Blastocystis spp. and Other Intestinal Parasites in Polish Soldiers Deployed to Lebanon and Iraq
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Study Area
2.4. Stool Examination
2.5. Molecular Assays for Blastocystis Identification
2.6. Statistical Methods
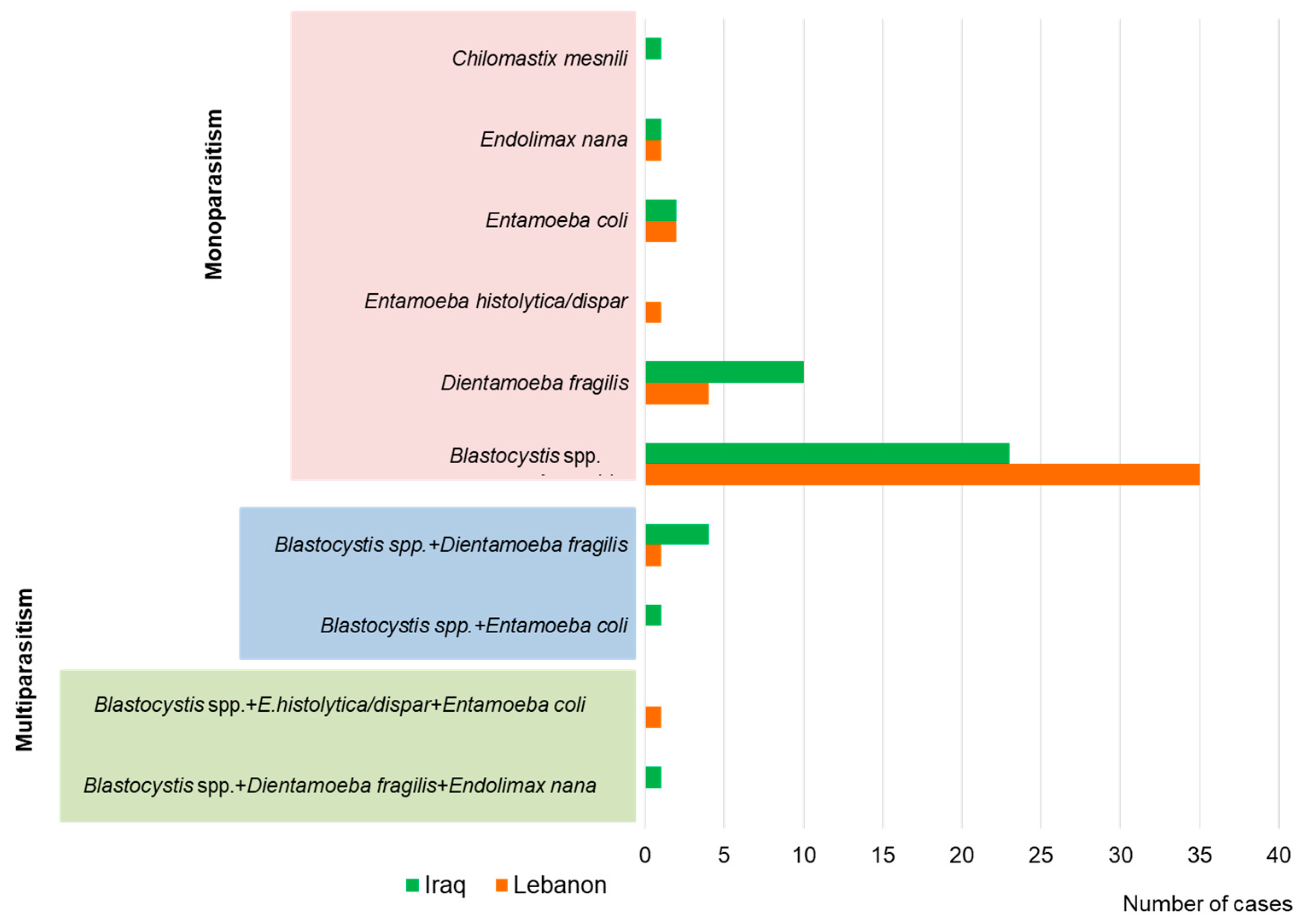
3. Results
4. Discussion
4.1. Prevalence of Blastocystis spp.
4.2. Co-Infection with Blastocystis spp.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilkman, K.; Pakkanen, S.H.; Lääveri, T.; Siikamäki, H.; Kantele, A. Travelers’ health problems and behavior: Prospective study with post-travel follow-up. BMC Infect. Dis. 2016, 16, 328. [Google Scholar] [CrossRef]
- Dunn, N.; Okafor, C.N.; Knizel, J.E. Travelers diarrhea (nursing). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sanchez, J.L.; Gelnett, J.; Petruccelli, B.P.; Defraites, R.F.; Taylor, D.N. Diarrheal disease incidence and morbidity among United States military personnel during short-term missions overseas. Am. J. Trop. Med. Hyg. 1998, 58, 299–304. [Google Scholar] [CrossRef]
- ten Hove, R.J.; van Esbroeck, M.; Vervoort, T.; van den Ende, J.; van Lieshout, L.; Verweij, J.J. Molecular diagnostics of intestinal parasites in returning travellers. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1045–1053. [Google Scholar] [CrossRef]
- Gefen-Halevi, S.; Biber, A.; Gazit, Z.; Amit, S.; Belausov, N.; Keller, N.; Smollan, G.; Schwartz, E. Persistent abdominal symptoms in returning travellers: Clinical and molecular findings. J. Travel Med. 2022, 29, taac011. [Google Scholar] [CrossRef]
- Jelinek, T.; Peyerl, G.; Löscher, T.; von Sonnenburg, F.; Nothdurft, H.D. The role of Blastocystis hominis as a possible intestinal pathogen in travellers. J. Infect. 1997, 35, 63–66. [Google Scholar] [CrossRef]
- Alfellani, M.A.; Stensvold, C.R.; Vidal-Lapiedra, A.; Onuoha, E.S.; Fagbenro-Beyioku, A.F.; Clark, C.G. Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop. 2013, 126, 11–18. [Google Scholar] [CrossRef]
- Caradonna, T.; Marangi, M.; Del Chierico, F.; Ferrari, N.; Reddel, S.; Bracaglia, G.; Normanno, G.; Putignani, L.; Giangaspero, A. Detection and prevalence of protozoan parasites in ready-to-eat packaged salads on sale in Italy. Food Microbiol. 2017, 67, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Clark, C.G. Molecular Identification and subtype analysis of Blastocystis. Curr. Protoc. Microbiol. 2016, 43, 20A.2.1–20A.2.10. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Velázquez, L.; Morán, P.; Serrano-Vázquez, A.; Portillo-Bobadilla, T.; González, E.; Pérez-Juárez, H.; Hernández, E.; Partida-Rodríguez, O.; Nieves-Ramírez, M.; Padilla, A.; et al. The regulatory function of Blastocystis spp. on the immune inflammatory response in the gut microbiome. Front. Cell. Infect. Microbiol. 2022, 12, 967724. [Google Scholar] [CrossRef] [PubMed]
- Kosik-Bogacka, D.; Lepczyńska, M.; Kot, K.; Szkup, M.; Łanocha-Arendarczyk, N.; Dzika, E.; Grochans, E. Prevalence, subtypes and risk factors of Blastocystis spp. infection among pre- and perimenopausal women. BMC Infect. Dis. 2021, 21, 1125. [Google Scholar] [CrossRef] [PubMed]
- Łanocha, A.; Łanocha-Arendarczyk, N.; Wilczyńska, D.; Zdziarska, B.; Kosik-Bogacka, D. Protozoan intestinal parasitic infection in patients with hematological malignancies. J. Clin. Med. 2022, 11, 2847. [Google Scholar] [CrossRef]
- Rudzińska, M.; Sikorska, K. Epidemiology of Blastocystis infection: A review of data from Poland in relation to other reports. Pathogens 2023, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Darwish, B.; Aboualchamat, G.; Al Nahhas, S. Molecular characterization of Blastocystis subtypes in symptomatic patients from the southern region of Syria. PLoS ONE 2023, 18, e0283291. [Google Scholar] [CrossRef]
- Downs, J.W.; Putnam, S.D.; Rockabrand, D.M.; El Okla, G.; Mostafa, M.; Monteville, M.R.; Antosek, L.E.; Herbst, J.; Tribble, D.R.; Riddle, M.S.; et al. A cross-sectional analysis of clinical presentations of and risk factors for enteric protozoan Infections in an Active Duty Population during Operation Iraqi Freedom. Trop. Dis. Travel Med. Vaccines 2015, 1, 2. [Google Scholar] [CrossRef][Green Version]
- Duda, A.; Kosik-Bogacka, D.; Lanocha-Arendarczyk, N.; Kołodziejczyk, L.; Lanocha, A. The prevalence of Blastocystis hominis and other protozoan parasites in soldiers returning from peacekeeping missions. Am. J. Trop. Med. Hyg. 2015, 92, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Stark, D.; Barratt, J.; Chan, D.; Ellis, J.T. Dientamoeba fragilis, the neglected trichomonad of the human bowel. Clin. Microbiol. Rev. 2016, 29, 553–580. [Google Scholar] [CrossRef] [PubMed]
- Cacciò, S.M. Molecular epidemiology of Dientamoeba fragilis. Acta Trop. 2018, 184, 73–77. [Google Scholar] [CrossRef]
- Wong, Z.W.; Faulder, K.; Robinson, J.L. Does Dientamoeba fragilis cause diarrhea? A systematic review. Parasitol. Res. 2018, 117, 971–980. [Google Scholar] [CrossRef]
- Clark, C.G.; van der Giezen, M.; Alfellani, M.A.; Stensvold, C.R. Chapter One—Recent developments in Blastocystis research. In Advances in Parasitology; Rollinson, D., Ed.; Academic Press: London, UK, 2013; pp. 1–32. [Google Scholar]
- Khaled, S.; Gantois, N.; Ayoubi, A.; Even, G.; Sawant, M.; El Houmayraa, J.; Nabot, M.; Benamrouz-Vanneste, S.; Chabé, M.; Certad, G.; et al. Blastocystis sp. prevalence and subtypes distribution amongst Syrian refugee communities living in north Lebanon. Microorganisms 2021, 9, 184. [Google Scholar] [CrossRef]
- Korzeniewski, K. Travel health prevention. Int. Marit. Health 2017, 68, 238–244. [Google Scholar] [CrossRef]
- Korzeniewski, K. The epidemiological situation in Iraq. Przegl. Epidemiol. 2006, 60, 845–855. [Google Scholar] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standard for Antimicrobial Susceptibility Testing; Document M100-S15; CLSI: Wayne, PA, USA, 2005. [Google Scholar]
- Garcia, L.S. Intestinal Protozoa: Amebae, Diagnostic Medical Parasitology; Garcia, L.S., Ed.; ASM Press: Washington, DC, USA, 2007; pp. 27–30. [Google Scholar]
- Korzeniewski, K.; Smoleń, A.; Augustynowicz, A.; Lass, A. Diagnostics of intestinal parasites in light microscopy among the population of children in eastern Afghanistan. Ann. Agric. Environ. Med. 2016, 23, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Scicluna, S.M.; Tawari, B.; Clark, C.G. DNA barcoding of Blastocystis. Protist 2006, 157, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Wu, Z.; Kimata, I.; Iseki, M.; Ali, I.K.; Hossain, M.B.; Zaman, V.; Haque, R.; Takahashi, Y. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol. Res. 2004, 92, 22–29. [Google Scholar] [PubMed]
- Scanlan, P.D.; Stensvold, C.R.; Cotter, P.D. Development and application of a Blastocystis subtype-specific PCR assay reveals that mixed-subtype infections are common in a healthy human population. Appl. Environ. Microbiol. 2015, 81, 4071–4076. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.T.; El-Bali, M.A.; Mohamed, A.A.; Abdel-Fatah, M.A.; El-Malky, M.A.; Mowafy, N.M.; Zaghlool, D.A.; Bakri, R.A.; Al-Harthi, S.A. Subtyping of Blastocystis sp. isolated from symptomatic and asymptomatic individuals in Makkah, Saudi Arabia. Parasites Vectors 2017, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- El Safadi, D.; Meloni, D.; Poirier, P.; Osman, M.; Cian, A.; Gaayeb, L.; Wawrzyniak, I.; Delbac, F.; El Alaoui, H.; Delhaes, L.; et al. Molecular epidemiology of Blastocystis in Lebanon and correlation between subtype 1 and gastrointestinal symptoms. Am. J. Trop. Med. Hyg. 2013, 88, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Riabi, T.; Haghighi, A.; Mirjalali, H.; Mohammad Ali Gol, S.; Karamati, S.A.; Ghasemian, M.; Bahadori Monfared, A.; Aghamohammadi, E.; Zojaji, H. Study of prevalence, distribution and clinical significance of Blastocystis isolated from two medical centers in Iran. Gastroenterol. Hepatol. Bed Bench 2017, 10 (Suppl. S1), S102–S107. [Google Scholar]
- Abdulwahhab, I.G. Phylogenetic tree of Blastocystis hominis in Iraqi children in Salah AL-Deen province, Iraq. Ann. Parasitol. 2022, 68, 391–398. [Google Scholar]
- Attah, A.O.; Sanggari, A.; Li, L.I.; Nik Him, N.A.I.I.; Ismail, A.H.; Meor Termizi, F.H. Blastocystis occurrence in water sources worldwide from 2005 to 2022: A review. Parasitol. Res. 2023, 122, 1–10. [Google Scholar] [CrossRef]
- Wadi, W.F.; Rathi, M.H.; Molan, A.L. The possible link between intestinal parasites and irritable bowel syndrome (IBS) in Diyala Province, Iraq. Ann. Parasitol. 2021, 67, 505–513. [Google Scholar]
- Hijjawi, N.; Zahedi, A.; Ryan, U. Molecular characterization of Entamoeba, Blastocystis and Cryptosporidium species in stool samples collected from Jordanian patients suffering from gastroenteritis. Trop. Parasitol. 2021, 11, 122–125. [Google Scholar]
- Jadallah, K.A.; Nimri, L.F.; Ghanem, R.A. Protozoan parasites in irritable bowel syndrome: A case-control study. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 201–207. [Google Scholar] [CrossRef]
- Jaran, A.S. Prevalence and seasonal variation of human intestinal parasites in patients attending hospital with abdominal symptoms in Northern Jordan. East. Mediterr. Health J. 2017, 22, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Palasuwan, A.; Palasuwan, D.; Mahittikorn, A.; Chiabchalard, R.; Combes, V.; Popruk, S. Subtype distribution of Blastocystis in communities along the Chao Phraya River, Thailand. Korean J. Parasitol. 2016, 54, 455–460. [Google Scholar] [CrossRef]
- Osman, M.; El Safadi, D.; Cian, A.; Benamrouz, S.; Nourrisson, C.; Poirier, P.; Pereira, B.; Razakandrainibe, R.; Pinon, A.; Lambert, C.; et al. Prevalence and risk factors for intestinal protozoan infections with Cryptosporidium, Giardia, Blastocystis and Dientamoeba among schoolchildren in Tripoli, Lebanon. PLoS Negl. Trop. Dis. 2016, 10, e0004496. [Google Scholar]
- Pietrzak-Makyła, B.; Korzeniewski, K.; Gładysz, P.; Lass, A. Detection and molecular characterization of Blastocystis species in Polish soldiers stationed in the Republic of Kosovo. Int. J. Mol. Sci. 2023, 24, 14100. [Google Scholar] [CrossRef] [PubMed]
- Leelayoova, S.; Rangsin, R.; Taamasri, P.; Naaglor, T.; Thathaisong, U.; Mungthin, M. Evidence of waterborne transmission of Blastocystis hominis. Am. J. Trop. Med. Hyg. 2004, 70, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Monteville, M.R.; Riddle, M.S.; Baht, U.; Putnam, S.D.; Frenck, R.W.; Brooks, K.; Moustafa, M.; Bland, J.; Sanders, J.W. Incidence, etiology, and impact of diarrhea among deployed US military personnel in support of Operation Iraqi Freedom and Operation Enduring Freedom. Am. J. Trop. Med. Hyg. 2006, 75, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008, 21, 639–665. [Google Scholar] [CrossRef] [PubMed]
- Greige, S.; El Safadi, D.; Bécu, N.; Gantois, N.; Pereira, B.; Chabé, M.; Benamrouz-Vanneste, S.; Certad, G.; El Hage, R.; Chemaly, M.; et al. Prevalence and subtype distribution of Blastocystis sp. isolates from poultry in Lebanon and evidence of zoonotic potential. Parasites Vectors 2018, 11, 389. [Google Scholar] [CrossRef]
- Hussein, E.M.; Hussein, A.M.; Eida, M.M.; Atwa, M.M. Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol. Res. 2008, 102, 853–860. [Google Scholar] [CrossRef]
- Salehi, R.; Haghighi, A.; Stensvold, C.R.; Kheirandish, F.; Azargashb, E.; Raeghi, S.; Kohansal, C.; Bahrami, F. Prevalence and subtype identification of Blastocystis isolated from humans in Ahvaz, Southwestern Iran. Gastroenterol. Hepatol. Bed Bench 2017, 10, 235–241. [Google Scholar]
- Hammood, A.M.; Ahmed, B.A.; Salman, Y.J. Blastocystis hominis detection among gastrointestinal disorders’ patients in Kirkuk Province using three different laboratory methods. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 883–901. [Google Scholar] [CrossRef]
- Forsell, J.; Granlund, M.; Samuelsson, L.; Koskiniemi, S.; Edebro, H.; Evengård, B. High occurrence of Blastocystis sp. subtypes 1-3 and Giardia intestinalis assemblage B among patients in Zanzibar, Tanzania. Parasites Vectors 2016, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, S.; Rafiei, A.; Saki, J.; Alizadeh, K. Prevalence and genotype characterization of Blastocystis hominis Among the Baghmalek People in Southwestern Iran in 2013–2014. Jundishapur J. Microbiol. 2015, 8, e23930. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Mirza, H.; Teo, J.D.; Wu, B.; Macary, P.A. Current views on the clinical relevance of Blastocystis spp. Curr. Infect. Dis. Rep. 2010, 12, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Lepczyńska, M.; Chen, W.C.; Dzika, E. Mysterious chronic urticaria caused by Blastocystis spp.? Int. J. Dermatol. 2016, 55, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Sarzhanov, F.; Dogruman-Al, F.; Santin, M.; Maloney, J.G.; Gureser, A.S.; Karasartova, D.; Taylan-Ozkan, A. Investigation of neglected protists Blastocystis sp. and Dientamoeba fragilis in immunocompetent and immunodeficient diarrheal patients using both conventional and molecular methods. PLoS Negl. Trop. Dis. 2021, 15, e0009779. [Google Scholar] [CrossRef] [PubMed]
- Oyofo, B.A.; Peruski, L.F.; Ismail, T.F.; el-Etr, S.H.; Churilla, A.M.; Wasfy, M.O.; Petruccelli, B.P.; Gabriel, M.E. Enteropathogens associated with diarrhea among military personnel during Operation Bright Star 96, in Alexandria, Egypt. Mil. Med. 1997, 162, 396–400. [Google Scholar] [CrossRef]
- Hamzé, M.; Naja, M.; Mallat, H. Biological analysis of workers in the food sector in north Lebanon. East. Mediterr. Health J. 2008, 14, 1425–1434. [Google Scholar] [PubMed]
- Piubelli, C.; Soleymanpoor, H.; Giorli, G.; Formenti, F.; Buonfrate, D.; Bisoffi, Z.; Perandin, F. Blastocystis prevalence and subtypes in autochthonous and immigrant patients in a referral centre for parasitic infections in Italy. PLoS ONE 2019, 14, e0210171. [Google Scholar] [CrossRef]
- Barratt, J.L.; Harkness, J.; Marriott, D.; Ellis, J.T.; Stark, D. A review of Dientamoeba fragilis carriage in humans: Several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes 2011, 2, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Jirků, M.; Kašparová, A.; Lhotská, Z.; Oborník, M.; Brožová, K.; Petrželková, K.J.; Samaš, P.; Kadlecová, O.; Stensvold, C.R.; Jirků, K. A cross-sectional study on the occurrence of the intestinal protist, Dientamoeba fragilis, in the gut-healthy volunteers and their animals. Int. J. Mol. Sci. 2022, 23, 15407. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Lebanon | Iraq | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M | F | T | M | F | T | M | F | T | |
| M/F: n (%) | 185 | 21 | 206 | 209 | 11 | 220 | 394 | 32 | 426 |
| Age (years): | |||||||||
| <35 | 53 | 11 | 64 | 52 | 5 | 57 | 105 | 16 | 121 |
| 35–45 | 98 | 9 | 107 | 108 | 4 | 112 | 206 | 13 | 219 |
| >46 | 34 | 1 | 35 | 49 | 2 | 51 | 83 | 3 | 86 |
| Species | Prevalence of Infection | Intensity of Infection (n/%) | ||
|---|---|---|---|---|
| + | ++ | +++ | ||
| Lebanon | ||||
| Blastocystis spp. | 17% | 17/48.5 | 11/31.4 | 7/20.0 |
| D. fragilis | 1.9% | 2/50.0 | 2/50.0 | - |
| Iraq | ||||
| Blastocystis spp. | 10.5% | 5/21.7 | 8/34.8 | 10/43.5 |
| D. fragilis | 4.5% | 5/50.0 | 4/40.0 | 1/10.0 |
| Soldiers Returning from: | Subtype of Blastocystis spp. | |||
|---|---|---|---|---|
| ST1 (n) | ST2 (n) | ST3 (n) | Unknown (n) | |
| single infection | ||||
| Lebanon | 5 | 7 | 14 | 5 |
| Iraq | 11 | 2 | 7 | - |
| co-infection with | ||||
| Blastocystis spp. and D. fragilis | ||||
| Lebanon | - | - | 1 | - |
| Iraq | 1 | - | 2 | - |
| Blastocystis spp. and D. fragilis and E. nana | ||||
| Lebanon | - | - | 1 | |
| Blastocystis spp. and Entamoeba histolytica/dispar and E. coli | ||||
| Lebanon | - | 1 | - | |
| Blastocystis spp. and E. coli | ||||
| Lebanon | 1 | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosik-Bogacka, D.I.; Korzeniewski, K.; Łanocha-Arendarczyk, N.; Korycińska, J.; Lepczyńska, M.; Dzika, E.; Marchelek-Myśliwiec, M. Blastocystis spp. and Other Intestinal Parasites in Polish Soldiers Deployed to Lebanon and Iraq. Pathogens 2024, 13, 271. https://doi.org/10.3390/pathogens13030271
Kosik-Bogacka DI, Korzeniewski K, Łanocha-Arendarczyk N, Korycińska J, Lepczyńska M, Dzika E, Marchelek-Myśliwiec M. Blastocystis spp. and Other Intestinal Parasites in Polish Soldiers Deployed to Lebanon and Iraq. Pathogens. 2024; 13(3):271. https://doi.org/10.3390/pathogens13030271
Chicago/Turabian StyleKosik-Bogacka, Danuta Izabela, Krzysztof Korzeniewski, Natalia Łanocha-Arendarczyk, Joanna Korycińska, Małgorzata Lepczyńska, Ewa Dzika, and Małgorzata Marchelek-Myśliwiec. 2024. "Blastocystis spp. and Other Intestinal Parasites in Polish Soldiers Deployed to Lebanon and Iraq" Pathogens 13, no. 3: 271. https://doi.org/10.3390/pathogens13030271
APA StyleKosik-Bogacka, D. I., Korzeniewski, K., Łanocha-Arendarczyk, N., Korycińska, J., Lepczyńska, M., Dzika, E., & Marchelek-Myśliwiec, M. (2024). Blastocystis spp. and Other Intestinal Parasites in Polish Soldiers Deployed to Lebanon and Iraq. Pathogens, 13(3), 271. https://doi.org/10.3390/pathogens13030271








