Measurement of Feline Alpha-1 Acid Glycoprotein in Serum and Effusion Using an ELISA Method: Analytical Validation and Diagnostic Role for Feline Infectious Peritonitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. AGP Measurement
2.2. Intra-Assay Precision
2.3. Inter-Assay Precision
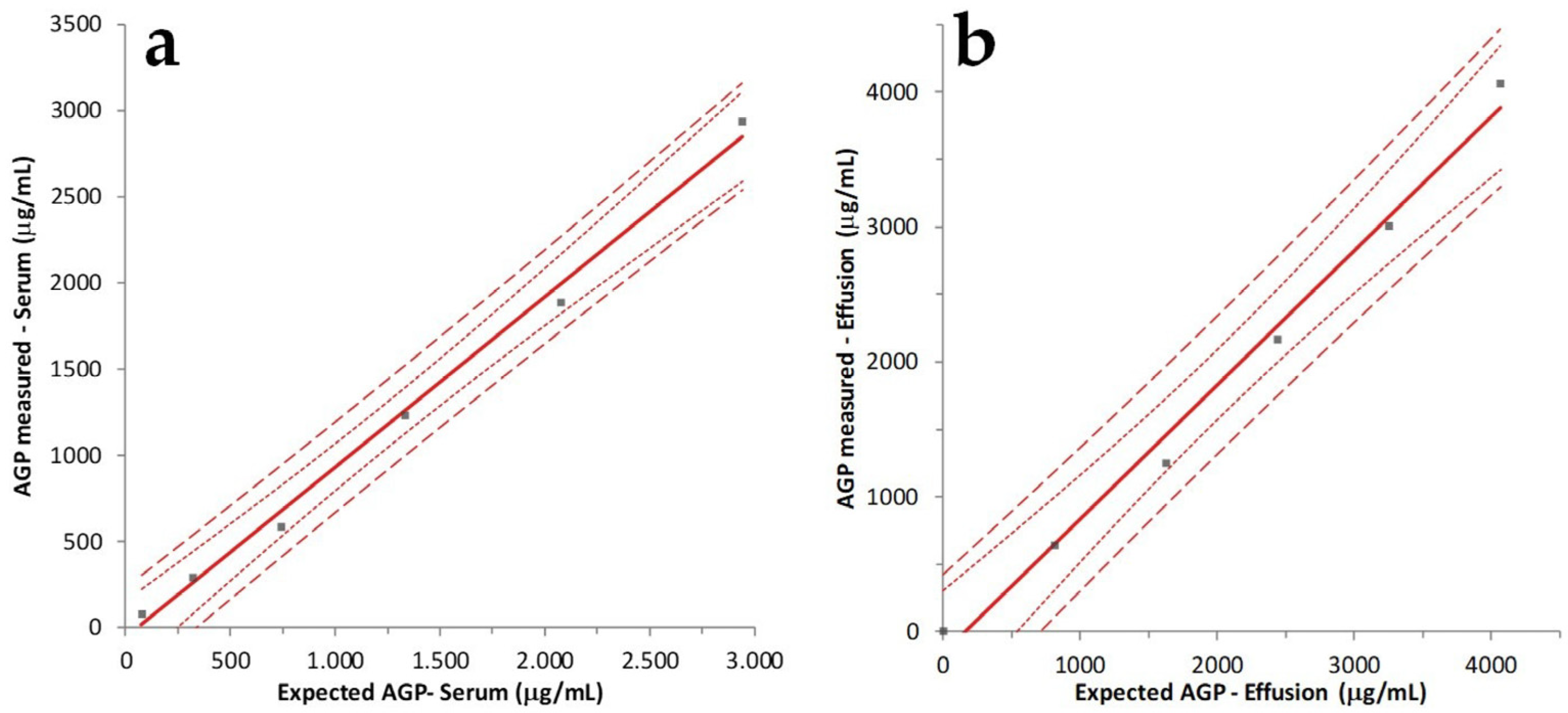
2.4. Accuracy Determination by Linearity under Dilution (LUD)
2.5. Comparison of Results Obtained in Cats with and without FIP
- -
- Routine hematology performed using an ADVIA 2120 (Siemens Healthineers, Dublin, Ireland);
- -
- Complete clinical chemistry panel run on a Beckman AU5800 (Beckman Coulter, Tokyo, Japan) with special emphasis on the concentrations of total protein, albumin, creatinine, and urea;
- -
- Serum protein electrophoresis performed on a Capillarys 2 (Sebia, France);
- -
- Physicochemical analysis of the effusion, including the macroscopic appearance of the fluid, refractometric estimation of specific gravity, and total nucleated cell count (TNCC) obtained with an ADVIA 2120 counter, as well as spectrophotometric measurement of total bilirubin (3,5-dichlorophenyldiazonium tetrafluoroborate method), cholesterol (esterase/peroxidase method), triglycerides (glycerol phosphate oxidase method), urea (urease method), creatinine (Jaffe reaction), total protein (biuret method), albumin (bromocresol green method), and LDH (enzymatic–kinetic method) using the Beckman instrument mentioned above and reagents provided by the manufacturer of the instrument;
- -
- Cytology of the effusion determined through microscopic analysis of smeared and cytocentrifuged specimens stained with May Grunwald-Giemsa and analyzed by board-certified clinical pathologists;
- -
- After RNA extraction using the QIAsymphony® DSP Virus/Pathogen Mini Kit (Qiagen S.p.A., Milan, Italy), real-time PCR (Reliance One-Step Multiplex RT-qPCR Supermix, Bio-Rad Laboratories Srl, Segrate, Milan, Italy) was performed to detect feline coronavirus RNA using the primers and amplification protocol previously described in the literature [33].
- -
- FIP: 20 cases on which all the following changes were present: hyperproteinemia, hypoalbuminemia, inverted A/G ratio, serum protein electrophoresis characterized by increased α2 and γ-globulin (polyclonal peak), effusions macroscopically consistent with FIP (yellowish and thick, possibly with fibrin clots) and characterized by high SG (>1.015), high protein content (>20 g/L), albumin:globulin ratio <1.0, low cellularity (<5 × 109/L), high LDH/TNCC ratio (>0.62), cytology consistent with FIP (non-degenerated neutrophils, absence of bacteria, low numbers of macrophages and lymphocytes, and presence of a proteinaceous granular background), and positive RT-PCR for FCoV [11,13,14,15,16].
- -
- Not FIP: 15 cases on which macroscopical, physicochemical, and cytological analyses of the fluid were not consistent with FIP; RT-PCR for FCoV was negative; and cytology revealed the presence of a disease other than FIP (e.g., neoplastic, septic, or lymphocyte-rich effusions/chylous effusions), possibly associated with chemical findings suggestive of another condition (e.g., low protein content or high triglyceride content).
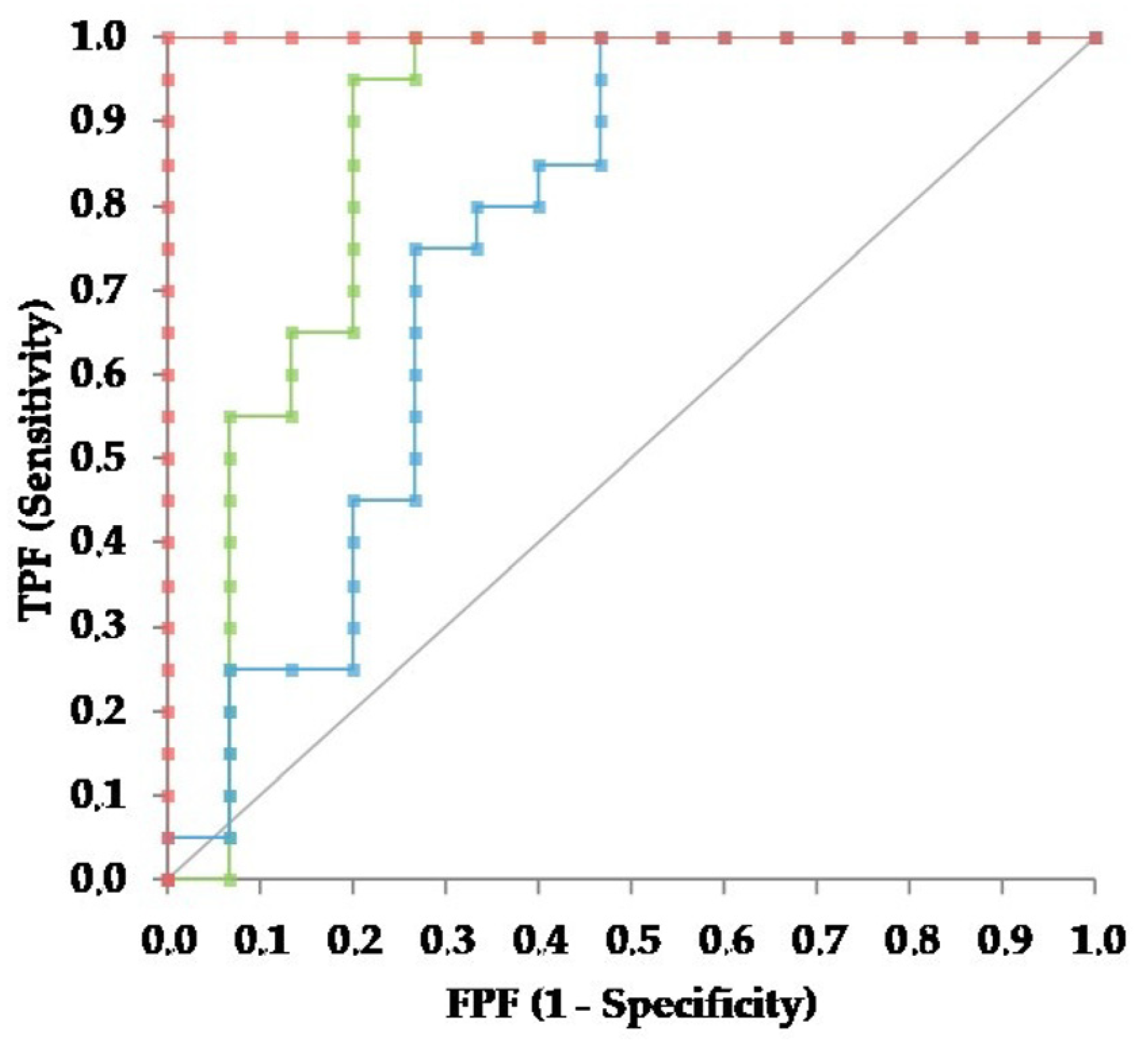
2.6. Statistical Analysis
- -
- True positives: samples from cats with FIP that had values higher than the operating point;
- -
- False positives: samples from cats without FIP that had values higher than the operating point;
- -
- False negatives: samples from cats with FIP that had values lower than the operating point;
- -
- True negatives: samples from cats without FIP that had values lower than the operating point.
3. Results
3.1. Precision and Accuracy of AGP Measurement
3.2. Group Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, B.G.; Perron, M.; Murakami, E.; Bauer, K.; Park, Y.; Eckstrand, C.; Liepnieks, M.; Pedersen, N.C. The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies. Vet. Microbiol. 2018, 219, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Delaplace, M.; Huet, H.; Gambino, A.; Le Poder, S. Feline coronavirus antivirals: A Review. Pathogens 2021, 10, 1150. [Google Scholar] [CrossRef]
- Tasker, S.; Addie, D.D.; Egberink, H.; Hofmann-Lehmann, R.; Hosie, M.J.; Truyen, U.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Feline infectious peritonitis: European Advisory Board on Cat Diseases guidelines. Viruses 2023, 15, 1847. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C.; Perron, M.; Bannasch, M.; Montgomery, E.; Murakami, E.; Liepnieks, M.; Liu, H.J. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J. Feline Med. Surg. 2019, 21, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Coggins, S.J.; Norris, J.M.; Malik, R.; Govendir, M.; Hall, E.J.; Kimble, B.; Thompson, M.F. Outcomes of treatment of cats with feline infectious peritonitis using parenterally administered remdesivir, with or without transition to orally administered GS-441524. J. Vet. Intern. Med. 2023, 37, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Zwicklbauer, K.; Krentz, D.; Bergmann, M.; Felten, S.; Dorsch, R.; Fischer, A.; Hofmann-Lehmann, R.; Meli, M.L.; Spiri, A.M.; Alberer, M.; et al. Long-term follow-up of cats in complete remission after treatment of feline infectious peritonitis with oral GS-441524. J. Feline Med. Surg. 2023, 25, 1098612X231183250. [Google Scholar] [CrossRef] [PubMed]
- Allinder, M.; Tynan, B.; Martin, C.; Furbish, A.; Austin, G.; Bartges, J.; Lourenço, B.N. Uroliths composed of antiviral compound GS-441524 in 2 cats undergoing treatment for feline infectious peritonitis. J. Vet. Intern. Med. 2024, 38, 370–374. [Google Scholar] [CrossRef]
- Taylor, S.S.; Coggins, S.; Barker, E.N.; Gunn-Moore, D.; Jeevaratnam, K.; Norris, J.M.; Hughes, D.; Stacey, E.; MacFarlane, L.; O’Brien, C.; et al. Retrospective study and outcome of 307 cats with feline infectious peritonitis treated with legally sourced veterinary compounded preparations of remdesivir and GS-441524 (2020–2022). J. Feline Med. Surg. 2023, 25, 1098612X231194460. [Google Scholar] [CrossRef]
- Fischer, Y.; Sauter-Louis, C.; Hartmann, K. Diagnostic accuracy of the Rivalta test for feline infectious peritonitis. Vet. Clin. Pathol. 2012, 41, 558–567. [Google Scholar] [CrossRef]
- Giordano, A.; Stranieri, A.; Rossi, G.; Paltrinieri, S. High diagnostic accuracy of the Sysmex XT-2000iV delta total nucleated cells on effusions for feline infectious peritonitis. Vet. Clin. Pathol. 2015, 44, 295–302. [Google Scholar] [CrossRef]
- Stranieri, A.; Giordano, A.; Paltrinieri, S.; Giudice, C.; Cannito, V.; Lauzi, S. Comparison of the performance of laboratory tests in the diagnosis of feline infectious peritonitis. J. Vet. Diagn. Investig. 2018, 30, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Tasker, S. Diagnosis of feline infectious peritonitis: Update on evidence supporting available tests. J. Feline Med. Surg. 2018, 20, 228–243. [Google Scholar] [CrossRef]
- Romanelli, P.; Paltrinieri, S.; Bonfanti, U.; Castaman, M.G.; Monza, E.; Bertazzolo, W. Utility of the ratio between lactate dehydrogenase (LDH) activity and total nucleated cell counts in effusions (LDH/TNCC ratio) for the diagnosis of feline infectious peritonitis (FIP). Animals 2022, 12, 2262. [Google Scholar] [CrossRef] [PubMed]
- Riemer, F.; Kuehner, K.A.; Ritz, S.; Sauter-Louis, C.; Hartmann, K. Clinical and laboratory features of cats with feline infectious peritonitis--a retrospective study of 231 confirmed cases (2000–2010). J. Feline Med. Surg. 2016, 18, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Stranieri, A.; Giordano, A.; Bo, S.; Braghiroli, C.; Paltrinieri, S. Frequency of electrophoretic changes consistent with feline infectious peritonitis in two different time periods (2004–2009 vs. 2013–2014). J. Feline Med. Surg. 2017, 19, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Felten, S.; Weider, K.; Doenges, S.; Gruendl, S.; Matiasek, K.; Hermanns, W.; Mueller, E.; Matiasek, L.; Fischer, A.; Weber, K.; et al. Detection of feline coronavirus spike gene mutations as a tool to diagnose feline infectious peritonitis. J. Feline Med. Surg. 2017, 19, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Felten, S.; Leutenegger, C.M.; Balzer, H.-J.; Pantchev, N.; Matiasek, K.; Wess, G.; Egberink, H.; Hartmann, K. Sensitivity and specificity of a real-time reverse transcriptase polymerase chain reaction detecting feline coronavirus mutations in effusion and serum/plasma of cats to diagnose feline infectious peritonitis. BMC Vet. Res. 2017, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Doenges, S.J.; Weber, K.; Dorsch, R.; Fux, R.; Hartmann, K. Comparison of real-time reverse transcriptase polymerase chain reaction of peripheral blood mononuclear cells, serum and cell-free body cavity effusion for the diagnosis of feline infectious peritonitis. J. Feline Med. Surg. 2017, 19, 344–350. [Google Scholar] [CrossRef]
- Longstaff, L.; Porter, E.; Crossley, V.J.; Hayhow, S.E.; Helps, C.R.; Tasker, S. Feline coronavirus quantitative reverse transcriptase polymerase chain reaction on effusion samples in cats with and without feline infectious peritonitis. J. Feline Med. Surg. 2017, 19, 240–245. [Google Scholar] [CrossRef]
- Günther, S.; Felten, S.; Wess, G.; Hartmann, K.; Weber, K. Detection of feline coronavirus in effusions of cats with and without feline infectious peritonitis using loop-mediated isothermal amplification. J. Virol. Methods 2018, 256, 32–36. [Google Scholar] [CrossRef]
- Felten, S.; Hartmann, K. Diagnosis of feline infectious peritonitis: A review of the current literature. Viruses 2019, 11, 1068. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.; Eckersall, P.D.; Addie, D.D.; Lawrence, C.E.; Jarrett, O. Value of alpha 1-acid glycoprotein in the diagnosis of feline infectious peritonitis. Vet. Rec. 1997, 141, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Spagnolo, V.; Colombo, A.; Paltrinieri, S. Changes in some acute phase protein and immunoglobulin concentrations in cats affected by feline infectious peritonitis or exposed to feline coronavirus infection. Vet. J. 2004, 167, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Hazuchova, K.; Held, S.; Neiger, R. Usefulness of acute phase proteins in differentiating between feline infectious peritonitis and other diseases in cats with body cavity effusions. J. Feline Med. Surg. 2017, 19, 809–816. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Giordano, A.; Tranquillo, V.; Guazzetti, S. Critical assessment of the diagnostic value of feline alpha1-acid glycoprotein for feline infectious peritonitis using the likelihood ratios approach. J. Vet. Diagn. Investig. 2007, 19, 266–272. [Google Scholar] [CrossRef]
- Giori, L.; Giordano, A.; Giudice, C.; Grieco, V.; Paltrinieri, S. Performances of different diagnostic tests for feline infectious peritonitis in challenging clinical cases. J. Small Anim. Pract. 2011, 52, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G. Acute phase proteins in cats: Diagnostic and prognostic role, future directions, and analytical challenges. Vet. Clin. Pathol. 2023, 52 (Suppl. S1), 37–49. [Google Scholar] [CrossRef]
- Bence, L.M.; Addie, D.D.; Eckersall, P.D. An immunoturbidimetric assay for rapid quantitative measurement of feline alpha-1-acid glycoprotein in serum and peritoneal fluid. Vet. Clin. Pathol. 2005, 34, 335–341. [Google Scholar] [CrossRef]
- Hazuchova, K.; Held, S.; Klemm, I.; Bauer, N. Simplified Light’s criteria and acute phase proteins reflect aetiology of feline body cavity effusions better than the traditional classification scheme. Animals 2023, 13, 1918. [Google Scholar] [CrossRef]
- Thalmeier, S.; Güssow, A.; Häuser, M.K.; Bauer, N.; Hazuchova, K. Cat alpha-1-acid glycoprotein enzyme-linked immunosorbent assay: Performance characteristics and reference intervals. J. Feline Med. Surg. 2023, 25, 1098612X231162836. [Google Scholar] [CrossRef]
- Addie, D.D.; Silveira, C.; Aston, C.; Brauckmann, P.; Covell-Ritchie, J.; Felstead, C.; Fosbery, M.; Gibbins, C.; Macaulay, K.; McMurrough, J.; et al. Alpha-1 acid glycoprotein reduction differentiated recovery from remission in a small cohort of cats treated for feline infectious peritonitis. Viruses 2022, 14, 744. [Google Scholar] [CrossRef] [PubMed]
- Waugh, E.M.; Haining, H.; Harvie, J.; Ridyard, A.E.; Eckersall, P.D. Validation of an automated immunoturbidimetric assay for feline serum amyloid A. BMC Vet. Res. 2022, 18, 359. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, E.; Mari, V.; Losurdo, M.; Lanave, G.; Trotta, A.; Dowgier, G.; Colaianni, M.L.; Zatelli, A.; Elia, G.; Buonavoglia, D.; et al. Discrepancies between feline coronavirus antibody and nucleic acid detection in effusions of cats with suspected fe-line infectious peritonitis. Res. Vet. Sci. 2019, 125, 421–424. [Google Scholar] [CrossRef]
- Kjelgaard-Hansen, M.; Jensen, A.L. Subjectivity in defining quality specifications for quality control and test validation. Vet. Clin. Pathol. 2010, 39, 133–135. [Google Scholar] [CrossRef]
- Gardner, I.A.; Greiner, M. Receiver-operating characteristic curves and likelihood ratios: Improvements over traditional methods for the evaluation and application of veterinary clinical pathology tests. Vet. Clin. Pathol. 2006, 35, 8–17. [Google Scholar] [CrossRef]
- Probo, M.; Valenti, V.; Venco, L.; Paltrinieri, S.; Lavergne, E.; Trumel, C.; Bertazzolo, W. Pleural lymphocyte-rich transudates in cats. J. Feline Med. Surg. 2018, 20, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Westgard, J.O. Method validation. In Basic Method Validation, 2nd ed.; Westgard, J.O., Ed.; Westgard QC: Madison, WI, USA, 2003; pp. 156–157. [Google Scholar]
- Tecles, F.; Muñoz, A.; Cerón, J.J.; Freeman, K. The number of replicates, and pooling versus individual measurements for analytical imprecision calculations: Does it matter? Vet. Clin. Pathol. 2020, 49, 112–118. [Google Scholar] [CrossRef]
- Ricós, C.; Alvarez, V.; Cava, F.; García-Lario, J.V.; Hernández, A.; Jiménez, C.V.; Minchinela, J.; Perich, C.; Simón, M. Current databases on biological variation: Pros, cons and progress. Scand. J. Clin. Lab. Investig. 1999, 59, 491–500. [Google Scholar] [CrossRef]
- Hayashi, Y.; Matsuda, R.; Maitani, T.; Imai, K.; Nishimura, W.; Ito, K.; Maeda, M. Precision, limit of detection and range of quantitation in competitive ELISA. Anal. Chem. 2004, 76, 1295–1301. [Google Scholar] [CrossRef]
- Joelsson, D.; Moravec, P.; Troutman, M.; Pigeon, J.; DePhillips, P. Optimizing ELISAs for precision and robustness using laboratory automation and statistical design of experiments. J. Immunol. Methods 2008, 337, 35–41. [Google Scholar] [CrossRef]
- Masseyeff, R.F. Standardization of immunoassays. Ann. Ist. Super Sanita. 1991, 27, 427–436. [Google Scholar]
- Sharma, K.; Fultariya, L.; Reddy Mallimala, P.; Shah, K.; Sharma, V. Comparison of the efficacy of Light’s criteria with serum-effusion albumin gradient and pleural effusion glucose. Cureus 2023, 15, e43319. [Google Scholar] [CrossRef] [PubMed]
- Upragarin, N.; Landman, W.J.; Gaastra, W.; Gruys, E. Extrahepatic production of acute phase serum amyloid A. Histol. Histopathol. 2005, 20, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Kalmovarin, N.; Friedrichs, W.E.; O’Brien, H.V.; Linehan, L.A.; Bowman, B.H.; Yang, F. Extrahepatic expression of plasma protein genes during inflammation. Inflammation 1991, 15, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Kjelgaard-Hansen, M.; Christensen, M.B.; Lee, M.H.; Jensen, A.L.; Jacobsen, S. Serum amyloid A isoforms in serum and synovial fluid from spontaneously diseased dogs with joint diseases or other conditions. Vet. Immunol. Immunopathol. 2007, 117, 296–301. [Google Scholar] [CrossRef]
- Lecchi, C.; Dilda, F.; Sartorelli, P.; Ceciliani, F. Widespread expression of SAA and Hp RNA in bovine tissues after evaluation of suitable reference genes. Vet. Immunol. Immunopathol. 2012, 145, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Lecchi, C.; Sauerwein, H.; Mielenz, M.; Häußler, S.; Restelli, L.; Giudice, C.; Ceciliani, F. Expression of α1-acid glycoprotein and lipopolysaccharide binding protein in visceral and subcutaneous adipose tissue of dairy cattle. Vet. J. 2015, 203, 223–227. [Google Scholar] [CrossRef]
- Albert, A. On the use and computation of likelihood ratios in clinical chemistry. Clin. Chem. 1982, 28, 1113–1119. [Google Scholar] [CrossRef]

| Specimen | Sample | Intra-Assay | Inter-Assay | ||
|---|---|---|---|---|---|
| AGP (μg/mL) | CV | AGP (μg/mL) | CV | ||
| Serum | Duplicate samples | 151.7–4244.4 | 5.4% (3.2–7.8%) | 226.9–4474.2 | 10.1% (6.4–14.6%) |
| Low pool | 180.1 | 13.5% | 225.4 | 10.2% | |
| High pool | 2727.7 | 8.6% | 2865.4 | 13.4% | |
| Effusion | Duplicate samples | 96.7–4316.6 | 5.9% (3.0–7.1%) | 87.0–4207.8 | 10.6% (3.7–13.9%) |
| Low pool | 196.3 | 9.2% | 217.2 | 10.4% | |
| High pool | 3828.5 | 12.8% | 3674.6 | 9.2% | |
| Group | Serum AGP (μg/mL) | Effusion AGP (μg/mL) | Effusion/Serum AGP |
|---|---|---|---|
| FIP (n = 20) | 2239 ± 1283 (1968) 1216–3371 (405–4428) | 1736 ± 832 (1717) 1011–2379 (549–3166) | 1.08 ± 1.43 (0.75) 0.63–0.98 (0.46–7.09) |
| Not FIP (n = 15) | 1173 ± 1397 (296) 246–1963 (165–4254) | 679 ± 1042 (233) 165–566 (103–4099) | 0.75 ± 0.46 (0.60) 0.50–1.03 (0.04–2.00) |
| p = 0.009 | p < 0.001 | p = 0.243 |
| Group | TNCC × 10³/μL | Serum AGP (μg/mL) | Effusion AGP (μg/mL) | Effusion/Serum AGP |
|---|---|---|---|---|
| Chylous (n = 4) | 14.4 ± 10.7 (9.7) 8.0–22.4 (7.9–30.3) | 219 ± 41 (228) 184–252 (165–257) | 168 ± 45 (180) 134–198 (103–209) | 0.78 ± 0.24 (0.78) 0.58–0.98 (0.49–1.07) |
| Hemorrhagic (n = 1) | 2.0 | 990 | 434 | 0.44 |
| LRT (n = 1) | 0.8 | 192 | 233 | 1.21 |
| Neoplastic (n = 6) | 238.3–499.1 (41.1) 17.6–170.1 (1.3–1255.9) | 1127 ± 1557 (560) 277–1142 (249–4254) | 310 ± 182 (257) 159–451 (156–592) | 0.70 ± 0.67 (0.54) 0.38–0.76 (0.04–2.00) |
| Septic (n = 3) | 322.8 ± 41.0 (344.4) 286.9–347.7 (275.4–348.4) | 2926 ± 815 (2840) 2272–3624 (2158–3780) | 2329 ± 1540 (1590) 1347–3681 (1299–4099) | 0.75 ± 0.29(0.60) 0.57–1.00 (0.56–1.08) |
| Diagnostic Indicator | Serum AGP | Effusion AGP | Effusion/Serum AGP |
|---|---|---|---|
| 100%Se | <438 μg/mL | <296 μg/mL | <0.44 |
| 100% Sp | >4099 μg/mL | >4254 μg/mL | >2.00 |
| Maximum LR+ | 8.50 (>1590 μg/mL) | 3.75 (>3780.3 μg/mL) | 1.80 (>0.71) |
| Optimal Se/Sp | 707 μg/mL (Se 80%; Sp 80%) | 990 μg/mL (Se 75%; Sp 73%) | 0.69 (Se 60%; Sp 60%) |
| Max Youden index | 0.750 (592 μg/mL) (Se 96%, Sp 80%) | 0.533 (296 μg/mL) (Se 100%, Sp 53%) | 0.367 (0.56) (Se 90%, Sp 47%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanelli, P.; Bertazzolo, W.; Prisciandaro, A.; Leone, A.; Bonfanti, U.; Paltrinieri, S. Measurement of Feline Alpha-1 Acid Glycoprotein in Serum and Effusion Using an ELISA Method: Analytical Validation and Diagnostic Role for Feline Infectious Peritonitis. Pathogens 2024, 13, 289. https://doi.org/10.3390/pathogens13040289
Romanelli P, Bertazzolo W, Prisciandaro A, Leone A, Bonfanti U, Paltrinieri S. Measurement of Feline Alpha-1 Acid Glycoprotein in Serum and Effusion Using an ELISA Method: Analytical Validation and Diagnostic Role for Feline Infectious Peritonitis. Pathogens. 2024; 13(4):289. https://doi.org/10.3390/pathogens13040289
Chicago/Turabian StyleRomanelli, Pierpaolo, Walter Bertazzolo, Andrea Prisciandaro, Andrea Leone, Ugo Bonfanti, and Saverio Paltrinieri. 2024. "Measurement of Feline Alpha-1 Acid Glycoprotein in Serum and Effusion Using an ELISA Method: Analytical Validation and Diagnostic Role for Feline Infectious Peritonitis" Pathogens 13, no. 4: 289. https://doi.org/10.3390/pathogens13040289
APA StyleRomanelli, P., Bertazzolo, W., Prisciandaro, A., Leone, A., Bonfanti, U., & Paltrinieri, S. (2024). Measurement of Feline Alpha-1 Acid Glycoprotein in Serum and Effusion Using an ELISA Method: Analytical Validation and Diagnostic Role for Feline Infectious Peritonitis. Pathogens, 13(4), 289. https://doi.org/10.3390/pathogens13040289






