Dysregulated Glucuronidation of Bilirubin Exacerbates Liver Inflammation and Fibrosis in Schistosomiasis Japonica through the NF-κB Signaling Pathway
Abstract
1. Introduction
2. Methods
2.1. Analysis of Blood Biochemical Examination Data for Patients with Advanced Schistosomiasis
2.2. Cell Culture
2.3. Experimental Animals
2.4. Preparation of Cercariae
2.5. Soluble Egg Antigen (SEA) Preparation
2.6. CCK-8 Method to Detect Cell Proliferation
2.7. Detection of Apoptosis by Flow Cytometry
2.8. ELISA for Serum Concentrations of Conjugated and Unconjugated Bilirubin
2.9. Histopathological Examination of Liver Samples
2.10. The mRNA Expression Levels of the Genes Were Assessed Using Fluorescence Quantitative PCR
2.11. Western Blot Assays
2.12. Data Processing and Statistical Analysis
3. Results
3.1. Changes and Correlation Analysis of Total Bilirubin and Indicators of Liver Injury and Liver Fibrosis in Patients with Advanced Schistosomiasis
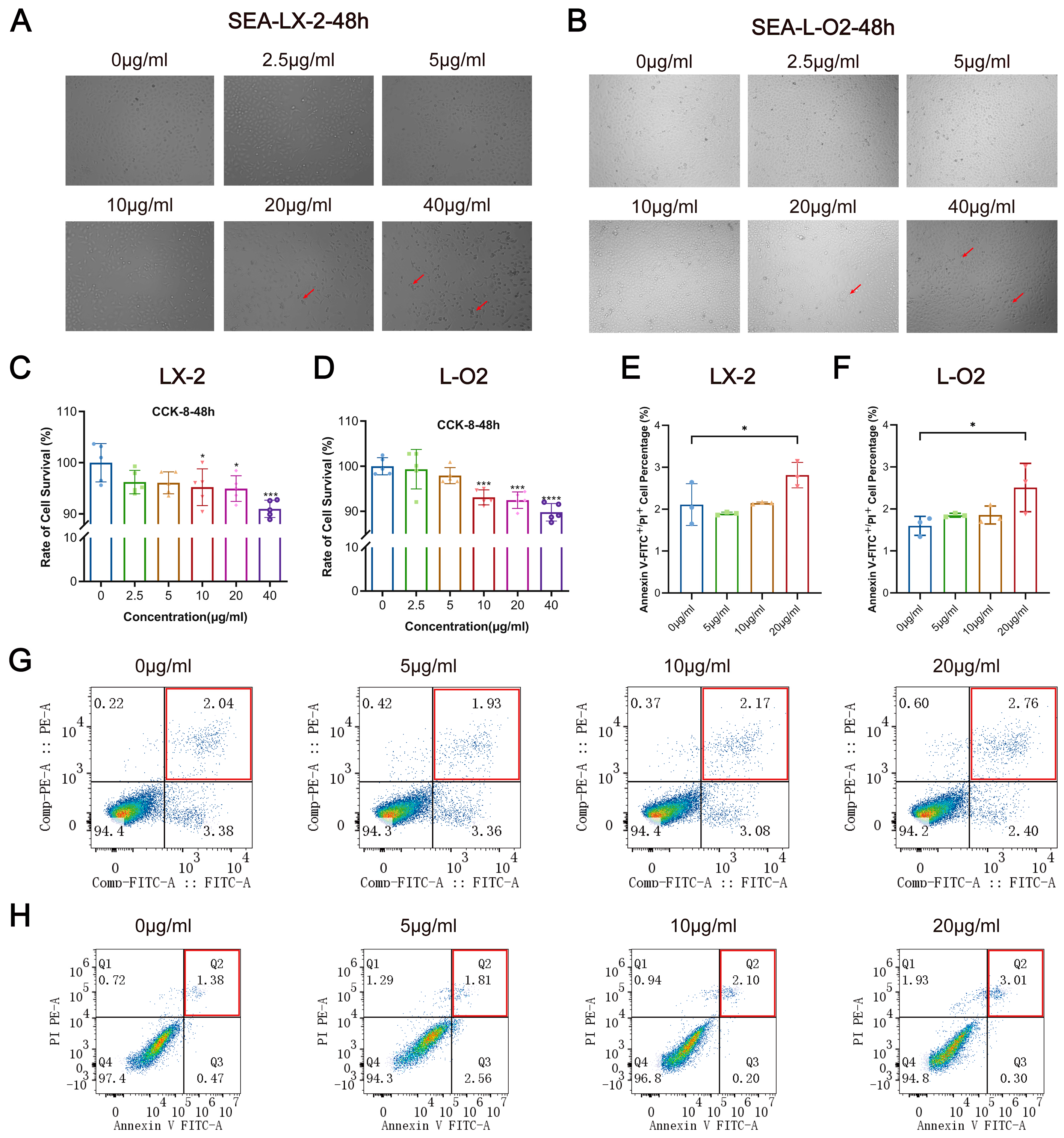
3.2. SEA Inhibits LX-2 and L-O2 Cell Proliferation and Promotes Apoptosis In Vitro
3.3. SEA Inhibits the mRNA Expression of UGT1A1 in LX-2 Cells and L-O2 Cells In Vitro
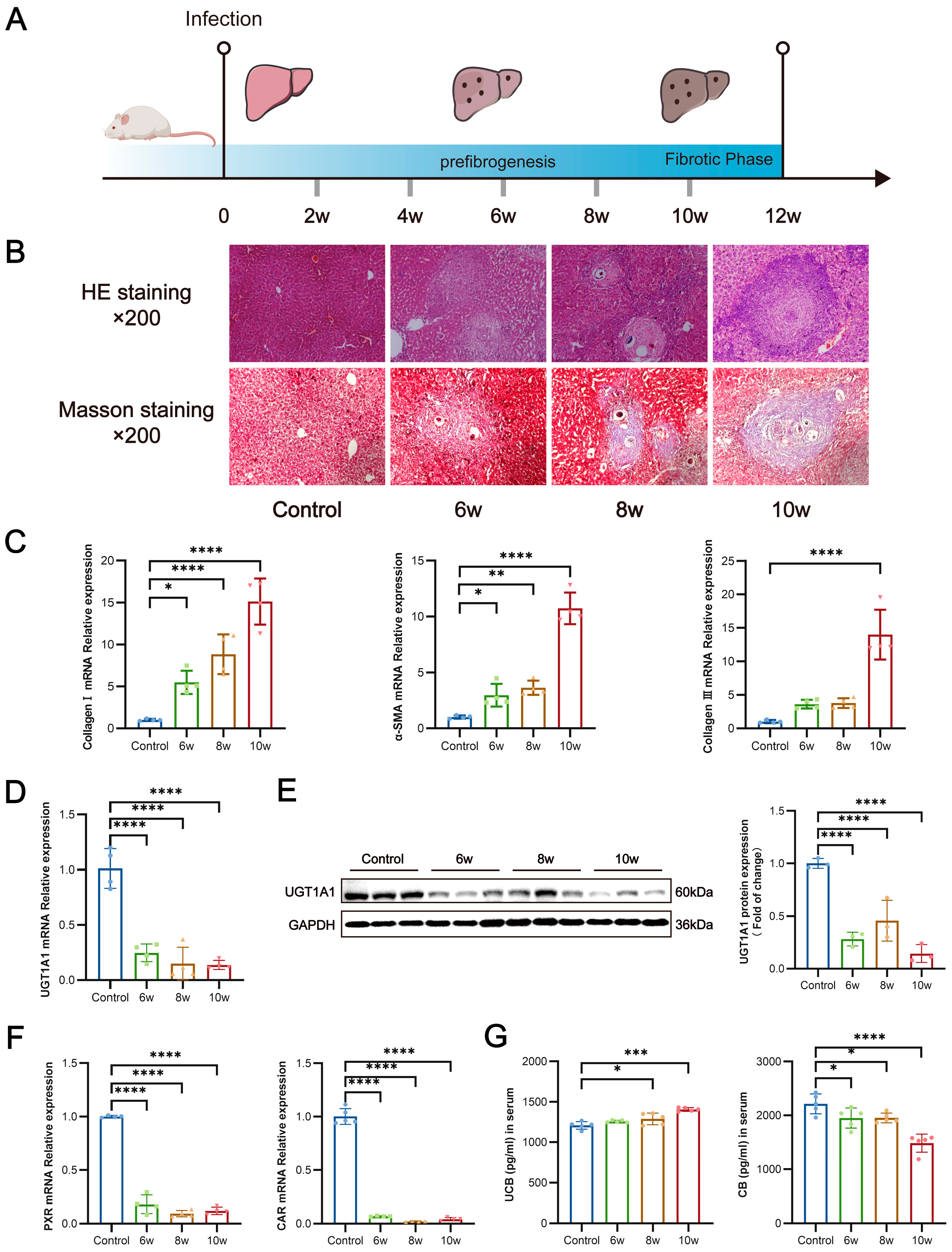
3.4. S. japonicum Infection Suppresses Hepatic UGT1A1 Expression, Thereby Impairing Glucuronidation of Bilirubin
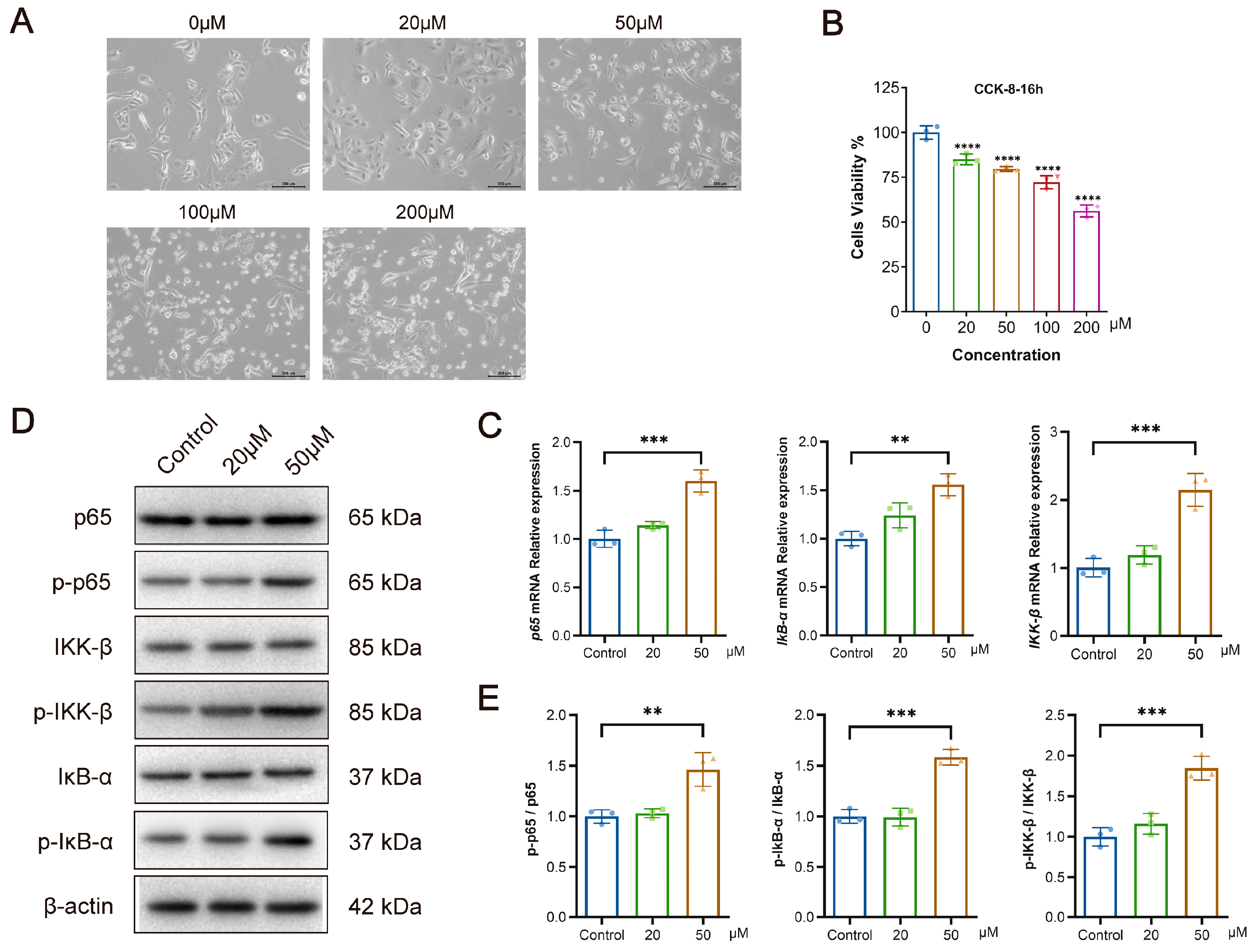
3.5. Bilirubin Inhibits the Proliferation of L-O2 Cells While Activating the NF-κB Signaling Pathway In Vitro
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef]
- Ross, A.G.; Chau, T.N.; Inobaya, M.T.; Olveda, R.M.; Li, Y.; Harn, D.A. A new global strategy for the elimination of schistosomiasis. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2017, 54, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Deng, Y.; Liu, Y.; Wang, Y.; Hu, W.; Huang, Y.; Yang, K. A retrospective analysis of schistosomiasis related literature from 2011–2020: Focusing on the next decade. Acta Trop. 2022, 238, 106750. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Liu, K.; Xiong, C.; Cao, P.; Tao, J. New detection method in experimental mice for schistosomiasis: ClinProTool and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Parasitol. Res. 2016, 115, 4173–4181. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, J.; Xu, Y.; Xiong, C.; Tong, D.; Hu, N.; Yang, H. Xiaochaihu decorction relieves liver fibrosis caused by Schistosoma japonicum infection via the HSP47/TGF-β pathway. Parasites Vectors 2020, 13, 254. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Bergquist, R.; Cai, P.; Ranasinghe, S.; Tebeje, B.M.; You, H. Schistosomiasis—From immunopathology to vaccines. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 355–371. [Google Scholar]
- Li, C.; Ren, G.; Deng, W.; Li, S.; Hu, B.; Shi, Y.; Wang, Y.; Dong, S.; Zhang, N.; Zheng, Y. Prevalence and incidence of advanced schistosomiasis and risk factors for case fatality in Hunan Province, China. Acta Trop. 2021, 217, 105862. [Google Scholar] [CrossRef]
- Dong, P.; Mei, C.; Yang, Y.; Zhou, Y.; Xu, Y.; Song, L.; Yu, C. Blocking BAFF Alleviates Hepatic Fibrosis in Schistosoma japonicum-Infected Mice. Pathogens 2023, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, L.; Hu, Y.; Yi, J.; Wan, L.; Shen, Y.; Liu, S.; Zhou, X.; Cao, J. Higher frequency of circulating Vδ1 γδT cells in patients with advanced schistosomiasis. Parasite Immunol. 2021, 43, e12871. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Feng, A.; Huang, Y. Research and control of advanced schistosomiasis japonica in China. Parasitol. Res. 2015, 114, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Jia, K.; He, J.; Shi, C.; Fang, M.; Song, L.; Zhang, P.; Zhao, Y.; Fu, J.; Li, S. Establishment of the tree shrew as an alcohol-induced Fatty liver model for the study of alcoholic liver diseases. PLoS ONE 2015, 10, e0128253. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.N.; Ferreira, D.W.; More, V.R.; Lake, A.D.; Lu, Z.; Manautou, J.E.; Slitt, A.L.; Cherrington, N.J. Altered UDP-glucuronosyltransferase and sulfotransferase expression and function during progressive stages of human nonalcoholic fatty liver disease. Drug Metab. Dispos. 2013, 41, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zheng, R.; Xie, P.; Guo, Q.; Ji, H.; Li, T. Dysregulation of UDP-glucuronosyltransferases in CCl4 induced liver injury rats. Chem. Biol. Interact. 2020, 325, 109115. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.G.; Nair, P.C.; Haines, A.Z.; McKinnon, R.A.; Mackenzie, P.I.; Meech, R. The UGTome: The expanding diversity of UDP glycosyltransferases and its impact on small molecule metabolism. Pharmacol. Ther. 2019, 204, 107414. [Google Scholar] [CrossRef] [PubMed]
- Allain, E.P.; Rouleau, M.; Lévesque, E.; Guillemette, C. Emerging roles for UDP-glucuronosyltransferases in drug resistance and cancer progression. Br. J. Cancer 2020, 122, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, X.; Chen, Y.; Zhang, L.-S.; Zhang, Y.-R.; Ji, D.-R.; Liu, S.-M.; Jia, M.-Z.; Zhu, Y.-H.; Qi, Y.-F. STAT3 promotes schistosome-induced liver injury by inflammation, oxidative stress, proliferation, and apoptosis signal pathway. Infect. Immun. 2021, 89, e00309–e00320. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Q.; Zhao, L.; Xiong, C.; Xu, Y.; Dong, X.; Wen, Y.; Cao, J. UHPLC-MS-Based Metabolomics Analysis Reveals the Process of Schistosomiasis in Mice. Front. Microbiol. 2020, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Plise, E.; Cheong, J.; Ho, Q.; Lin, M. Evaluating the in vitro inhibition of UGT1A1, OATP1B1, OATP1B3, MRP2, and BSEP in predicting drug-induced hyperbilirubinemia. Mol. Pharm. 2013, 10, 3067–3075. [Google Scholar] [CrossRef]
- Vítek, L. Bilirubin as a signaling molecule. Med. Res. Rev. 2020, 40, 1335–1351. [Google Scholar] [CrossRef] [PubMed]
- Iyanagi, T. Molecular mechanism of phase I and phase II drug-metabolizing enzymes: Implications for detoxification. Int. Rev. Cytol. 2007, 260, 35–112. [Google Scholar] [PubMed]
- Surendran, S.P.; Thomas, R.G.; Moon, M.J.; Park, R.; Lee, J.H.; Jeong, Y.Y. A bilirubin-conjugated chitosan nanotheranostics system as a platform for reactive oxygen species stimuli-responsive hepatic fibrosis therapy. Acta Biomater. 2020, 116, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Laskar, A.A.; Khan, M.A.; Rahmani, A.H.; Fatima, S.; Younus, H. Thymoquinone, an active constituent of Nigella sativa seeds, binds with bilirubin and protects mice from hyperbilirubinemia and cyclophosphamide-induced hepatotoxicity. Biochimie 2016, 127, 205–213. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, A.F. Bilirubin toxicity to human erythrocytes: A more sanguine view. Pediatrics 2007, 120, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, P.; Fallahi, M.; Kazemian, M.; Taleghani, N.T.; Nouripour, S.; Radfar, M. Bilirubin induced encephalopathy. Iran. J. Child Neurol. 2020, 14, 7. [Google Scholar] [PubMed]
- Liu, D.; Yu, Q.; Li, Z.; Zhang, L.; Hu, M.; Wang, C.; Liu, Z. UGT1A1 dysfunction increases liver burden and aggravates hepatocyte damage caused by long-term bilirubin metabolism disorder. Biochem. Pharmacol. 2021, 190, 114592. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, G.; Kurian, D.; Xu, M.; Dai, Y.; Zhou, Y.; Xu, Y.; Wang, J.; Zhang, Y.; Gao, Q. Proteomic patterns as biomarkers for the early detection of schistosomiasis japonica in a rabbit model. Int. J. Mass Spectrom. 2011, 299, 191–195. [Google Scholar] [CrossRef]
- Zhu, T.; Xue, Q.; Liu, Y.; Xu, Y.; Xiong, C.; Lu, J.; Yang, H.; Zhang, Q.; Huang, Y. Analysis of Intestinal Microflora and Metabolites From Mice With DSS-Induced IBD Treated With Schistosoma Soluble Egg Antigen. Front. Cell Dev. Biol. 2021, 9, 777218. [Google Scholar] [CrossRef]
- Gao, X.; Yang, X.; Zhang, B. Neuroprotection of taurine against bilirubin-induced elevation of apoptosis and intracellular free calcium ion in vivo. Toxicol. Mech. Methods 2011, 21, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, B.; Azuelos, I.; Anastasiou, D.; Chambers, R.C. Fibrometabolism—An emerging therapeutic frontier in pulmonary fibrosis. Sci. Signal. 2021, 14, eaay1027. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhang, J.; Chen, H.; Nie, H.; Miller, H.; Gong, Q.; Liu, C. T Lymphocyte-Mediated Liver Immunopathology of Schistosomiasis. Front. Immunol. 2020, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.P.; Ramm, G.A.; Robinson, M.W.; McManus, D.P.; Gobert, G.N. Schistosome-induced fibrotic disease: The role of hepatic stellate cells. Trends Parasitol. 2018, 34, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Meng, H.-Y.; Liu, S.-M.; Wang, Y.; Yang, X.-X.; Lu, F.; Wang, H.-Y. Identification of key metabolic changes during liver fibrosis progression in rats using a urine and serum metabolomics approach. Sci. Rep. 2017, 7, 11433. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yin, X.; Zhang, L.; Yao, M.; Wei, D.; Wu, Y. Serum proteomic profiling in patients with advanced Schistosoma japonicum-induced hepatic fibrosis. Parasites Vectors 2021, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, Y.; Huang, Y.; Sun, F.; Tian, H.; Hu, N.; Shi, L.; Hua, H. Identification of newly developed advanced schistosomiasis with MALDI-TOF mass spectrometry and ClinProTools analysis. Parasite 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-H.; Shiels, R.G.; Lang, C.A.; Seyed Khoei, N.; Bulmer, A.C. Diagnostic criteria and contributors to Gilbert’s syndrome. Crit. Rev. Clin. Lab. Sci. 2018, 55, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, M.; Zhang, L.; Teng, X.; Chen, X.; Zhou, X.; Ma, Z.; Qi, L.; Wang, P. Differences in UGT1A1 gene mutations and pathological liver changes between Chinese patients with Gilbert syndrome and Crigler-Najjar syndrome type II. Medicine 2017, 96, e8620. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, X.; Liu, S.; Zhuang, L.; Wang, M.; Wang, Y.; Xin, Y.; Xuan, S. Relationship of circulating total bilirubin, UDP-glucuronosyltransferases 1A1 and the development of non-alcoholic fatty liver disease: A cross-sectional study. BMC Gastroenterol. 2022, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yu, Q.; Ning, Q.; Liu, Z.; Song, J. The relationship between UGT1A1 gene & various diseases and prevention strategies. Drug Metab. Rev. 2022, 54, 1–21. [Google Scholar] [PubMed]
- Tukey, R.H.; Strassburg, C.P. Human UDP-glucuronosyltransferases: Metabolism, expression, and disease. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 581. [Google Scholar] [CrossRef] [PubMed]
- Mimche, S.M.; Nyagode, B.A.; Merrell, M.D.; Lee, C.-M.; Prasanphanich, N.S.; Cummings, R.D.; Morgan, E.T. Hepatic cytochrome P450s, phase II enzymes and nuclear receptors are downregulated in a Th2 environment during Schistosoma mansoni infection. Drug Metab. Dispos. 2014, 42, 134–140. [Google Scholar] [CrossRef]
- Manhães-Rocha, D.A.; Conte, F.P.; Fidalgo-Neto, A.A.; De-Oliveira, A.C.; Ribeiro-Pinto, L.F.; Paumgartten, F.J. Alterations of hepatic microsomal enzymes in the early phase of murine schistosomiasis. Acta Trop. 2005, 95, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.A.; Musaigwa, F.; Mosala, P.; Berkiks, I.; Brombacher, F. Type 2 immunity: A two-edged sword in schistosomiasis immunopathology. Trends Immunol. 2022, 43, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Shiu, T.-Y.; Huang, T.-Y.; Huang, S.-M.; Shih, Y.-L.; Chu, H.-C.; Chang, W.-K.; Hsieh, T.-Y. Nuclear factor κB down-regulates human UDP-glucuronosyltransferase 1A1: A novel mechanism involved in inflammation-associated hyperbilirubinaemia. Biochem. J. 2013, 449, 761–770. [Google Scholar] [CrossRef]
- Sugatani, J. Function, genetic polymorphism, and transcriptional regulation of human UDP-glucuronosyltransferase (UGT) 1A1. Drug Metab. Pharmacokinet. 2013, 28, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Jiang, Y.; Chen, P.; Fan, X.; Li, D.; Liu, A.; Ma, X.; Xie, W.; Liu, P.; Gonzalez, F.J. Schisandrol B protects against cholestatic liver injury through pregnane X receptors. Br. J. Pharmacol. 2017, 174, 672–688. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, L.; Zheng, R.; Wang, X.; Li, L.; Ji, H.; Hu, Q. Picroside II protects against cholestatic liver injury possibly through activation of farnesoid X receptor. Phytomedicine 2020, 68, 153153. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-X.; Ding, Y.; Zhang, Y.; Ma, N.-H.; Shi, F.; Kang, P.; Cai, Z.-Z.; Zhang, T. Yinchenhao decoction ameliorates alpha-naphthylisothiocyanate induced intrahepatic cholestasis in rats by regulating phase II metabolic enzymes and transporters. Front. Pharmacol. 2018, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, B.; Ye, T.; Wang, Y.; Xia, D.; Qian, J. Physiological concentrations of bilirubin control inflammatory response by inhibiting NF-κB and inflammasome activation. Int. Immunopharmacol. 2020, 84, 106520. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver—Linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Ben-Neriah, Y.; Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 2011, 12, 715–723. [Google Scholar] [CrossRef] [PubMed]


| TBIL (2–20 μmol/L) | ALT (5–45 U/L) | AST (5–45 U/L) | HA (<100 ng/mL) | LN (<50 ng/mL) | PIIIP (<30 ng/mL) | CIV (<30 ng/mL) | |
|---|---|---|---|---|---|---|---|
| Median | 12.8 | 14.2 | 29 | 154.59 | 68.71 | 14.15 | 25.14 |
| Upper quartile | 8.65 | 8 | 22.05 | 82.39 | 38.28 | 7.15 | 17.36 |
| Lower quartile | 19.9 | 24 | 38.08 | 304.83 | 157.92 | 23.00 | 48.33 |
| Number of positives | 180 | 37 | 123 | 497 | 443 | 89 | 311 |
| Total number of tests | 745 | 745 | 745 | 745 | 745 | 745 | 745 |
| Positivity rate | 24.16% | 4.97% | 16.51% | 66.71% | 59.46% | 11.95% | 41.74% |
| ALT | AST | LN | HA | PIIIP | CIV | |
|---|---|---|---|---|---|---|
| r | 0.433 | 0.416 | 0.349 | 0.023 | −0.277 | 0.311 |
| p | <0.001 | <0.001 | <0.001 | 0.528 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Q.; Wang, Y.; Liu, Y.; Hua, H.; Zhou, X.; Xu, Y.; Zhang, Y.; Xiong, C.; Liu, X.; Yang, K.; et al. Dysregulated Glucuronidation of Bilirubin Exacerbates Liver Inflammation and Fibrosis in Schistosomiasis Japonica through the NF-κB Signaling Pathway. Pathogens 2024, 13, 287. https://doi.org/10.3390/pathogens13040287
Xue Q, Wang Y, Liu Y, Hua H, Zhou X, Xu Y, Zhang Y, Xiong C, Liu X, Yang K, et al. Dysregulated Glucuronidation of Bilirubin Exacerbates Liver Inflammation and Fibrosis in Schistosomiasis Japonica through the NF-κB Signaling Pathway. Pathogens. 2024; 13(4):287. https://doi.org/10.3390/pathogens13040287
Chicago/Turabian StyleXue, Qingkai, Yuyan Wang, Yiyun Liu, Haiyong Hua, Xiangyu Zhou, Yongliang Xu, Ying Zhang, Chunrong Xiong, Xinjian Liu, Kun Yang, and et al. 2024. "Dysregulated Glucuronidation of Bilirubin Exacerbates Liver Inflammation and Fibrosis in Schistosomiasis Japonica through the NF-κB Signaling Pathway" Pathogens 13, no. 4: 287. https://doi.org/10.3390/pathogens13040287
APA StyleXue, Q., Wang, Y., Liu, Y., Hua, H., Zhou, X., Xu, Y., Zhang, Y., Xiong, C., Liu, X., Yang, K., & Huang, Y. (2024). Dysregulated Glucuronidation of Bilirubin Exacerbates Liver Inflammation and Fibrosis in Schistosomiasis Japonica through the NF-κB Signaling Pathway. Pathogens, 13(4), 287. https://doi.org/10.3390/pathogens13040287







