher, C.R.; Streicker, D.G.; Schnell, M.J. The spread and evolution of rabies virus: Conquering new frontiers. Nat. Rev. Microbiol. 2018, 16, 241–255. [Google Scholar] [CrossRef]
- Khan, G.; Perveen, N. Monkeypox: Past, Present, and Future. Adv. Exp. Med. Biol. 2024, 1451, 1–20. [Google Scholar] [CrossRef]
- Eisfeld, A.J.; Biswas, A.; Guan, L.; Gu, C.; Maemura, T.; Trifkovic, S.; Wang, T.; Babujee, L.; Dahn, R.; Halfmann, P.J.; et al. Pathogenicity and transmissibility of bovine H5N1 influenza virus. Nature 2024, 633, 426–432. [Google Scholar] [CrossRef]
- Wang, C.-X.; Xiu, L.-S.; Hu, Q.-Q.; Lee, T.-C.; Liu, J.; Shi, L.; Zhou, X.-N.; Guo, X.-K.; Hou, L.; Yin, K. Advancing early warning and surveillance for zoonotic diseases under climate change: Interdisciplinary systematic perspectives. Adv. Clim. Chang. Res. 2023, 14, 814–826. [Google Scholar] [CrossRef]
- Authored by the members of the One Health High-Level Expert Panel (OHHLEP); Markotter, W.; Mettenleiter, T.C.; Adisasmito, W.B.; Almuhairi, S.; Barton Behravesh, C.; Bilivogui, P.; Bukachi, S.A.; Casas, N.; Cediel Becerra, N.; et al. Prevention of zoonotic spillover: From relying on response to reducing the risk at source. PLoS Pathog. 2023, 19, e1011504. [Google Scholar] [CrossRef]
- Hulme, P.E. Advancing One Biosecurity to Address the Pandemic Risks of Biological Invasions. Bioscience 2021, 71, 708–721. [Google Scholar] [CrossRef]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tambo, E.; Djuikoue, I.C.; Tazemda, G.K.; Fotsing, M.F.; Zhou, X.N. Early stage risk communication and community engagement (RCCE) strategies and measures against the coronavirus disease 2019 (COVID-19) pandemic crisis. Glob. Health J. 2021, 5, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Hassan, O.A.; de Balogh, K.; Winkler, A.S. One Health early warning and response system for zoonotic diseases outbreaks: Emphasis on the involvement of grassroots actors. Vet. Med. Sci. 2023, 9, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.M.; Klunk, J.; Harbeck, M.; Devault, A.; Waglechner, N.; Sahl, J.W.; Enk, J.; Birdsell, D.N.; Kuch, M.; Lumibao, C.; et al. Yersinia pestis and the plague of Justinian 541-543 AD: A genomic analysis. Lancet Infect. Dis. 2014, 14, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Theves, C.; Crubezy, E.; Biagini, P. History of Smallpox and Its Spread in Human Populations. Microbiol. Spectr. 2016, 4, 161–172. [Google Scholar] [CrossRef]
- Khan, F.; Ali, M. The last case of smallpox. Lancet Infect. Dis. 2018, 18, 1318. [Google Scholar] [CrossRef]
- Berche, P. The Spanish flu. Presse Med. 2022, 51, 104127. [Google Scholar] [CrossRef]
- Xu, R.; Ekiert, D.C.; Krause, J.C.; Hai, R.; Crowe, J.E., Jr.; Wilson, I.A. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 2010, 328, 357–360. [Google Scholar] [CrossRef]
- Simonsen, L.; Spreeuwenberg, P.; Lustig, R.; Taylor, R.J.; Fleming, D.M.; Kroneman, M.; Van Kerkhove, M.D.; Mounts, A.W.; Paget, W.J.; Teams, G.L.C. Global mortality estimates for the 2009 Influenza Pandemic from the GLaMOR project: A modeling study. PLoS Med. 2013, 10, e1001558. [Google Scholar] [CrossRef]
- Peiris, J.S.; de Jong, M.D.; Guan, Y. Avian influenza virus (H5N1): A threat to human health. Clin. Microbiol. Rev. 2007, 20, 243–267. [Google Scholar] [CrossRef]
- Sah, R.; Mohanty, A.; Rohilla, R.; Mehta, R.; Leon-Figueroa, D.A.; Barboza, J.J.; Chattu, V.K.; Padhi, B.K. Human death due to H5N1 amid the COVID-19 pandemic and Mpox outbreak: A call for action. Int. J. Surg. 2023, 109, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Reperant, L.A.; Grenfell, B.T.; Osterhaus, A.D. Quantifying the risk of pandemic influenza virus evolution by mutation and re-assortment. Vaccine 2015, 33, 6955–6966. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Merilainen, P.; Lindh, E.; Kitajima, M.; Osterlund, P.; Ikonen, N.; Savolainen-Kopra, C.; Pitkanen, T. Avian Influenza outbreaks: Human infection risks for beach users—One health concern and environmental surveillance implications. Sci. Total Environ. 2024, 943, 173692. [Google Scholar] [CrossRef] [PubMed]
- Pangesti, K.N.A.; Abd El Ghany, M.; Walsh, M.G.; Kesson, A.M.; Hill-Cawthorne, G.A. Molecular epidemiology of respiratory syncytial virus. Rev. Med. Virol. 2018, 28, e1968. [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Laupland, K.B.; Valiquette, L. Ebola virus disease. Can. J. Infect. Dis. Med. Microbiol. 2014, 25, 128–129. [Google Scholar] [CrossRef]
- Safari, S.; Baratloo, A.; Rouhipour, A.; Ghelichkhani, P.; Yousefifard, M. Ebola Hemorrhagic Fever as a Public Health Emergency of International Concern; a Review Article. Emergency 2015, 3, 3–7. [Google Scholar]
- Mylne, A.Q.; Pigott, D.M.; Longbottom, J.; Shearer, F.; Duda, K.A.; Messina, J.P.; Weiss, D.J.; Moyes, C.L.; Golding, N.; Hay, S.I. Mapping the zoonotic niche of Lassa fever in Africa. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Garry, R.F. Lassa fever—The road ahead. Nat. Rev. Microbiol. 2023, 21, 87–96. [Google Scholar] [CrossRef]
- Cherry, J.D. The chronology of the 2002-2003 SARS mini pandemic. Paediatr. Respir. Rev. 2004, 5, 262–269. [Google Scholar] [CrossRef]
- Holmes, E.C.; Goldstein, S.A.; Rasmussen, A.L.; Robertson, D.L.; Crits-Christoph, A.; Wertheim, J.O.; Anthony, S.J.; Barclay, W.S.; Boni, M.F.; Doherty, P.C.; et al. The origins of SARS-CoV-2: A critical review. Cell 2021, 184, 4848–4856. [Google Scholar] [CrossRef] [PubMed]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Bellini, W.J.; Rota, P.A.; Harcourt, B.H.; Tamin, A.; Lam, S.K.; Ksiazek, T.G.; Rollin, P.E.; Zaki, S.R.; Shieh, W.; et al. Nipah virus: A recently emergent deadly paramyxovirus. Science 2000, 288, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Hossain, M.J.; Sultana, S.; Homaira, N.; Khan, S.U.; Rahman, M.; Gurley, E.S.; Rollin, P.E.; Lo, M.K.; Comer, J.A.; et al. Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector Borne Zoonotic Dis. 2012, 12, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Debiasi, R.L.; Tyler, K.L. West Nile virus meningoencephalitis. Nat. Clin. Pract. Neurol. 2006, 2, 264–275. [Google Scholar] [CrossRef]
- Wang, H.R.; Liu, T.; Gao, X.; Wang, H.B.; Xiao, J.H. Impact of climate change on the global circulation of West Nile virus and adaptation responses: A scoping review. Infect. Dis. Poverty 2024, 13, 38. [Google Scholar] [CrossRef]
- Khairullah, A.R.; Kurniawan, S.C.; Hasib, A.; Silaen, O.S.M.; Widodo, A.; Effendi, M.H.; Ramandinianto, S.C.; Moses, I.B.; Riwu, K.H.P.; Yanestria, S.M. Tracking lethal threat: In-depth review of rabies. Open Vet. J. 2023, 13, 1385–1399. [Google Scholar] [CrossRef]
- Bastos, V.; Pacheco, V.; Rodrigues, E.D.L.; Moraes, C.N.S.; Nobile, A.L.; Fonseca, D.L.M.; Souza, K.B.S.; do Vale, F.Y.N.; Filgueiras, I.S.; Schimke, L.F.; et al. Neuroimmunology of rabies: New insights into an ancient disease. J. Med. Virol. 2023, 95, e29042. [Google Scholar] [CrossRef]
- Townsend, S.E.; Lembo, T.; Cleaveland, S.; Meslin, F.X.; Miranda, M.E.; Putra, A.A.; Haydon, D.T.; Hampson, K. Surveillance guidelines for disease elimination: A case study of canine rabies. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 249–261. [Google Scholar] [CrossRef]
- Bhatia, B.; Feldmann, H.; Marzi, A. Kyasanur Forest Disease and Alkhurma Hemorrhagic Fever Virus-Two Neglected Zoonotic Pathogens. Microorganisms 2020, 8, 1406. [Google Scholar] [CrossRef]
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.Y.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA 2013, 110, 8399–8404. [Google Scholar] [CrossRef] [PubMed]
- Luby, S.P.; Gurley, E.S.; Hossain, M.J. Transmission of human infection with Nipah virus. Clin. Infect. Dis. 2009, 49, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Wongnak, P.; Thanapongtharm, W.; Kusakunniran, W.; Karnjanapreechakorn, S.; Sutassananon, K.; Kalpravidh, W.; Wongsathapornchai, K.; Wiratsudakul, A. A ‘what-if’ scenario: Nipah virus attacks pig trade chains in Thailand. BMC Vet. Res. 2020, 16, 300. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Dhama, K.; Chakraborty, S.; Tiwari, R.; Natesan, S.; Khandia, R.; Munjal, A.; Vora, K.S.; Latheef, S.K.; Karthik, K.; et al. Nipah virus: Epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies—A comprehensive review. Vet. Q. 2019, 39, 26–55. [Google Scholar] [CrossRef]
- MacMahon, K.L.; Delaney, L.J.; Kullman, G.; Gibbins, J.D.; Decker, J.; Kiefer, M.J. Protecting poultry workers from exposure to avian influenza viruses. Public Health Rep. 2008, 123, 316–322. [Google Scholar] [CrossRef]
- Hollenbeck, J.E. Interaction of the role of Concentrated Animal Feeding Operations (CAFOs) in Emerging Infectious Diseases (EIDS). Infect. Genet. Evol. 2016, 38, 44–46. [Google Scholar] [CrossRef]
- Olivero, J.; Fa, J.E.; Real, R.; Marquez, A.L.; Farfan, M.A.; Vargas, J.M.; Gaveau, D.; Salim, M.A.; Park, D.; Suter, J.; et al. Recent loss of closed forests is associated with Ebola virus disease outbreaks. Sci. Rep. 2017, 7, 14291. [Google Scholar] [CrossRef]
- Ortiz, D.I.; Piche-Ovares, M.; Romero-Vega, L.M.; Wagman, J.; Troyo, A. The Impact of Deforestation, Urbanization, and Changing Land Use Patterns on the Ecology of Mosquito and Tick-Borne Diseases in Central America. Insects 2021, 13, 20. [Google Scholar] [CrossRef]
- Ali, S.; Gugliemini, O.; Harber, S.; Harrison, A.; Houle, L.; Ivory, J.; Kersten, S.; Khan, R.; Kim, J.; LeBoa, C.; et al. Environmental and Social Change Drive the Explosive Emergence of Zika Virus in the Americas. PLoS Negl. Trop. Dis. 2017, 11, e0005135. [Google Scholar] [CrossRef]
- Wilk-da-Silva, R.; Prist, P.R.; Medeiros-Sousa, A.R.; Laporta, G.Z.; Mucci, L.F.; Marrelli, M.T. The role of forest fragmentation in yellow fever virus dispersal. Acta Trop. 2023, 245, 106983. [Google Scholar] [CrossRef]
- Keatts, L.O.; Robards, M.; Olson, S.H.; Hueffer, K.; Insley, S.J.; Joly, D.O.; Kutz, S.; Lee, D.S.; Chetkiewicz, C.B.; Lair, S.; et al. Implications of Zoonoses From Hunting and Use of Wildlife in North American Arctic and Boreal Biomes: Pandemic Potential, Monitoring, and Mitigation. Front. Public Health 2021, 9, 627654. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.M.; Li, R.; Ling, J.; Grace, D.; Nguyen-Viet, H.; Lindahl, J.F. Live and Wet Markets: Food Access versus the Risk of Disease Emergence. Trends Microbiol. 2021, 29, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, N.D.; Switzer, W.M.; Carr, J.K.; Bhullar, V.B.; Shanmugam, V.; Tamoufe, U.; Prosser, A.T.; Torimiro, J.N.; Wright, A.; Mpoudi-Ngole, E.; et al. Naturally acquired simian retrovirus infections in central African hunters. Lancet 2004, 363, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Courgnaud, V.; Abela, B.; Auzel, P.; Pourrut, X.; Bibollet-Ruche, F.; Loul, S.; Liegeois, F.; Butel, C.; Koulagna, D.; et al. Risk to human health from a plethora of simian immunodeficiency viruses in primate bushmeat. Emerg. Infect. Dis. 2002, 8, 451–457. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, R. Wildlife trade is likely thesource of SARS-CoV-2. Science 2022, 377, 925–926. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Tambyah, P.A. SARS: Responding to an unknown virus. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 589–595. [Google Scholar] [CrossRef]
- Iyaniwura, S.A.; Ringa, N.; Adu, P.A.; Mak, S.; Janjua, N.Z.; Irvine, M.A.; Otterstatter, M. Understanding the impact of mobility on COVID-19 spread: A hybrid gravity-metapopulation model of COVID-19. PLoS Comput. Biol. 2023, 19, e1011123. [Google Scholar] [CrossRef]
- Bajardi, P.; Poletto, C.; Ramasco, J.J.; Tizzoni, M.; Colizza, V.; Vespignani, A. Human mobility networks, travel restrictions, and the global spread of 2009 H1N1 pandemic. PLoS ONE 2011, 6, e16591. [Google Scholar] [CrossRef]
- Costard, S.; Wieland, B.; de Glanville, W.; Jori, F.; Rowlands, R.; Vosloo, W.; Roger, F.; Pfeiffer, D.U.; Dixon, L.K. African swine fever: How can global spread be prevented? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef]
- Jacobsen, K.H.; Aguirre, A.A.; Bailey, C.L.; Baranova, A.V.; Crooks, A.T.; Croitoru, A.; Delamater, P.L.; Gupta, J.; Kehn-Hall, K.; Narayanan, A.; et al. Lessons from the Ebola Outbreak: Action Items for Emerging Infectious Disease Preparedness and Response. Ecohealth 2016, 13, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Schwalbe, N. Primary health care: A cornerstone of pandemic prevention, preparedness, response, and recovery. Lancet 2023, 401, 1847. [Google Scholar] [CrossRef] [PubMed]
- Umar, T.P.; Kadir, A.; Mohammed, Y.A.; Setti, M.O. Healthcare system preparedness for the next pandemic beyond COVID-19 situation. J. Prev. Med. Hyg. 2022, 63, E493–E494. [Google Scholar] [CrossRef] [PubMed]
- Leal Filho, W.; Ternova, L.; Parasnis, S.A.; Kovaleva, M.; Nagy, G.J. Climate Change and Zoonoses: A Review of Concepts, Definitions, and Bibliometrics. Int. J. Environ. Res. Public Health 2022, 19, 893. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites. Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef]
- Lamy, K.; Tran, A.; Portafaix, T.; Leroux, M.D.; Baldet, T. Impact of regional climate change on the mosquito vector Aedes albopictus in a tropical island environment: La Reunion. Sci. Total Environ. 2023, 875, 162484. [Google Scholar] [CrossRef]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef]
- Carey, C. The impacts of climate change on the annual cycles of birds. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 3321–3330. [Google Scholar] [CrossRef]
- Dalpasso, A.; Seglie, D.; Eusebio Bergo, P.; Ciraci, A.; Compostella, M.; Laddaga, L.; Manica, M.; Marino, G.; Pandolfo, I.; Soldato, G.; et al. Effects of temperature and precipitation changes on shifts in breeding phenology of an endangered toad. Sci. Rep. 2023, 13, 14573. [Google Scholar] [CrossRef]
- Cordes, L.S.; Blumstein, D.T.; Armitage, K.B.; CaraDonna, P.J.; Childs, D.Z.; Gerber, B.D.; Martin, J.G.A.; Oli, M.K.; Ozgul, A. Contrasting effects of climate change on seasonal survival of a hibernating mammal. Proc. Natl. Acad. Sci. USA 2020, 117, 18119–18126. [Google Scholar] [CrossRef]
- Muzembo, B.A.; Ntontolo, N.P.; Ngatu, N.R.; Khatiwada, J.; Suzuki, T.; Wada, K.; Kitahara, K.; Ikeda, S.; Miyoshi, S.I. Misconceptions and Rumors about Ebola Virus Disease in Sub-Saharan Africa: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 4714. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Galvani, A.P. Exacerbation of measles mortality by vaccine hesitancy worldwide. Lancet Glob. Health 2023, 11, e478–e479. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.H.; Sun, Z.; Kruk, M.E. Association between infrastructure and observed quality of care in 4 healthcare services: A cross-sectional study of 4,300 facilities in 8 countries. PLoS Med. 2017, 14, e1002464. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.S. Unveiling the ABCs: Identifying India’s Healthcare Service Gaps. Cureus 2023, 15, e42398. [Google Scholar] [CrossRef] [PubMed]
- Buseh, A.G.; Stevens, P.E.; Bromberg, M.; Kelber, S.T. The Ebola epidemic in West Africa: Challenges, opportunities, and policy priority areas. Nurs. Outlook 2015, 63, 30–40. [Google Scholar] [CrossRef]
- Roodenbeke, E.D.; Lucas, S.; Rouzaut, A.; Bana, F. Outreach Services as a Strategy to Increase Access to Health Workers in Remote and Rural Areas: Increasing Access to Health Workers in Rural and Remote Areas; WHO Guidelines Approved by the Guidelines Review Committee: Geneva, Swizterland, 2011. [Google Scholar]
- Moten, A.; Schafer, D.F.; Montgomery, E. A prescription for health inequity: Building public health infrastructure in resource-poor settings. J. Glob. Health 2012, 2, 020302. [Google Scholar] [CrossRef]
- Han, H.J.; Wen, H.L.; Zhou, C.M.; Chen, F.F.; Luo, L.M.; Liu, J.W.; Yu, X.J. Bats as reservoirs of severe emerging infectious diseases. Virus Res. 2015, 205, 1–6. [Google Scholar] [CrossRef]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef]
- Han, B.A.; Schmidt, J.P.; Bowden, S.E.; Drake, J.M. Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 7039–7044. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, Z.; Li, S.; Yin, H. A Review on Zoonotic Pathogens Associated with Non-Human Primates: Understanding the Potential Threats to Humans. Microorganisms 2023, 11, 246. [Google Scholar] [CrossRef]
- Tomori, O.; Oluwayelu, D.O. Domestic Animals as Potential Reservoirs of Zoonotic Viral Diseases. Annu. Rev. Anim. Biosci. 2023, 11, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Kahn, R.E.; Richt, J.A. The pig as a mixing vessel for influenza viruses: Human and veterinary implications. J. Mol. Genet. Med. 2008, 3, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Curlin, J.; Remling-Mulder, L.; Moriarty, R.; Goff, K.; O’Connor, S.; Stenglein, M.; Marx, P.; Akkina, R. Cross-Species Transmission and Evolution of SIV Chimpanzee Progenitor Viruses Toward HIV-1 in Humanized Mice. Front. Microbiol. 2020, 11, 1889. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Govindarajulu, M.; Parise, R.S.; Neel, L.; Shankar, T.; Patel, S.; Lowery, P.; Smith, F.; Dhanasekaran, M.; Moore, T. Emerging SARS-CoV-2 Variants: A Review of Its Mutations, Its Implications and Vaccine Efficacy. Vaccines 2021, 9, 1195. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Rodriguez, I.; Buitrago-Garcia, D.; Simancas-Racines, D.; Zambrano-Achig, P.; Del Campo, R.; Ciapponi, A.; Sued, O.; Martinez-Garcia, L.; Rutjes, A.W.; Low, N.; et al. False-negative results of initial RT-PCR assays for COVID-19: A systematic review. PLoS ONE 2020, 15, e0242958. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Jameel, S.; Sarkar, S. India’s Battle against COVID-19: Progress and Challenges. Am. J. Trop. Med. Hyg. 2020, 103, 1343–1347. [Google Scholar] [CrossRef]
- Akinyemi, J.O.; Agunbiade, M.O.; Salawu, M.M.; Eniade, O.D.; Yaya, S.; Fawole, O.I. Perceptions of COVID-19 transmission risk and testing readiness in rural Southwest Nigeria. Sci. Afr. 2022, 17, e01334. [Google Scholar] [CrossRef]
- Morse, S.S.; Mazet, J.A.; Woolhouse, M.; Parrish, C.R.; Carroll, D.; Karesh, W.B.; Zambrana-Torrelio, C.; Lipkin, W.I.; Daszak, P. Prediction and prevention of the next pandemic zoonosis. Lancet 2012, 380, 1956–1965. [Google Scholar] [CrossRef]
- Zumla, A.; Rao, M.; Wallis, R.S.; Kaufmann, S.H.; Rustomjee, R.; Mwaba, P.; Vilaplana, C.; Yeboah-Manu, D.; Chakaya, J.; Ippolito, G.; et al. Host-directed therapies for infectious diseases: Current status, recent progress, and future prospects. Lancet Infect. Dis. 2016, 16, e47–e63. [Google Scholar] [CrossRef]
- Fahrni, M.L.; Ismail, I.A.; Refi, D.M.; Almeman, A.; Yaakob, N.C.; Saman, K.M.; Mansor, N.F.; Noordin, N.; Babar, Z.U. Management of COVID-19 vaccines cold chain logistics: A scoping review. J. Pharm. Policy Pract. 2022, 15, 16. [Google Scholar] [CrossRef]
- Feyisa, D. Cold Chain Maintenance and Vaccine Stock Management Practices at Public Health Centers Providing Child Immunization Services in Jimma Zone, Oromia Regional State, Ethiopia: Multi-Centered, Mixed Method Approach. Pediatr. Health Med. Ther. 2021, 12, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.A.; Workneh, B.D.; Kahissay, M.H. Knowledge, attitude and practice of vaccinators and vaccine handlers on vaccine cold chain management in public health facilities, Ethiopia: Cross-sectional study. PLoS ONE 2021, 16, e0247459. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate change increases cross-species viral transmission risk. Nature 2022, 607, 555–562. [Google Scholar] [CrossRef] [PubMed]
- The United States Agency for International Development Emerging Pandemic Threats PREDICT Project. Global Detection of Emerging Wildlife Viral Zoonoses. In Fowler’s Zoo and Wild Animal Medicine Current Therapy; Elsevier: Amsterdam, The Netherlands, 2018; Volume 9, pp. 110–116. [Google Scholar] [CrossRef]
- Esposito, M.M.; Turku, S.; Lehrfield, L.; Shoman, A. The Impact of Human Activities on Zoonotic Infection Transmissions. Animals 2023, 13, 1646. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, H.; Asif, A.; Fatima, M.; Rehman, Y. Potential role of viral metagenomics as a surveillance tool for the early detection of emerging novel pathogens. Arch. Microbiol. 2021, 203, 865–872. [Google Scholar] [CrossRef]
- Carroll, D.; Daszak, P.; Wolfe, N.D.; Gao, G.F.; Morel, C.M.; Morzaria, S.; Pablos-Mendez, A.; Tomori, O.; Mazet, J.A.K. The Global Virome Project. Science 2018, 359, 872–874. [Google Scholar] [CrossRef]
- Herrmann, J.; Blumenstock, J.S. Global health security: Training a public health workforce to combat international and domestic threats. J. Public Health Manag. Pract. 2014, 20 (Suppl. S5), S118–S119. [Google Scholar] [CrossRef]
- The Lancet Infectious, D. Addressing the global health security agenda. Lancet Infect. Dis. 2014, 14, 257. [Google Scholar] [CrossRef]
- Gongal, G. One Health approach in the South East Asia region: Opportunities and challenges. Curr. Top. Microbiol. Immunol. 2013, 366, 113–122. [Google Scholar] [CrossRef]
- Reaser, J.K.; Witt, A.; Tabor, G.M.; Hudson, P.J.; Plowright, R.K. Ecological countermeasures for preventing zoonotic disease outbreaks: When ecological restoration is a human health imperative. Restor. Ecol. 2021, 29, e13357. [Google Scholar] [CrossRef]
- Barbier, E.B. Habitat loss and the risk of disease outbreak. J. Environ. Econ. Manag. 2021, 108, 102451. [Google Scholar] [CrossRef] [PubMed]
- Stull, J.W.; Bjorvik, E.; Bub, J.; Dvorak, G.; Petersen, C.; Troyer, H.L. 2018 AAHA Infection Control, Prevention, and Biosecurity Guidelines. J. Am. Anim. Hosp. Assoc. 2018, 54, 297–326. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.M.; Wieland, B.; Knight, G.M.; Lines, J.; Naylor, N.R. The effectiveness of biosecurity interventions in reducing the transmission of bacteria from livestock to humans at the farm level: A systematic literature review. Zoonoses Public Health 2021, 68, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, J.H.; Rajamaki, B.; Ijaz, S.; Sauni, R.; Toomey, E.; Blackwood, B.; Tikka, C.; Ruotsalainen, J.H.; Kilinc Balci, F.S. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst. Rev. 2020, 5, CD011621. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 521–547. [Google Scholar] [CrossRef]
| Year | Disease | Pathogen | Place | Reservoir Hosts | Insect Vectors | Severity (Approximately) |
|---|---|---|---|---|---|---|
| 1347–1353 | Bubonic Plague | Yersinia pestis | Europe, Asia, North Africa | Rodents (primarily rats) | Fleas, human body lice | Pandemic, estimated 25–200 million deaths [10,11] |
| 1918–1920 | Spanish Flu | Influenza A virus (H1N1) | Global | Pigs | None | Pandemic, estimated 17–50 million deaths [5] |
| 1957–1958 | Asian Flu | Influenza A virus (H2N2) | Global | Ducks, geese | None | Pandemic, 1 million deaths [12] |
| 1968–1970 | Hong Kong Flu | Influenza A virus (H3N2) | Global | Pigs, birds | None | Pandemic, 1–4 million deaths [13] |
| 2003–2004 | SARS | SARS-CoV-1 | China, Hong Kong, Taiwan, Canada | Bats, civets | None | 8098 cases, 774 deaths [14] |
| 2009–2010 | Swine Flu | Influenza A virus (H1N1) | Global | Pigs | None | Pandemic, 151,700–575,400 deaths [15] |
| 2012-present | MERS | MERS-CoV | Middle East, South Korea | Bats, camels | None | 2499 cases, 858 deaths (as of 2019) [16] |
| 1997-present | Avian Flu | Influenza A virus (H5N1) | Asia, Africa, Middle East, Europe | Birds | None | 863 cases, 456 deaths (as of 2022) [17] |
| 2001-present | Nipah | Nipah virus | Bangladesh, India, Malaysia | Bats, pigs | None | Outbreaks with high mortality rates [18] |
| 2003–2004 | Avian Flu | Influenza A virus (H7N7) | Netherlands | Birds | None | 89 confirmed cases, 1 death [19] |
| 2013-present | Avian Flu | Influenza A virus (H7N9) | China | Birds | None | 1568 cases, 616 deaths [20] |
| 2014–2016 | Ebola | Ebola virus | West Africa | Bats, primates | None | 28,616 cases, 11,310 deaths [21] |
| 2015–2016 | Zika | Zika virus | Americas, Oceania | Primates | Mosquitoes | 500,000 cases estimated [22] |
| 2019-present | COVID-19 | SARS-CoV-2 | Global | Bats, pangolins (suspected) | None | Ongoing pandemic, over 6.8 million deaths (as of May 2023) [23] |
| 1999-present | West Nile | West Nile virus | Africa, Europe, Middle East, North America, West Asia | Birds | Mosquitoes | Varies by outbreak; in US: 51,607 cases, 2369 deaths (till 2019) [24] |
| 1931-present | Rift Valley fever | Rift Valley fever virus | Africa, Middle East | Livestock (cattle, sheep, goats) | Mosquitoes | Periodic outbreaks; can cause significant livestock losses and human illness [25] |
| Ancient-present | Rabies | Rabies virus | Global | Mammals (dogs, bats, etc.) | None | ~59,000 deaths annually worldwide [26] |
| 1970-present | Mpox (formerly Monkeypox) | Monkeypox virus | Central and West Africa, with outbreaks globally | Rodents, primates | None | 2022–2023 global outbreak: >85,000 cases, 89 deaths [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatia, B.; Sonar, S.; Khan, S.; Bhattacharya, J. Pandemic-Proofing: Intercepting Zoonotic Spillover Events. Pathogens 2024, 13, 1067. https://doi.org/10.3390/pathogens13121067
Bhatia B, Sonar S, Khan S, Bhattacharya J. Pandemic-Proofing: Intercepting Zoonotic Spillover Events. Pathogens. 2024; 13(12):1067. https://doi.org/10.3390/pathogens13121067
Chicago/Turabian StyleBhatia, Bharti, Sudipta Sonar, Seema Khan, and Jayanta Bhattacharya. 2024. "Pandemic-Proofing: Intercepting Zoonotic Spillover Events" Pathogens 13, no. 12: 1067. https://doi.org/10.3390/pathogens13121067
APA StyleBhatia, B., Sonar, S., Khan, S., & Bhattacharya, J. (2024). Pandemic-Proofing: Intercepting Zoonotic Spillover Events. Pathogens, 13(12), 1067. https://doi.org/10.3390/pathogens13121067







