Abstract
Cercarial dermatitis (CD), or “Swimmer’s itch” as it is also known, is a waterborne illness caused by a blood fluke from the family Schistosomatidae. It occurs when cercariae of trematode species that do not have humans as their definitive host accidentally penetrate human skin (in an aquatic environment) and trigger allergic symptoms at the site of contact. It is an emerging zoonosis that occurs through water and is often overlooked during differential diagnosis. Some of the factors contributing to the emergence of diseases like CD are related to global warming, which brings about climate change, water eutrophication, the colonization of ponds by snails susceptible to the parasite, and sunlight exposure in the summer, associated with migratory bird routes. Therefore, with the increase in tourism, especially at fluvial beaches, it is relevant to analyze the current epidemiological scenario of CD in European countries and the potential regions at risk.
1. Introduction
Many water-borne diseases are emerging due to various factors. Among these emerging diseases is cercarial dermatitis (CD), a snail-borne zoonosis that can occur in both fresh and marine waters, resulting from the penetration of trematode cercariae into human skin [1,2]. One factor for this emergence is the increase in leisure and work activities in cercaria-infested freshwater bodies, where intermediate host snail habitats are present [1,3]. Other factors include climate change, which contributes to changes in aquatic bird and freshwater snail populations.
The first scientific reports using the term CD in Europe occurred around the 1930s, shortly after the name was coined in the United States in 1928 [4]. Nevertheless, this disease has acquired the popular name “swimmer’s itch”, reflecting the main symptom and how it is acquired [5]. The parasite invades the dermis, causing severe itching and other allergic reactions, such as oedema and erythema of the affected area [1,6]. CD is caused by Schistosomatidae in general, including the genus Schistosoma (the causative agents of schistosomiasis], but a large proportion of the species described in European studies are avian schistosomes [7], reflecting the high prevalence of infection in birds [8], which are able to penetrate human skin instead of their definitive host but do not complete their life cycle.
CD has global distribution, especially due to the migratory patterns of birds, which increases its risk of emergence in areas where no cases have been previously reported [9]. Most reported CD cases, including in Europe, and thus the primary focus of research, relate to freshwater bodies. However, studies conducted in other countries, including the USA, have highlighted cases of CD occurring in seawater [10]. In Africa and Asia, although species of the genus Schistosoma are the most studied due to their great impact on human health, other genera of Schistosomatidae have also been reported as responsible for CD cases [11,12]. Furthermore, avian parasite species primarily described in Europe have recently been reported elsewhere, such as Iran [12], with dispersion associated with migratory birds, but most species are reportedly confined to the European continent possibly due to parasite–intermediate host specificity [13].
Recently, some European countries have reported an increase in the number of CD cases, with new cases in locations with no previous records of infection [14]. The etiological agents were primarily parasite species of the genus Trichobilhazia, which have lymnaeid intermediate hosts [8]. Underreporting is a problem due to possible missed or overlooked diagnoses [7], making it difficult to measure the full economic impact [8], although the tourism industry emerges as a visibly affected sector due to beach closures [15].
This review focuses on the current epidemiological pattern of CD in Europe, including its broader impact on European countries, with a brief reference to the CD scenario outside the continent, based on an extensive review of international published papers on this subject.
2. Etiologic Agents
Avian schistosomes are commonly distributed worldwide, and the prevalence of infection in intermediate host snails may vary amongst localities and years, but no correlation was observed with factors such as latitude [8]. They are commonly associated with freshwater habitats such as lakes, ponds, and rivers, where birds and humans come into contact with water [1]. Globally, the main described etiological agents of CD are trematodes of the genus Trichobilharzia, but clinical manifestations in humans can be due to other avian schistosomes, such as genera Dendritobilharzia, Gigantobilharzia, Allobilharzia, Austrobilharzia, Anserobilharzia, Bilharziella, Macrobilharzia, Ornithobilharzia, and Jilinobilharzia [1,16]. Although the genus Schistosoma can also cause CD in some parts of the world [1], in this review, we will focus on avian schistosomes (mostly of the genus Trichobilharzia) as the main agent of CD in Europe.
In Europe, CD primarily occurs in freshwater bodies and arises from species of the genus Trichobilharzia [1,17], which rely on freshwater snails as intermediate hosts and birds as definitive hosts [2]. The cercariae of this group had been classified as Cercaria ocellata by La Valette (1855), a name that persisted for some time. However, bird parasites originally obtained from a given snail species were not always able to infect other snail species. This observed intermediate host specificity led to the discovery that CD agents belonged to different species, and the name C. ocellata was abandoned in Europe [18]. Recognized CD etiological agents on the European continent include the species Trichobilharzia szidati [19], Trichobilharzia regenti [20], Trichobilharzia franki [21], Trichobilharzia physellae (Austria) [22], Trichobilharzia anseri (Iceland) [23], Trichobilharzia mergi (Iceland) [24], and Trichobilharzia salmanticensis [25], the latter reported in only a few studies. One characteristic shared among the cercariae of this group is the presence of two pigmented eye spots and the furcocercarial shape (bifurcated tail), which enhance their swimming capacity and rapid tissue penetration [18].
The most prevalent species in Europe (Table 1) have been shown to exhibit specificity with snail hosts, such as T. szidati with lymnaeids Lymnaea stagnalis and Radix sp., T. franki with L. stagnalis and Radix sp., T. regenti with Radix sp. [1,2,18], and T. physellae with the physid Physa acuta [1,22]. Additionally, Bilharziela polonica demonstrates high specificity with the planorbid Planorbarius corneus [22,26].

Table 1.
Molecular sequences of some schistosomatids that can cause cercarial dermatitis deposited in the GenBank (Europe).
3. Intermediate and Definitive Hosts
The most commonly observed intermediate hosts are freshwater snails, such as planorbids, lymnaeids, and physids [7]. Lymnaea stagnalis is the most frequently reported intermediate host of avian schistosomes in Europe, with widespread geographical distribution across this continent [60]. Infection of this snail species with T. szidati has different effects in juveniles and adults: juvenile forms have their sexual development compromised, while adults increase their oviposition rate, seemingly due to disruption of snail neuroendocrine control [61]. Notably, a single infected L. stagnalis can generate CD cases, as it can release over 30,000 cercariae in one day [60]. In Europe, the epidemiology of human CD is significantly influenced by the prevalence of avian schistosomes in freshwater snails, with recorded prevalence rates ranging from 0.05% to over 50% [8].
Parasite and snail host specificity occurs through various mechanisms. Molecules released by snails may function as pheromones, attracting the parasites to suitable host snails [8,62]. In instances where miracidia successfully penetrate the snail’s mucosa, the host’s immunological response is primarily mediated by hemocytes through encapsulation of the parasite [8]. However, the parasite employs various strategies to evade the snail’s immune system, including mimicry or hemocyte activity suppression, thereby ensuring its successful development to the cercarial phase [8,63,64].
The definitive hosts of Trichobilharzia sp. are waterfowl, primarily Anseriform birds [18,65], of which, in Europe, the most common species are Anas platyrhynchos (mallard), Anas crecca (common teal), Anas clypeata (northern shoveller), Aythya fuligula (tufted duck), Cygnus olor (mute swan), Anser anser (greylag goose), and Mergus merganser (goosander). The migratory behaviour of these species facilitates the dissemination of avian schistosomes, notably the Trichobilharzia group, along their routes [23,45]. In experimental studies on the development of infection by T. regenti in the definitive host (ducks) and in a mouse model [20], it was observed that, after penetrating the skin of ducks and mice (mammal model), this species tends to reside in peripheral nerves, passing through the meninges and potentially causing neural symptoms, including leg paralysis [20,66]. The parasites end up in the nasal cavity, where oviposition occurs, as eggs (mature and immature) as well as miracidia that hatched outside the water were observed in the nasal cavity of the duck model [66].
Among migratory birds, the Egyptian goose has recently adapted to several European countries and it has been found with infections by other platyhelminths, including some trematodes not previously described [67,68]. This bird species is common in certain African countries, such as Rwanda, where it has been described as hosting species such as Trichobilharzia spinulata [69,70]. Another Anatid species introduced to Europe is Oxyura jamaicensis, originating from the USA and now present in multiple European countries [71,72]. While it remains uncertain whether this bird species can serve as a definitive host for the Tricobilharzia group in Europe, the possibility cannot be ruled out, given its susceptibility to other trematodes and the known capacity of T. regenti to infect a large number of Anatids [1,73]. Although swimmer’s itch caused by T. regenti has not been described so far, the possibility is not excluded, considering that Anatids are already recognized as hosts for digenic trematodes. Adaptation of T. regenti to European aquatic environments could expand the pool of definitive hosts and increase the potential for infection of accidental hosts, including humans. This possibility is substantiated by experimental investigations, as migration of the parasite among organs has been observed even in mammalian models, including in the lungs [45].
4. Parasite Development, Biology, and Pathology
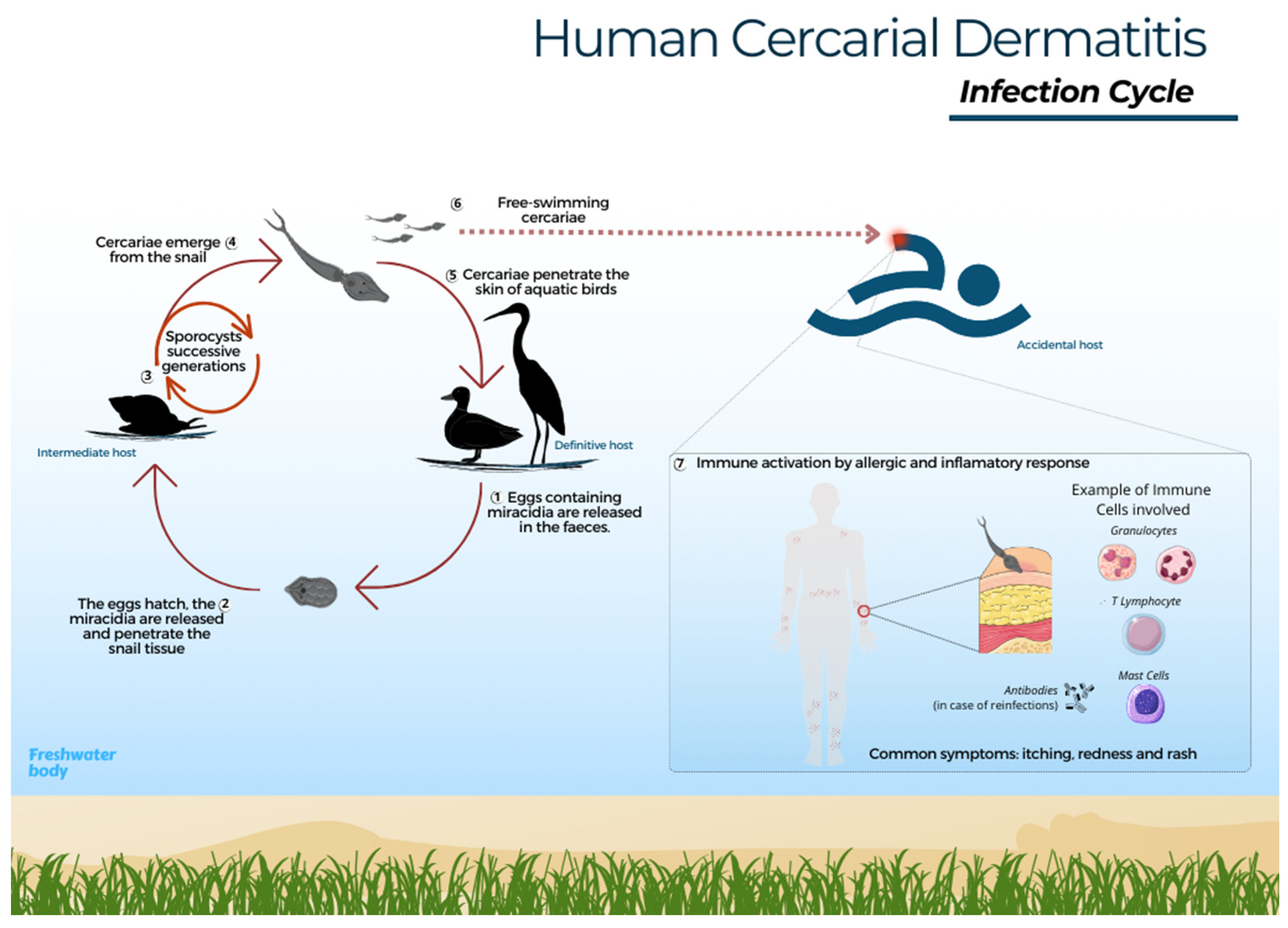
The parasite life cycle begins when the faeces of infected birds are released into water or near the shore, where the eggs hatch and the miracidia emerge and swim in search of a snail [18]. Trichobilharzia szidati miracidia survive approximately 20 h at 20 °C, while species of the genus Schistosoma last up to 16 h at 15 °C [19,74]. In general, species of the Trichobilharzia group have high specificity toward particular snail species [18]. Miracidia are attracted by glycoproteins present in the specific snail host and ultimately penetrate the cephalopodal region [18,75]. After miracidia penetration, the sporocyst mother, and sometimes the sporocyst daughter, stage develop and eventually produce cercariae [8,76]. Upon release from the snail, the cercarial lifespan depends on water temperature and can last up to a full day [77]. Thus, at the first opportunity to invade any dermal tissue, avian or mammalian, these cercariae begin a mechanical and chemical process of penetration by losing their tail and releasing enzymes to facilitate skin perforation (Figure 1) [13,78].
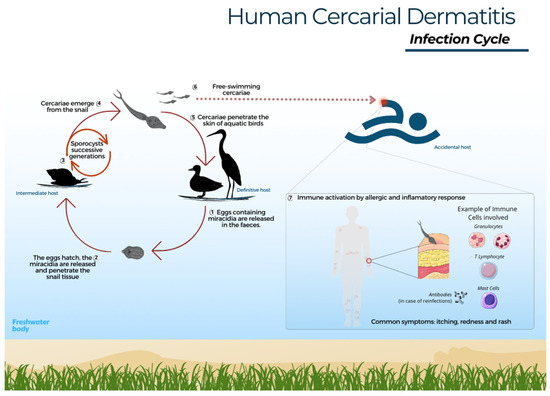
Figure 1.
Human cercarial dermatitis – infection cycle. 1- Eggs found in the faeces of infected aquatic birds hatch into miracidia upon contact with water. 2- Miracidia then seek out a specific snail host and penetrate its mucosa. 3- Within the snail, the cycle progresses through the sporocyst phase and subsequent generations; 4- Emergence of infectious cercariae. 5- These cercariae penetrate the skin of the definitive avian host, shedding their tail. Then, schistosomula migrate through blood vessels to various organs, where they develop into adult forms, initiating the sexual phase. 6- Free-swimming cercariae may penetrate human skin, leading to dermatitis. 7- The immune system responds with an allergic and inflammatory reaction, involving the recruitment of neutrophils, mast cells, eosinophils, and T lymphocytes; these cells release cytokines to regulate the inflammatory process and aid in elimination of the parasite.
In birds, schistosomatid larvae can be observed in visceral or nasal locations, depending on the species. In other words, the cercariae develop into schistosomula and migrate through the bloodstream to the organs, and in some species, even to the central nervous system toward the nasal cavity, and after maturing into adults, they begin their reproductive phase [1,79].
When cercariae come into contact with humans, an accidental host, a hypersensitivity reaction occurs, followed by an inflammatory response. Upon reinfection, there may be a subsequent release of antibodies and interleukins to help eliminate the parasite [13,80]. In other words, the immune system responds by activating cells and defence pathways against invasion. Some insights about the infection in mammals have been achieved through experimental animal studies, where it was possible to observe the migration of cercariae to other organs [5]. Experimental studies in mice have shown that the cells involved in the response to, for example, T. regenti, are neutrophils, eosinophils, mast cells, and T lymphocytes, which, by releasing inflammatory mediators, cause itching and local tissue damage [17,80]. The immune response modulation aims to eliminate the invasive cercariae, leading to the characteristic symptoms of itching, redness, and rash associated with CD. Penetration by a large number of cercariae may cause increased pruritus and intensify the signs and symptoms of CD [13]. Research thus suggests that, in an accidental host, cercariae are eliminated by the immune system and die in the dermis; however, cases of entry into the blood vessels and migration between organs have been reported in experimental mammal models [17,81]. In the accidental host, the parasite does not differentiate sexually, and the cycle is interrupted.
5. Cercarial Dermatitis in Europe: What Do We Know?
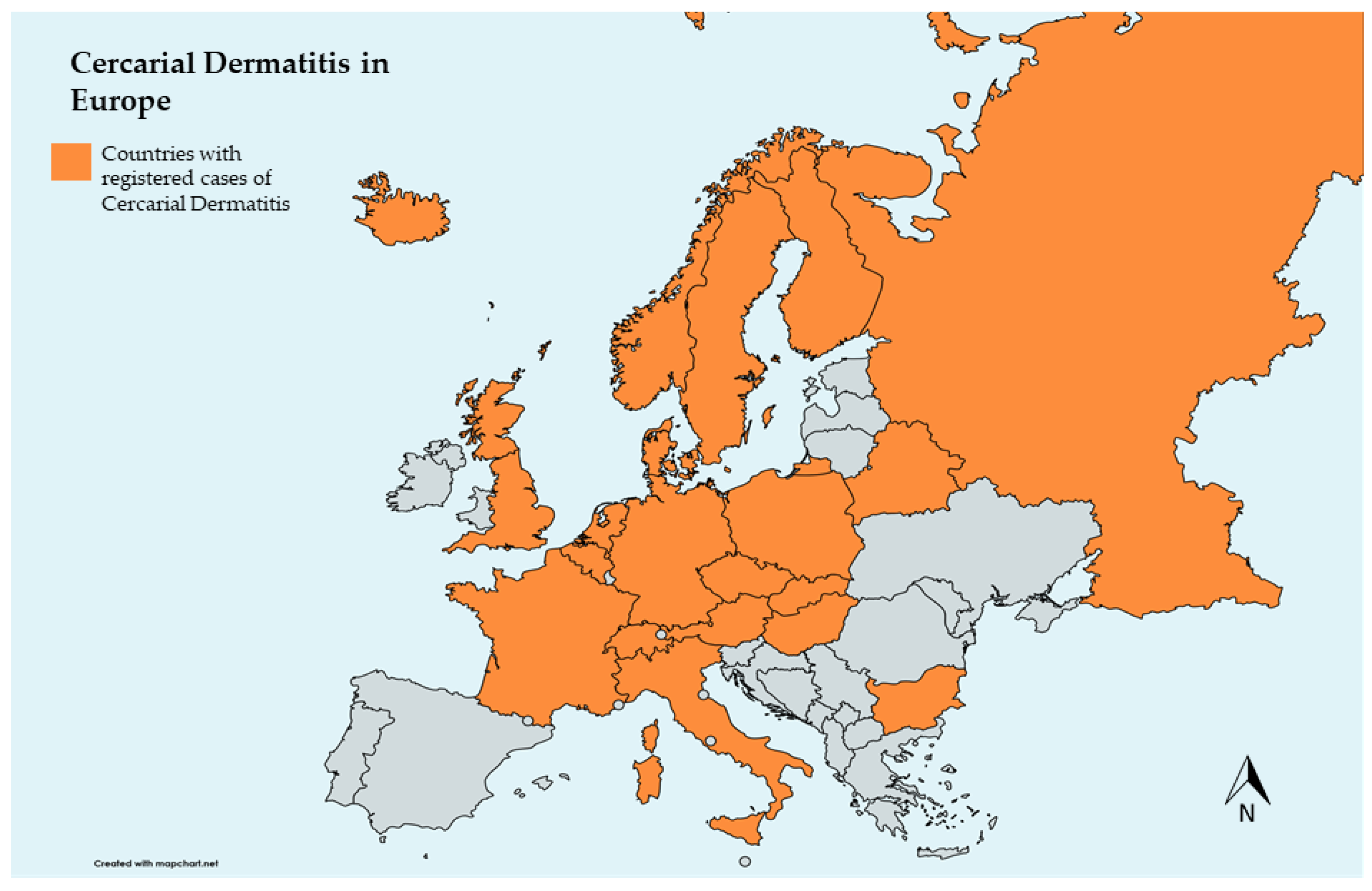
The emergence of diseases in Europe has been increasingly discussed due to their public health and economic impact [82], many of which are zoonotic [83]. With significantly higher tourist influx, notably to freshwater beaches, there has been a recent increase in CD case notifications in some European countries, with the majority occurring in the summer (Figure 2) [3,42,84,85]. Considering climate change and schistosomatid trematode developmental characteristics, European countries face a potential risk for CD increase [86]. Similarities between aquatic habitats used for recreation in Europe could facilitate the spread of these parasites, particularly, but not only, between neighboring countries due to the expansion of migratory birds toward northern regions [87]. In particular, rising water temperatures favor the spread of the parasite and consequent infection, whether in the definitive or accidental host [13]. Thus, CD is becoming an emerging, and sometimes reemerging disease that may have a negative impact, especially on tourism, bringing the need for control or mitigation measures. This impact may be underestimated due to undiagnosed cases. Therefore, it is important to encourage clinicians to obtain an inclusive medical history encompassing CD, encouraging its notification, to facilitate investigations in affected regions. The negative effects of CD on human health could be mitigated by enhancing freshwater users’ understanding of the infection and knowledge about risk areas, including water quality, snail presence, and bird populations [1].
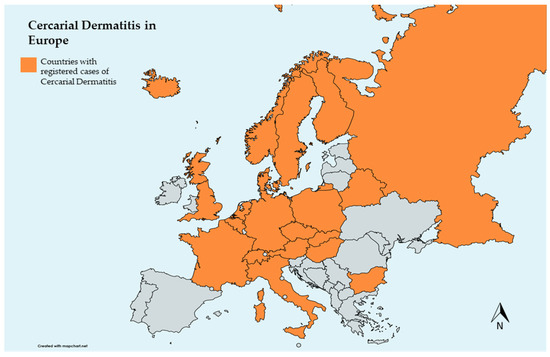
Figure 2.
Map of European countries that registered cases of cercarial dermatitis (bicontinental countries were excluded, except for Russia).
Studies conducted in Europe provide insights into the species involved in CD across the continent, encompassing birds, snails, and parasites [46]. Some investigations often employ molecular approaches, revealing similar species in neighbouring countries or even in those not sharing borders [28,88]. In general, the studies have consistently identified the prevalent intermediate hosts, specifically the genera Radix and Lymnaea, associated with causative agents such as T. szidati, T. franki, T. regenti, T. physellae (Austria), T. anseri (Iceland), T. mergi (Iceland), and B. polonica, as the primary contributors to CD in Europe [22,23,24,56,89]. The studies identified were not carried out in all European countries, but mainly in central and northern Europe, indicating significant gaps in the evaluation of the full extent of this zoonosis, maybe due to undiagnosed cases masking the true CD prevalence in other countries, such as Portugal. Research presents challenges, inclusively due to fluctuations in snail populations induced by environmental changes [86,90]; however, further research is crucial, particularly considering that some migratory bird populations are becoming resident in southern regions [91] with favourable climate conditions [92].
Despite the initial awareness of L. stagnalis infected with T. szidati in northern Polish lakes and subsequent human risk assessment tests in 2004 [93], molecular studies confirmed the presence of both T. szidati and T. regenti only several years later, from 2018 [54,94]. This finding allowed the characterization of snails (L. stagnalis, Radix balthica/labiata, Radix auricularia, and P. corneus) infected with digenic larvae (B. polonica and Trichobilharzia sp.) [89]. In Germany, studies on freshwater areas, such as Lake Baldeney, Ruhr River, and Lake Tunisee, confirmed the presence of T. franki in the latter, emphasizing the need for prior knowledge for preventive measures in recreational waters [21,79,95,96]. In the Netherlands, molecular analysis has been employed to detect the presence of Trichobilharzia sp. in water at recreational bathing sites, drawing attention to the potential correlation between snail research and environmental investigations [97,98], deepening our understanding of the CD scenario in the country.
Research conducted across European regions has elucidated the substantial role of avian species in CD dissemination. De Gentile et al. [99] already commented on the possibility of CD emergence in several countries. French investigations into bird migration patterns and their interactions with aquatic habitats, particularly at Lake Annecy, have led to various control interventions to mitigate the negative impact on tourism [42,100], such as bird hunting, given the diverse avifauna (migratory and non-migratory), and mechanical snail control [44]. However, other lakes, such as Lake Der-Chantecoq, experienced an exponential increase in the emergence of CD cases within a few years at the beginning of the 21st century [42]. Phylogenetic studies undertaken in France have led to a deeper understanding of parasite haplotype diversity, including in relation to other countries and continents, given the significant number of migratory birds in the region [23,44,46]. In Switzerland and France, Lake Geneva remains a potential source of CD due to its bird population and migratory fluxes, as they straddle the border, with potential infection points distributed throughout its length [58,101,102].
Certain regions have experienced outbreaks of CD, indicating its emerging potential. In Belgium, Lake Eau d’Heure has had numerous reported CD cases caused by T. franki, with R. auricularia identified as the intermediate host [6]. But recently, in Kampenhout, the increase of CD cases had led to the identification of T. regenti in the country, underscoring the importance of monitoring infected snails to avoid outbreaks [30]. The challenge of early Trichobilharzia detection in some countries, such as Belgium, may stem from the low abundance of intermediate hosts that may vary seasonally [5]. Slovakia presents a notable example, where evidence of the presence of T. franki was only obtained due to outbreak monitoring, specifically at Lake Košice [103]. Until then, studies in the country had been consistently integrated into central European investigations, focusing on the general exploration of trematodes in nations sharing bodies of freshwater [104].
Austria, like several other European countries, has experienced numerous cases of CD, although limited studies had been conducted by the end of the 20th century [105]. Efforts were made to estimate the risk in humans through population questionnaires, providing a valuable means of information dissemination to the community [106]. Molecular studies have since revealed evidence of T. szidati and T. franki in the country, with L. stagnalis and Radix sp. serving as intermediate hosts, respectively. Additionally, the presence of T. physellae has been confirmed, with the snail P. acuta acting as an intermediate host [22,27]. In Hungary, despite some reported cases, the disease has been generally neglected, and only recently have molecular studies been conducted to identify involved species and their distribution [2]. These studies found CD to be caused by Schistosoma turkestanicum, with deer as the definitive host, in the Danube River [107]. Furthermore, eggs and adults of B. polonica, Trichobilharzia sp., and Dendritobilharzia pulverulenta were found in organs from bird carcasses, confirming the presence of these trematodes in the country [2,40].
Belarus and Russia have centered their molecular studies around Lake Naroch, located in Belarus [49,74]. The lake, identified as a hotspot with a high number of CD cases, has implemented extensive mollusc elimination measures, particularly targeting L. stagnalis [108]. Similarly, Italy has faced outbreaks at various lakes since the initial identification of the infection in the country, leading to the molecular discovery of T. franki in Radix sp. snails at Lake Vico [52]. The significance of monitoring infection was demonstrated by subsequent outbreaks involving the same trematode species occurring in Lake Albano years later [14].
In countries such as Spain and Portugal, which have water sources that can be considered at risk, studies focused only on human and livestock trematodes [109,110]. Both countries have faced outbreaks of urinary schistosomiasis, Portugal in the 1920s in the Algarve region [111] and Spain, recently, with autochthonous cases in Almería [112]. However, at the end of the 20th century, Simón Vicente and Simón Martin referred to CD cases in Salamanca caused by T. salmanticensis [25]. Soldanová et al. [5] points out that the greater number of studies in some countries compared to others may be because their research centers are more focused on CD, which would explain why countries with the same characteristics have few or no studies on this subject.
6. An Overview of CD Outside Europe
Countries outside Europe may exhibit similar CD agents and challenges but also differences, as follows. Both the USA and Canada use the cercariometry approach for detection of species involved in CD [113,114], which is not a tool commonly used in Europe. These countries also feature the presence of Lymnaea sp., but, in contrast, Physa sp. is also an important intermediate host, including T. physellae [35,115]. Canada faces challenges with neglected diagnoses of CD; however, people actively participate in citizen science studies, aiding in understanding and mapping the disease’s distribution, as well as predicting its prevalence [116]. In South America, where CD cases are relatively underreported, molecular methodologies are the predominant detection standard [117]. This is exemplified by Ebbs et al. [88], who, in studies of ducks of the genus Anas migrating from South Africa, New Zealand, and Argentina to North America, observed a considerable infection rate with Trichobilharzia querquedulae and showed that strains were not geographically restricted, punctuating its widespread dissemination. The importance of monitoring avian migratory routes was also confirmed by Ashrafi et al. [12], who documented the emergence of T. franki in Iran and molecularly matched the parasite genotype thus far previously exclusive to Europe. As in Europe, various studies have reinforced the need to understand the role of freshwater snails in the development of fluke diseases, such as CD, and devise measures to control the disease without adversely affecting fauna. Climatic factors have also emerged as a major concern, directly and indirectly influencing pathogens and hosts, thereby impacting the prevalence and distribution of CD [92].
7. Concluding Remarks
The economic impacts attributed to CD in Europe predominantly affect the tourism sector, particularly with the growing number of individuals engaging in recreational water activities [42,79]. Depending on the level of cercaria infestation in water bodies, certain fluvial beaches may temporarily close as a protective measure until conditions allow for their reopening [89,103]. It is essential to consider the potential occupational risks associated with CD, particularly associated with farming, notably rice fields, where intermediate and vertebrate hosts are present. These farmer workers can experience prolonged and direct exposure to parasites over consecutive hours and days, increasing the likelihood of work absenteeism due to allergic manifestations [1]. This scenario reflects situations observed in Asian countries where CD endemicity is primarily attributed to continuous exposure among farmer workers [11,118]. In response to outbreaks, countries are conducting investigations to assess the CD situation, implementing measures that range from questionnaire-based surveys [106] to cohort follow-up [102], and exploring the potential use of commercially available skin creams as protection for bathers, fishers, and workers [119].
CD is an emerging and re-emerging zoonotic disease in many countries, and in the current context of climate change and global warming, the study of this disease becomes even more relevant. Preventive and control measures should aim to sensitize and educate the population about waterborne diseases without inducing panic to the extent of interrupting water use. Tourism and rice culture, significant regional economic sectors, underscore the importance of maintaining healthy environments by demonstrating concern for both human and animal health through monitoring natural waterbodies. Enhanced collaboration between European countries and institutions investing in knowledge and CD control measures is advisable. In essence, a One Health approach is crucial, whereby molecular detection, characterization, and potential preventive measures can yield more accurate prevalence figures and facilitate more effective interventions.
Author Contributions
Conceptualization, S.B. and M.T.B.; methodology, S.B.; M.C. and M.T.B.; software, M.T.B.; writing—original draft preparation, S.B. and M.T.B.; writing—review and editing, S.B., M.C., P.M.F., I.L.M. and M.T.B.; supervision, S.B.; project administration, M.C.; funding acquisition, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by Portuguese Foundation for Science and Technology for funds to GHTM—UID/04413/2020 and LA-REAL—LA/P/0117/2020 and Project PTDC-2022.01349.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Horák, P.; Mikeš, L.; Lichtenbergová, L.; Skála, V.; Soldánová, M.; Brant, S.V. Avian Schistosomes and Outbreaks of Cercarial Dermatitis. Clin. Microbiol. Rev. 2015, 28, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Juhász, A.; Majoros, G.; Cech, G. Threat of cercarial dermatitis in Hungary: A first report of Trichobilharzia franki from the mallard (Anas platyrhynchos) and European ear snail (Radix auricularia) using molecular methods. Parasitol. Parasites Wildl. 2022, 18, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, K.; Nouroosta, A.; Sharifdini, M.; Mahmoudi, M.R.; Rahmati, B.; Brant, S.V. Genetic diversity of an avian nasal schistosome causing cercarial dermatitis in the Black Sea-Mediterranean migratory route. Parasitol. Res. 2018, 117, 3821–3833. [Google Scholar] [CrossRef] [PubMed]
- Cort, W.W. Studies on Schistosome Dermatitis. Am. J. Epidemiol. 1950, 52, 251–307. [Google Scholar] [CrossRef]
- Soldánová, M.; Selbach, C.; Kalbe, M.; Kostadinova, A.; Sures, B. Swimmer’s itch: Etiology, impact, and risk factors in Europe. Trends Parasitol. 2013, 29, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Caron, Y.; Cabaraux, A.; Marechal, F.; Losson, B. Swimmer’s Itch in Belgium: First Recorded Outbreaks, Molecular Identification of the Parasite Species and Intermediate Hosts. Vector-Borne Zoonotic Dis. 2017, 17, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Brant, S.V.; Loker, E.S. Discovery-based studies of schistosome diversity stimulate new hypotheses about parasite biology. Trends Parasitol. 2013, 29, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Horák, P.; Kolářová, L. Snails, waterfowl and cercarial dermatitis. Freshw. Biol. 2011, 56, 779–790. [Google Scholar] [CrossRef]
- Hoeffler, D.F. Cercarial Dermatitis. Arch. Environ. Health Int. J. 1974, 29, 225–229. [Google Scholar] [CrossRef]
- Brant, S.V.; Cohen, A.N.; James, D.; Hui, L.; Hom, A.; Loker, E.S. Cercarial Dermatitis Transmitted by Exotic Marine Snail. Emerg. Infect. Dis. 2010, 16, 1357–1365. [Google Scholar] [CrossRef]
- Rao, V.G.; Dash, A.P.; Agrawal, M.C.; Yadav, R.S.; Anvikar, A.R.; Vohra, S.; Bhondeley, M.K.; Ukey, M.J.; Das, S.K.; Minocha, R.K.; et al. Cercarial dermatitis in central India: An emerging health problem among tribal communities. Ann. Trop. Med. Parasitol. 2007, 101, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, K.; Sharifdini, M.; Darjani, A.; Brant, S.V. Migratory routes, domesticated birds and cercarial dermatitis: The distribution of Trichobilharzia franki in Northern Iran. Parasite 2021, 28, 4. [Google Scholar] [CrossRef] [PubMed]
- Kolářová, L.; Horák, P.; Skírnisson, K.; Marečková, H.; Doenhoff, M. Cercarial Dermatitis, a Neglected Allergic Disease. Clin. Rev. Allergy Immunol. 2013, 45, 63–74. [Google Scholar] [CrossRef] [PubMed]
- De Liberato, C.; Berrilli, F.; Bossù, T.; Magliano, A.; Montalbano Di Filippo, M.; Di Cave, D.; Sigismondi, M.; Cannavacciuolo, A.; Scaramozzino, P. Outbreak of swimmer’s itch in Central Italy: Description, causative agent and preventive measures. Zoonoses Public Health 2019, 66, 377–381. [Google Scholar] [CrossRef]
- Kolářová, L. Schistosomes causing cercarial dermatitis: A mini-review of current trends in systematics and of host specificity and pathogenicity. Folia Parasitol. 2007, 54, 81–87. [Google Scholar] [CrossRef]
- Lashaki, E.K.; Teshnizi, S.H.; Gholami, S.; Fakhar, M.; Brant, S.V.; Dodangeh, S. Global prevalence status of avian schistosomes: A systematic review with meta-analysis. Parasite Epidemiol. Control 2020, 9, e00142. [Google Scholar] [CrossRef] [PubMed]
- Macháček, T.; Turjanicová, L.; Bulantová, J.; Hrdý, J.; Horák, P.; Mikeš, L. Cercarial dermatitis: A systematic follow-up study of human cases with implications for diagnostics. Parasitol. Res. 2018, 117, 3881–3895. [Google Scholar] [CrossRef]
- Horák, P.; Kolářová, L.; Adema, C.M. Biology of the schistosome genus Trichobilharzia. Adv. Parasitol. 2002, 52, 155–233. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, W. Biologie und Entwicklung von Trichobilharzia szidati n. sp. (Trematoda, Schistosomatidae), einem Erreger von Dermatitis beim Menschen. Z. Parasitenkd. 1952, 15, 203–266. [Google Scholar] [CrossRef]
- Horák, P.; Dvořák, J.; Kolářová, L.; Trefil, L. Trichobilharzia regenti, a pathogen of the avian and mammalian central nervous systems. Parasitology 1999, 119, 577–581. [Google Scholar] [CrossRef]
- Müller, V.; Kimmig, P. Trichobilharzia franki n. sp.—The cause of swimmer’s dermatitis in southwest German dredged lakes. Appl. Parasitol. 1994, 35, 12–31. Available online: https://pubmed.ncbi.nlm.nih.gov/8173581/ (accessed on 14 December 2023). [PubMed]
- Helmer, N.; Blatterer, H.; Hörweg, C.; Reier, S.; Sattmann, H.; Schindelar, J.; Szucsich, N.U.; Haring, E. First Record of Trichobilharzia physellae (Talbot, 1936) in Europe, a Possible Causative Agent of Cercarial Dermatitis. Pathogens 2021, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Jouet, D.; Kolářová, L.; Patrelle, C.; Ferté, H.; Skírnisson, K. Trichobilharzia anseri n. sp. (Schistosomatidae: Digenea), a new visceral species of avian schistosomes isolated from greylag goose (Anser anser L.) in Iceland and France. Infect. Genet. Evol. 2015, 34, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kolářová, L.; Skírnisson, K.; Ferté, H.; Jouet, D. Trichobilharzia mergi sp. nov. (Trematoda: Digenea: Schistosomatidae), a visceral schistosome of Mergus serrator (L.) (Aves: Anatidae). Parasitol. Int. 2013, 62, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Simon Martin, F.; Simon Vicente, F. The life cycle of Trichobilharzia salmanticensis n. sp. (Digenea: Schistosomatidae), related to cases of human dermatitis. Res. Rev. Parasitol. 1999, 59, 13–18. [Google Scholar] [CrossRef]
- Rizevsky, S.V.; Cherviakovsky, E.M.; Kurchenko, V.P. Molecular taxonomic identification of Schistosomatidae from Naroch Lake and Polonevichi Lake in Belarus. Biochem. Syst. Ecol. 2011, 39, 14–21. [Google Scholar] [CrossRef]
- Reier, S.; Haring, E.; Billinger, F.; Blatterer, H.; Duda, M.; Gorofsky, C.; Grasser, H.P.; Heinisch, W.; Hörweg, C.; Kruckenhauser, L.; et al. First confirmed record of Trichobilharzia franki Müller & Kimmig, 1994, from Radix auricularia (Linnaeus, 1758) for Austria. Parasitol. Res. 2020, 119, 4135–4141. [Google Scholar] [CrossRef] [PubMed]
- Semyenova, S.K.; Chrisanfova, G.G.; Guliaev, A.S.; Yesakova, A.P.; Ryskov, A.P. Structural and Population Polymorphism of RT-Like Sequences in Avian Schistosomes Trichobilharzia szidati (Platyhelminthes: Digenea: Schistosomatidae). Biomed. Res. Int. 2015, 2015, 315312. [Google Scholar] [CrossRef] [PubMed]
- Chrisanfova, G.; Mozharovskaya, L.; Zhukova, T.; Nefedova, D.; Semyenova, S. Non-coding Regions of Mitochondrial DNA and the cox1 Gene Reveal Genetic Variability Among Local Belarusian Populations of the Causative Agent of Cercarial Dermatitis, Bird Schistosome Trichobilharzia szidati (Digenea: Schistosomatidae). Acta Parasitol. 2021, 66, 1193–1203. [Google Scholar] [CrossRef]
- Schols, R.; Smitz, N.; Vanderheyden, A.; Huyse, T. Expanding the swimmer’s itch pool of the Benelux: A first record of the neurotropic Trichobilharzia regenti and potential link to human infection. Parasites Vectors. 2024, 17, 1. [Google Scholar] [CrossRef]
- Dvořák, J.; Vaňáčová, Š.; Hampl, V.; Flegr, J.; Horák, P. Comparison of European Trichobilharzia species based on ITS1 and ITS2 sequences. Parasitology 2002, 124, 307–313. [Google Scholar] [CrossRef]
- Lockyer, A.E.; Olson, P.D.; Østergaard, P.; Rollinson, D.; Johnston, D.A.; Attwood, S.W.; Southgate, V.R.; Horak, P.; Snyder, S.D.; Le, T.H.; et al. The phylogeny of the Schistosomatidae based on three genes with emphasis on the interrelationships of Schistosoma Weinland, 1858. Parasitology 2003, 126, 203–224. [Google Scholar] [CrossRef]
- Rudolfová, J.; Hampl, V.; Bayssade-Dufour, C.; Lockyer, A.E.; Littlewood, D.T.J.; Horák, P. Validity reassessment of Trichobilharzia species using Lymnaea stagnalis as the intermediate host. Parasitol. Res. 2005, 95, 79–89. [Google Scholar] [CrossRef]
- Rudolfová, J.; Littlewood, D.T.; Sitko, J.; Horák, P. Bird schistosomes of wildfowl in the Czech Republic and Poland. Folia Parasitol. 2007, 54, 88–93. [Google Scholar] [CrossRef]
- Brant, S.V.; Loker, E.S. Molecular Systematics of the Avian Schistosome Genus Trichobilharzia (Trematoda: Schistosomatidae) in North America. J. Parasitol. 2009, 95, 941–963. [Google Scholar] [CrossRef]
- Aldhoun, J.A.; Podhorský, M.; Holická, M.; Horák, P. Bird schistosomes in planorbid snails in the Czech Republic. Parasitol. Int. 2012, 61, 250–259. [Google Scholar] [CrossRef]
- Christiansen, A.Ø.; Olsen, A.; Buchmann, K.; Kania, P.W.; Nejsum, P.; Vennervald, B.J. Molecular diversity of avian schistosomes in Danish freshwater snails. Parasitol. Res. 2016, 115, 1027–1037. [Google Scholar] [CrossRef]
- Al-Jubury, A.; Duan, Y.; Kania, P.; Tracz, E.; Bygum, A.; Jørgensen, L.; Horák, P.; Buchmann, K. Avian schistosome species in Danish freshwater lakes: Relation to biotic and abiotic factors. J. Helminthol. 2021, 95, e22. [Google Scholar] [CrossRef]
- Lawton, S.P.; Lim, R.M.; Dukes, J.P.; Cook, R.T.; Walker, A.J.; Kirk, R.S. Identification of a major causative agent of human cercarial dermatitis, Trichobilharzia franki (Müller and Kimmig 1994), in southern England and its evolutionary relationships with other European populations. Parasites Vectors 2014, 7, 277. [Google Scholar] [CrossRef]
- Juhász, A.; Barlow, S.E.J.; Williams, H.; Johnson, B.; Walsh, N.D.; Cunningham, L.C.; Jones, S.; LaCourse, E.J.; Stothard, J.R. A report of Bilharziella polonica cercariae in Knowsley Safari, Prescot, United Kingdom, with notes on other trematodes implicated in human cercarial dermatitis. J. Helminthol. 2022, 96, e79. [Google Scholar] [CrossRef]
- Aldhoun, J.A.; Faltýnková, A.; Karvonen, A.; Horák, P. Schistosomes in the North: A unique finding from a prosobranch snail using molecular tools. Parasitol. Int. 2009, 58, 314–317. [Google Scholar] [CrossRef]
- Ferté, H.; Depaquit, J.; Carré, S.; Villena, I.; Léger, N. Presence of Trichobilharzia szidati in Lymnaea stagnalis and T. franki in Radix auricularia in northeastern France: Molecular evidence. Parasitol. Res. 2005, 95, 150–154. [Google Scholar] [CrossRef]
- Bayssade-Dufour, C.; Jouet, D.; Rudolfova, J.; Horák, P.; Ferté, H. Seasonal morphological variations in bird schistosomes. Parasite 2006, 13, 205–214. [Google Scholar] [CrossRef]
- Jouet, D.; Ferté, H.; Depaquit, J.; Rudolfová, J.; Latour, P.; Zanella, D.; Kaltenbach, M.L.; Léger, N. Trichobilharzia spp. in natural conditions in Annecy Lake, France. Parasitol. Res. 2008, 103, 51–58. [Google Scholar] [CrossRef]
- Jouet, D.; Ferté, H.; Hologne, C.; Kaltenbach, M.L.; Depaquit, J. Avian schistosomes in French aquatic birds: A molecular approach. J. Helminthol. 2009, 83, 181–189. [Google Scholar] [CrossRef]
- Jouet, D.; Skírnisson, K.; Kolářová, L.; Ferté, H. Final hosts and variability of Trichobilharzia regenti under natural conditions. Parasitol. Res. 2010, 107, 923–930. [Google Scholar] [CrossRef]
- Jouet, D.; Skírnisson, K.; Kolářová, L.; Ferté, H. Molecular diversity of Trichobilharzia franki in two intermediate hosts (Radix auricularia and Radix peregra): A complex of species. Infect. Genet. Evol. 2010, 10, 1218–1227. [Google Scholar] [CrossRef]
- Hertel, J.; Hamburger, J.; Haberl, B.; Haas, W. Detection of bird schistosomes in lakes by PCR and filter-hybridization. Exp. Parasitol. 2002, 101, 57–63. [Google Scholar] [CrossRef]
- Majoros, G.; Dán, Á.; Erdélyi, K. A natural focus of the blood fluke Orientobilharzia turkestanica (Skrjabin, 1913) (Trematoda: Schistosomatidae) in red deer (Cervus elaphus) in Hungary. Vet. Parasitol. 2010, 170, 218–223. [Google Scholar] [CrossRef]
- Kolářová, L.; Rudolfová, J.; Hampl, V.; Skírnisson, K. Allobilharzia visceralis gen. nov., sp. nov. (Schistosomatidae-Trematoda) from Cygnus cygnus (L.) (Anatidae). Parasitol. Int. 2006, 55, 179–186. [Google Scholar] [CrossRef]
- Aldhoun, J.A.; Kolářová, L.; Horák, P.; Skírnisson, K. Bird schistosome diversity in Iceland: Molecular evidence. J. Helminthol. 2009, 83, 173–180. [Google Scholar] [CrossRef]
- Cipriani, P.; Mattiucci, S.; Paoletti, M.; Scialanca, F.; Nascetti, G. Molecular evidence of Trichobilharzia franki Müller and Kimmig, 1994 (Digenea: Schistosomatidae) in Radix auricularia from Central Italy. Parasitol. Res. 2011, 109, 935–940. [Google Scholar] [CrossRef]
- Soldánová, M.; Georgieva, S.; Roháčová, J.; Knudsen, R.; Kuhn, J.A.; Henriksen, E.H.; Siwertsson, A.; Shaw, J.C.; Kuris, A.M.; Amundsen, P.A.; et al. Molecular analyses reveal high species diversity of trematodes in a sub-Arctic lake. Int. J. Parasitol. 2017, 47, 327–345. [Google Scholar] [CrossRef]
- Marszewska, A.; Strzała, T.; Cichy, A.; Dąbrowska, G.B.; Żbikowska, E. Agents of swimmer’s itch—Dangerous minority in the Digenea invasion of Lymnaeidae in water bodies and the first report of Trichobilharzia regenti in Poland. Parasitol. Res. 2018, 117, 3695–3704. [Google Scholar] [CrossRef]
- Stanicka, A.; Migdalski, Ł.; Zając, K.S.; Cichy, A.; Lachowska-Cierlik, D.; Żbikowska, E. The genus Bilharziella vs. other bird schistosomes in snail hosts from one of the major recreational lakes in Poland. Knowl. Manag. Aquat. Ecosyst. 2021, 2020, 12. [Google Scholar] [CrossRef]
- Korsunenko, A.V.; Chrisanfova, G.G.; Ryskov, A.P.; Movsessian, S.O.; Vasilyev, V.A.; Semyenova, S.K. Detection of European Trichobilharzia Schistosomes (T. franki, T. szidati, and T. regenti) Based on Novel Genome Sequences. J. Parasitol. 2010, 96, 802–806. [Google Scholar] [CrossRef]
- Korsunenko, A.; Chrisanfova, G.; Lopatkin, A.; Beer, S.A.; Voronin, M.; Ryskov, A.P.; Semyenova, S.K. Genetic differentiation of cercariae infrapopulations of the avian schistosome Trichobilharzia szidati based on RAPD markers and mitochondrial cox1 gene. Parasitol. Res. 2012, 110, 833–841. [Google Scholar] [CrossRef]
- Picard, D.; Jousson, O. Genetic variability among cecariae of the shistomatidae (Trematoda: Digenea) causing swimmer’s itch in Europe. Parasite 2001, 8, 237–242. [Google Scholar] [CrossRef]
- Webster, B.L.; Rudolfová, J.; Horák, P.; Littlewood, D.T.J. The complete mitochondrial genome of the bird Schistosome Trichobilharzia regenti (PLATYHELMINTHES: DIGENEA), causative agent of cercarial dermatitis. J. Parasitol. 2007, 93, 553–561. [Google Scholar] [CrossRef]
- Soldánová, M.; Selbach, C.; Sures, B. The Early Worm Catches the Bird? Productivity and Patterns of Trichobilharzia szidati Cercarial Emission from Lymnaea stagnalis. Knight M, editor. PLoS ONE 2016, 11, e0149678. [Google Scholar] [CrossRef]
- Skála, V.; Walker, A.J.; Horák, P. Snail defence responses to parasite infection: The Lymnaea stagnalis-Trichobilharzia szidati model. Dev. Comp. Immunol. 2020, 102, 103464. [Google Scholar] [CrossRef]
- Haas, W. Parasitic worms: Strategies of host finding, recognition and invasion. Zoology 2003, 106, 349–364. [Google Scholar] [CrossRef]
- Horák, P.; Kolářová, L. Molluscan and vertebrate immune responses to bird schistosomes. Parasite Immunol. 2005, 27, 247–255. [Google Scholar] [CrossRef]
- Horák, P.; Knaap, W.P.W. Lectins in snail-trematode immune interactions: A review. Folia Parasitol. 1997, 44, 161–172. Available online: https://folia.paru.cas.cz/ (accessed on 11 March 2024).
- Skírnisson, K.; Kolářová, L. Diversity of bird schistosomes in anseriform birds in Iceland based on egg measurements and egg morphology. Parasitol. Res. 2008, 103, 43–50. [Google Scholar] [CrossRef]
- Hrádková, K.; Horák, P. Neurotropic behaviour of Trichobilharzia regenti in ducks and mice. J. Helminthol. 2002, 76, 137–141. [Google Scholar] [CrossRef]
- Gyimesi, A.; Lensink, R. Egyptian Goose Alopochen aegyptiaca: An introduced species spreading in and from the Netherlands. Wildfowl 2012, 62, 128–145. Available online: https://wildfowl.wwt.org.uk/index.php/wildfowl/article/view/1331 (accessed on 16 January 2024).
- Fischer, E.F.; Recht, S.; Vélez, J.; Rogge, L.; Taubert, A.; Hermosilla, C.R. Occurrence of Gastrointestinal Parasites in Synanthropic Neozoan Egyptian Geese (Alopochen aegyptiaca, Linnaeus 1766) in Germany. Diversity 2023, 15, 388. [Google Scholar] [CrossRef]
- Fain, A. Nasal Trichobilharziasis: A New Avian Schistosomiasis. Nature 1956, 177, 389. [Google Scholar] [CrossRef]
- Appleton, C.C. The eggs of some blood-flukes (Trematoda: Schistosomatidae) from South African birds. S. Afr. J. Zool. 1982, 17, 147–150. [Google Scholar] [CrossRef][Green Version]
- Muñoz-Fuentes, V.; Green, A.J.; Sorenson, M.D.; Negro, J.J.; Vilà, C. The ruddy duck Oxyura jamaicensis in Europe: Natural colonization or human introduction? Mol. Ecol. 2006, 15, 1441–1453. [Google Scholar] [CrossRef]
- Anastácio, P.M.; Ribeiro, F.; Capinha, C.; Banha, F.; Gama, M.; Filipe, A.F.; Rebelo, R.; Sousa, R. Non-native freshwater fauna in Portugal: A review. Sci. Total Environ. 2019, 650, 1923–1934. [Google Scholar] [CrossRef]
- Padilla-Aguilar, P.; Romero-Callejas, E.; Osorio-Sarabia, D.; Pérez–Ponce de León, G.; Alcalá-Canto, Y. New records of helminth parasites of nine species of waterfowl in Mexico, and a checklist of the helminth fauna of Anatidae occurring in Mexican wetlands. J. Helminthol. 2020, 94, e176. [Google Scholar] [CrossRef]
- Jamieson, B.; Haas, W. Miracidium of Schistosoma. In Schistosoma: Biology, Pathology and Control, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 77–117. [Google Scholar] [CrossRef]
- Kalbe, M.; Haberl, B.; Haas, W. Snail Host Finding by Fasciola hepatica and Trichobilharzia ocellata: Compound Analysis of “Miracidia-Attracting Glycoproteins. Exp. Parasitol. 2000, 96, 231–242. [Google Scholar] [CrossRef]
- Nelwan, M.L. Schistosomiasis: Life Cycle, Diagnosis, and Control. Curr. Ther. Res. 2019, 91, 5–9. [Google Scholar] [CrossRef]
- Al-Jubury, A.; Kania, P.; Bygum, A.; Buchmann, K. Temperature and light effects on Trichobilharzia szidati cercariae with implications for a risk analysis. Acta Vet. Scand. 2020, 62, 54. [Google Scholar] [CrossRef]
- Auer, H.; Aspöck, H. Helminths and helminthoses in Central Europe: General overview and diseases caused by trematodes (flukes). Wien. Med. Wochenschr. 2014, 164, 405–413. [Google Scholar] [CrossRef]
- Selbach, C.; Soldánová, M.; Sures, B. Estimating the risk of swimmer’s itch in surface waters—A case study from Lake Baldeney, River Ruhr. Int. J. Hyg. Environ. Health 2016, 219, 693–699. [Google Scholar] [CrossRef]
- Kourilová, P.; Hogg, K.G.; Kolárová, L.; Mountford, A.P. Cercarial Dermatitis Caused by Bird Schistosomes Comprises Both Immediate and Late Phase Cutaneous Hypersensitivity Reactions. J. Immunol. 2004, 172, 3766–3774. [Google Scholar] [CrossRef]
- Horák, P.; Kolářová, L. Bird schistosomes: Do they die in mammalian skin? Trends Parasitol. 2001, 17, 66–69. [Google Scholar] [CrossRef]
- Semenza, J.C.; Paz, S. Climate change and infectious disease in Europe: Impact, projection and adaptation. Lancet Reg. Health—Eur. 2021, 9, 100230. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Tracz, E.; Al-Jubury, A.; Buchmann, K.; Bygum, A. Outbreak of Swimmer’s Itch in Denmark. Acta Derm. Venereol. 2019, 99, 1116–1120. [Google Scholar] [CrossRef]
- Fernandes, G.; de Castro, E.; Gomes, H. Water Resources and Tourism Development in Estrela Geopark Territory: Meaning and Contributions of Fluvial Beaches to Valorise the Destination. Eur. Countrys. 2020, 12, 551–567. [Google Scholar] [CrossRef]
- Stensgaard, A.S.; Vounatsou, P.; Sengupta, M.E.; Utzinger, J. Schistosomes, snails and climate change: Current trends and future expectations. Acta Trop. 2019, 190, 257–268. [Google Scholar] [CrossRef]
- Kolářová, L.; Horák, P.; Skírnisson, K. Methodical approaches in the identification of areas with a potential risk of infection by bird schistosomes causing cercarial dermatitis. J. Helminthol. 2010, 84, 327–335. [Google Scholar] [CrossRef]
- Ebbs, E.T.; Loker, E.S.; Davis, N.E.; Flores, V.; Veleizan, A.; Brant, S.V. Schistosomes with wings: How host phylogeny and ecology shape the global distribution of Trichobilharzia querquedulae (Schistosomatidae). Int. J. Parasitol. 2016, 46, 669–677. [Google Scholar] [CrossRef]
- Marszewska, A.; Cichy, A.; Heese, T.; Żbikowska, E. The real threat of swimmers’ itch in anthropogenic recreational water body of the Polish Lowland. Parasitol. Res. 2016, 115, 3049–3056. [Google Scholar] [CrossRef]
- Malone, J.B.; Nieto, P.; Tadesse, A. Biology-based mapping of vector-borne parasites by geographic information systems and remote sensing. Parassitologia 2006, 48, 77–79. Available online: https://pubmed.ncbi.nlm.nih.gov/16881402/ (accessed on 19 January 2024). [PubMed]
- Cotton, P.A. Avian migration phenology and global climate change. Proc. Natl. Acad. Sci. USA 2003, 100, 12219–12222. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Efectos del cambio climático en las helmintiasis animales y zoonóticas. Rev. Sci. Tech. L’oie 2008, 27, 443–457. [Google Scholar] [CrossRef]
- Żbikowska, E. Is there a potential danger of “swimmer’s itch in Poland? Parasitol. Res. 2002, 89, 59–62. [Google Scholar] [CrossRef]
- Korycińska, J.; Rybak-d’Obyrn, J.; Kubiak, D.; Kubiak, K.; Dzika, E. Dermatological and Molecular Evidence of Human Cercarial Dermatitis in North-Eastern Poland. Vector-Borne Zoonotic Dis. 2021, 21, 269–274. [Google Scholar] [CrossRef]
- Soldánová, M.; Selbach, C.; Sures, B.; Kostadinova, A.; Pérez-del-Olmo, A. Larval trematode communities in Radix auricularia and Lymnaea stagnalis in a reservoir system of the Ruhr River. Parasites Vectors 2010, 3, 56. [Google Scholar] [CrossRef]
- Strathmann, M.; Horstkott, M.; Koch, C.; Gayer, U.; Wingender, J. The River Ruhr—An urban river under particular interest for recreational use and as a raw water source for drinking water: The collaborative research project “Safe Ruhr”—Microbiological aspects. Int. J. Hyg. Environ. Health 2016, 219, 643–661. [Google Scholar] [CrossRef]
- Schets, F.M.; Lodder, W.J.; van Duynhoven, Y.T.H.P.; de Roda Husman, A.M. Cercarial dermatitis in the Netherlands caused by Trichobilharzia spp. J. Water Health 2008, 6, 187–195. [Google Scholar] [CrossRef]
- Schets, F.M.; Lodder, W.J.; De Roda Husman, A.M. Confirmation of the presence of Trichobilharzia by examination of water samples and snails following reports of cases of cercarial dermatitis. Parasitology 2010, 137, 77–83. [Google Scholar] [CrossRef]
- De Gentile, L.; Picot, H.; Bourdeau, P.; Bardet, R.; Kerjan, A.; Piriou, M.; Le Guennic, A.; Bayssade-Dufour, C.; Chabasse, D.; Mott, K. Cercarial dermatitis in Europe: A new public health problem? Bull. World Health Organ. 1996, 74, 159–163. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8706231 (accessed on 25 January 2024). [PubMed]
- Caumes, E.; Felder-Moinet, S.; Couzigou, C.; Darras-Joly, C.; Latour, P.; Léger, N. Failure of an ointment based on IR3535 (ethyl butylacetylaminopropionate) to prevent an outbreak of cercarial dermatitis during swimming races across Lake Annecy, France. Ann. Trop. Med. Parasitol. 2003, 97, 157–163. [Google Scholar] [CrossRef]
- Eklu-Natey, D.T.; Al-Khudri, M.; Gauthey, D.; Dubois, J.P.; Wüest, J.; Vaucher, C. Epidémiologie de la dermatite des baigneurs et morphologie de Trichobilharzia cf. ocellata dans le lac Léman. Rev. Suisse Zool. 1985, 92, 939–953. [Google Scholar] [CrossRef]
- Chamot, E.; Toscani, L.; Rougemont, A. Public health importance and risk factors for cercarial dermatitis associated with swimming in Lake Leman at Geneva, Switzerland. Epidemiol. Infect. 1998, 120, 305–314. [Google Scholar] [CrossRef]
- Gulyás, K.; Soldánová, M.; Orosová, M.; Oros, M. Confirmation of the presence of zoonotic Trichobilharzia franki following a human cercarial dermatitis outbreak in recreational water in Slovakia. Parasitol. Res. 2020, 119, 2531–2537. [Google Scholar] [CrossRef]
- Faltýnková, A.; Našincová, V.; Kablásková, L. Larval trematodes (Digenea) of planorbid snails (Gastropoda: Pulmonata) in Central Europe: A survey of species and key to their identification. Syst. Parasitol. 2008, 69, 155–178. [Google Scholar] [CrossRef]
- Allerberger, F.; Wotzer, G.; Dierich, M.P.; Moritz, C.; Fritsch, P.; Haas, W. Occurrence of swimmer’s itch in the Tyrol|Auftreten Von Badedermatitis in Tirol. Immun. Infekt. 1994, 22, 30–32. Available online: https://europepmc.org/article/med/7927463 (accessed on 16 January 2024).
- Hörweg, C.; Sattmann, H.; Auer, H. Cercarial dermatitis in Austria: Questionnaires as useful tools to estimate risk factors? Wien. Klin. Wochenschr. 2006, 118, 77–80. [Google Scholar] [CrossRef]
- Juhász, A.; Dán, Á.; Dénes, B.; Kucsera, I.; Danka, J.; Majoros, G. Egy ritka zoonosis: A Schistosoma turkestanicum vérmétely által okozott cercaria dermatitis Magyarországon. Orvosi Hetil. 2016, 157, 1579–1586. [Google Scholar] [CrossRef]
- Rizevsky, S.V.; Bodilovskaya, O.A.; Golubev, A.P.; Kurchenko, V.P. Capacity for long-term self-fertilization of the pulmonate mollusk Lymnaea stagnalis as a factor of preservation of human cercarial dermatitis foci. Dokl. Biol. Sci. 2012, 443, 109–112. [Google Scholar] [CrossRef]
- Arias, M.; Lomba, C.; Dacal, V.; Vázquez, L.; Pedreira, J.; Francisco, I.; Piñeiro, P.; Cazapal-Monteiro, C.; Suárez, J.L.; Díez-Baños, P.; et al. Prevalence of mixed trematode infections in an abattoir receiving cattle from northern Portugal and north-west Spain. Vet. Rec. 2011, 168, 408. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Human and Animal Fascioliasis: Origins and Worldwide Evolving Scenario. Clin. Microbiol. Rev. 2022, 35, e00088-19. [Google Scholar] [CrossRef]
- Fraga de Azevedo, J.; Da Silva, J.B.; Coito, A.D.; Coelho, M.F.; Colaco, A.A. Portuguese focus of schistosomiasis. An. Inst. Med. Trop. 1948, 5, 175–222. [Google Scholar] [CrossRef]
- Salas-Coronas, J.; Bargues, M.D.; Lozano-Serrano, A.B.; Artigas, P.; Martínez-Ortí, A.; Mas-Coma, S.; Merino-Salas, S.; Vivas-Pérez, J.I.A. Evidence of autochthonous transmission of urinary schistosomiasis in Almeria (southeast Spain): An outbreak analysis. Travel Med. Infect. Dis. 2021, 44, 102165. [Google Scholar] [CrossRef]
- Rudko, S.P.; McPhail, B.A.; Reimink, R.L.; Froelich, K.; Turnbull, A.; Hanington, P.C. Non-resident definitive host presence is sufficient to sustain avian schistosome populations. Int. J. Parasitol. 2022, 52, 305–315. [Google Scholar] [CrossRef]
- McPhail, B.A.; Froelich, K.; Reimink, R.L.; Hanington, P.C. Simplifying Schistosome Surveillance: Using Molecular Cercariometry to Detect and Quantify Cercariae in Water. Pathogens 2022, 11, 565. [Google Scholar] [CrossRef]
- Loker, E.S.; DeJong, R.J.; Brant, S.V. Scratching the Itch: Updated Perspectives on the Schistosomes Responsible for Swimmer’s Itch around the World. Pathogens 2022, 11, 587. [Google Scholar] [CrossRef]
- Gordy, M.A.; Cobb, T.P.; Hanington, P.C. Swimmer’s itch in Canada: A look at the past and a survey of the present to plan for the future. Environ. Health 2018, 17, 73. [Google Scholar] [CrossRef]
- Pinto, H.A.; Pulido-Murillo, E.A.; de Melo, A.L.; Brant, S.V. Putative new genera and species of avian schistosomes potentially involved in human cercarial dermatitis in the Americas, Europe and Africa. Acta Trop. 2017, 176, 415–420. [Google Scholar] [CrossRef]
- Gohardehi, S.; Fakhar, M.; Madjidaei, M. Avian Schistosomes and Human Cercarial Dermatitis in a Wildlife Refuge in Mazandaran Province, Northern Iran. Zoonoses Public Health 2013, 60, 442–447. [Google Scholar] [CrossRef]
- Wulff, C.; Haeberlein, S.; Haas, W. Cream formulations protecting against cercarial dermatitis by Trichobilharzia. Parasitol. Res. 2007, 101, 91–97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).