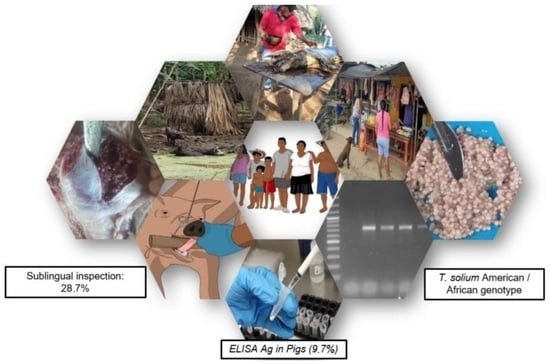
The Frequency of Porcine Cysticercosis and Factors Associated with Taenia solium Infection in the Municipality of Tuchín-Córdoba, Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Size
2.3. Sublingual Inspection
2.4. ELISA
2.5. Postmortem Inspection
2.6. Histopathological Analysis
2.7. DNA Extraction and PCR
2.8. Household Questionnaire
2.9. Statistical Analysis
3. Results
3.1. General Description of Pig Backyards
3.2. General Description of Population
3.3. Frequency of Porcine Cysticercosis
3.4. Risk Factors for Porcine T. solium Cysticercosis
4. Discussion
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases: Second WHO Report on Neglected Tropical Diseases; WHO: Geneva, Switzerland, 2013; XII, 140. Available online: https://apps.who.int/iris/handle/10665/77950 (accessed on 15 December 2023).
- Flisser, A. Cysticercosis: Neglected disease. Bol. Med. Hosp. Mex. 2011, 68, 138–145. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1665-11462011000200010&lng=pt (accessed on 15 August 2022).
- Póvoa, A.; Vieira, P.; Silva, A.; Pantazi, I.; Correia, J. Disseminated Cysticercosis. Eur. J. Case Rep. Intern. Med. 2021, 8, 002430. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, E. Teniasis/Cysticercosis: From conventional diagnosis to molecular diagnosis. Salus 2007, 11 (Suppl. S1), 57–61. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S1690-46482006000100001&lng=es (accessed on 15 December 2023).
- García, H.H.; Gonzalez, A.E.; Evans, C.A.; Gilman, R.H. Cysticercosis Working Group in Peru. Taenia solium cysticercosis. Lancet 2003, 362, 547–556. [Google Scholar] [CrossRef]
- Organización Panamericana de la Salud (OPS). Informe Primera Reunión Regional Sobre Control de Taenia solium en América Latina. Colombia. 2015. Available online: https://www.paho.org/hq/dmdocuments/2016/primera-reunion-regional-control-tena-solium-americas-2015.pdf (accessed on 15 December 2023).
- García, H.H.; González, A.E.; O´Neal, S.; Gilman, R.H. Grupo de Trabajo en Cisticercosis en Perú. Apuntes y recomendaciones para el establecimiento de programas de control de la teniasis / cisticercosis por Taenia solium en el Perú. Rev. Peru. Med. Exp. Salud Publica 2018, 35, 132–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kann, S.; Bruennert, D.; Hansen, J.; Mendoza, G.A.C.; Gonzalez, J.J.C.; Quintero, C.L.A.; Hanke, M.; Hagen, R.M.; Backhaus, J.; Frickmann, H. High Prevalence of Intestinal Pathogens in Indigenous in Colombia. J. Clin. Med. 2020, 9, 2786. [Google Scholar] [CrossRef]
- Rodríguez-Morales, A.J.; Yepes-Echeverri, M.C.; Acevedo-Mendoza, W.F.; Marín-Rincón, H.A.; Culquichicón, C.; Parra-Valencia, E.; Cardona-Ospina, J.A.; Flisser, A. Mapping the residual incidence of taeniasis and cysticercosis in Colombia, 2009-2013, using geographical information systems: Implications for public health and travel medicine. Travel Med. Infect Dis. 2018, 22, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Flórez, A.; Pastrán, S.M.; Vargas, N.S.; Beltrán, M.; Enriquez, Y.; Peña, A.; Muñoz, L. Cisticercosis en Colombia. Estudio de seroprevalencia 2008-2010. Acta Neurol. Colomb. 2013, 29, 73–86. [Google Scholar]
- Instituto Colombiano Agropecuario (ICA). Censo Nacional Porcino. 2021. Available online: https://www.ica.gov.co (accessed on 22 September 2022).
- Pawlowski, Z.; Allan, J.; Sarti, E. Control of Taenia solium taeniasis/cysticercosis: From research towards implementation. Int. J. Parasitol. 2005, 35, 1221–1232. [Google Scholar] [CrossRef]
- Organización Panamericana de la Salud. Pautas Operativas Para las Actividades de Control de la Teniasis y la Cisticercosis Causadas por Taenia solium; OPS: Washington, DC, USA, 2019; Available online: https://iris.paho.org/handle/10665.2/51660 (accessed on 22 August 2022).
- Departamento Administrativo Nacional de Estadística (DANE). Censo Nacional de Población y Vivienda. 2018. Available online: https://www.dane.gov.co/files/censo2018/informacion-tecnica/PERSONAS_DEMOGRAFICO_Cuadros_CNPV_2018.xlsx (accessed on 10 May 2022).
- Instituto Colombiano Agropecuario (ICA). Censo Pecuario Nacional. 2019. Available online: https://www.ica.gov.co/areas/pecuaria/servicios/epidemiologia-veterinaria/censos-2016/censo-2018 (accessed on 1 February 2022).
- Quintero, C.; Ruiz, L.; Ballut, C.; Moreno de Barco, N. Prevalencia de cisticercosis porcina en los municipios de Moñitos y Los Córdobas. MVZ 2000, 5, 9–22. Available online: https://imbiomed.com.mx/1/1/articulos.php?method=showDetail&id_articulo=40905&id_seccion=2714&id_ejemplar=4193&id_revista=162 (accessed on 15 December 2023).
- Thrusfield, M. Veterinary Epidemiology; Blackwell Science Ltd.: Oxford, UK, 1995; p. 624. [Google Scholar]
- Gonzalez, A.E.; Cama, V.; Gilman, R.H.; Tsang, V.C.; Pilcher, J.B.; Chavera, A.; Castro, M.; Montenegro, T.; Verastegui, M.; Miranda, E.; et al. Prevalence and comparison of serologic assays, necropsy, and tongue examination for the diagnosis of porcine cysticercosis in Peru. Am. J. Trop. Med. Hyg. 1990, 43, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de Sanidad Animal (OIE). Cisticercosis. Manual terrestre de la OIE. 2018. Available online: https://www.oie.int/fileadmin/Home/esp/Health_standards/tahm/3.09.05_Cisticercosis.pdf (accessed on 15 August 2022).
- Chembensofu, M.; Mwape, K.E.; Van Damme, I.; Hobbs, E.; Phiri, I.K.; Masuku, M.; Zulu, G.; Colston, A.; Willingham, A.L.; Devleesschauwer, B.; et al. Re-visiting the detection of porcine cysticercosis based on full carcass dissections of naturally Taenia solium infected pigs. Parasit. Vectors 2017, 10, 572. [Google Scholar] [CrossRef] [PubMed]
- Boa, M.E.; Kassuku, A.A.; Willingham, A.L., 3rd; Keyyu, J.D.; Phiri, I.K.; Nansen, P. Distribution and density of cysticerci of Taenia solium by muscle groups and organs in naturally infected local finished pigs in Tanzania. Vet. Parasitol. 2002, 106, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Allan, J.C.; Sato, M.O.; Nakao, M.; Sako, Y.; Nakaya, K.; Qiu, D.; Mamuti, W.; Craig, P.S.; Ito, A. DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J. Clin. Microbiol. 2004, 42, 548–553. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 5 March 2023).
- Phiri, I.; Dorny, P.; Gabriel, S.; Willingham, A.; Sikasunge, C.; Siziya, S.; Vercruysse, J. Assessment of routine inspection methods for porcine cysticercosis in Zambian village pigs. JHL 2006, 80, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de la Salud (OMS). Teniasis y Cisticercosis. 2020. Available online: https://www.who.int/es/news-room/fact-sheets/detail/taeniasis-cysticercosis (accessed on 19 September 2020).
- Forero, J.C.G.; Rodríguez, M.M.R.; Arteaga, L.R.V. Determinación de la seroprevalencia de cisticercosis porcina e identificación de teniasis humana en personas criadoras de cerdos en el área urbana del municipio de Coyaima Tolima. Rev. Med. 2017, 25, 31–45. [Google Scholar] [CrossRef]
- CystiTeam Group for Epidemiology and Modelling of Taenia solium Taeniasis/Cysticercosis. The World Health Organization 2030 goals for Taenia solium: Insights and perspectives from transmission dynamics modelling: CystiTeam Group for Epidemiology and Modelling of Taenia solium Taeniasis/Cysticercosis. Gates Open Res. 2019, 3, 1546. [Google Scholar] [CrossRef]
- Braae, U.C.; Magnussen, P.; Ndawi, B.; Harrison, W.; Lekule, F.; Johansen, M.V. Effect of repeated mass drug administration with praziquantel and track and treat of taeniosis cases on the prevalence of taeniosis in Taenia solium endemic rural communities of Tanzania. Acta Trop. 2017, 165, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Dorny, P.; Phiri, I.K.; Vercruysse, J.; Gabriël, S.; Willingham, A.L., III; Brandt, J.; Victor, B.; Speybroeck, N.; Berkvens, D. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. Int. J. Parasitol. 2004, 34, 569–576. [Google Scholar] [CrossRef]
- Nguekam, A.; Zoli, A.P.; Vondou, L.; Pouedet, S.M.; Assana, E.; Dorny, P.; Brandt, J.; Losson, B.; Geerts, S. Kinetics of circulating antigens in pigs experimentally infected with Taenia solium eggs. Vet. Parasitol. 2003, 111, 323–332. [Google Scholar] [CrossRef]
- Praet, N.; Kanobana, K.; Kabwe, C.; Maketa, V.; Lukanu, P.; Lutumba, P.; Polman, K.; Matondo, P.; Speybroeck, N.; Dorny, P.; et al. Taenia solium cysticercosis in the Democratic Republic of Congo: How does pork trade affect the transmission of the parasite? PLoS Negl. Trop. Dis. 2010, 4, e817. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.; Wilkins, P.; Dorny, P. Immunological and molecular diagnosis of cysticercosis. Pathog. Glob. Health 2012, 106, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.R.; Geerts, S.; De Deken, R.; Kumar, V.; Ceulemans, F.; Brijs, L.; Falla, N. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int. J. Parasitol. 1992, 22, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.J.; Joshua, G.W.; Wright, S.H.; Parkhouse, R.M. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol. 1989, 11, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Deckers, N.; Kanobana, K.; Silva, M.; Gonzalez, A.E.; García, H.H.; Gilman, R.H.; Dorny, P. Serological responses in porcine cysticercosis: A link with the parasitological outcome of infection. Int. J. Parasitol. 2008, 38, 1191–1198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poudel, I.; Sah, K.; Subedi, S.; Kumar Singh, D.; Kushwaha, P.; Colston, A.; Gauci, C.G.; Donadeu, M.; Lightowlers, M.W. Implementation of a practical and effective pilot intervention against transmission of Taenia solium by pigs in the Banke district of Nepal. PLoS Negl. Trop. Dis. 2019, 13, e0006838. [Google Scholar] [CrossRef]
- Acevedo-Nieto, E.C.; Pinto, P.S.A.; Silva, L.F.; Guimarães-Peixoto, R.P.M.; Santos, T.O.; Bevilacqua, P.D. Prevalence and risk factors for porcine cysticercosis in rural communities of eastern Minas Gerais, Brazil. Pesqui. Vet. Bras. 2017, 37, 905–910. [Google Scholar] [CrossRef]
- Rodríguez-Hidalgo, R.; Benítez-Ortiz, W.; Dorny, P.; Geerts, S.; Geysen, D.; Ron-Román, J.; Proaño-Pérez, P.; Chávez-Larrea, M.A.; Barrionuevo-Samaniego, M.; Celi-Erazo, M.; et al. Taeniosis-cysticercosis in man and animals in the Sierra of Northern Ecuador. Vet. Parasitol. 2003, 118, 51–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khaing, T.A.; Bawm, S.; Wai, S.S.; Htut, Y.; Htun, L.L. Epidemiological Survey on Porcine Cysticercosis in Nay Pyi Taw Area, Myanmar. J. Vet. Med. 2015, 2015, 340828. [Google Scholar] [CrossRef][Green Version]
- Singh, S.P.; Singh, B.B.; Kalambhe, D.G.; Pathak, D.; Aulakh, R.S.; Dhand, N.K. Prevalence and distribution of Taenia solium cysticercosis in naturally infected pigs in Punjab, India. PLoS Negl. Trop. Dis. 2018, 12, e0006960. [Google Scholar] [CrossRef]
- Sithole, M.I.; Bekker, J.L.; Tsotetsi-Khambule, A.M.; Mukaratirwa, S. Ineffectiveness of meat inspection in the detection of Taenia solium cysticerci in pigs slaughtered at two abattoirs in the Eastern Cape Province of South Africa. Vet. Parasitol. Reg. Stud. Rep. 2019, 17, 100299. [Google Scholar] [CrossRef]
- Da Silva, M.R.M.; Uyhara, C.N.S.; Silva, F.H.; Espindola, N.M.; Poleti, M.D.; Vaz, A.J.; Meirelles, F.V.; Maia, A.A.M. Cysticercosis in experimentally and naturally infected pigs: Parasitological and immunological diagnosis. Vet. Bras. 2012, 32, 297–302. [Google Scholar] [CrossRef]
- Lightowlers, M.W.; Assana, E.; Jayashi, C.M.; Gauci, C.G.; Donadeu, M. Sensitivity of partial carcass dissection for assessment of porcine cysticercosis at necropsy. Int. J. Parasitol. 2015, 45, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.O.; Sako, Y.; Nakao, M.; Yamasaki, H.; Nakaya, K.; Ito, A. Evaluation of Purified Taenia solium Glycoproteins and Recombinant Antigens in the Serologic Detection of Human and Swine Cysticercosis. J. Infect. Dis. 2006, 194, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Sreedevi, C.; Hafeez, M.; Kumar, P.A.; Rayulu, V.C.; Subramanyam, K.V.; Sudhakar, K. PCR test for detecting Taenia solium cysticercosis in pig carcasses. Trop. Anim. Health Prod. 2012, 44, 95–99. [Google Scholar] [CrossRef]
- Sarti Gutierrez, E.; Schantz, P.M.; Aguilera, J.; Lopez, A. Epidemiologic observations on porcine cysticercosis in a rural community of Michoacan State, Mexico. Vet. Parasitol. 1992, 41, 195–201. [Google Scholar] [CrossRef]
- Diaz, F.; Garcia, H.H.; Gilman, R.H.; Gonzales, A.E.; Castro, M.; Tsang, V.C.W.; Pilcher, J.B.; Vaswuez, L.E.; Lescano, M.; Carcamo, C.; et al. Cysticercosis Working Group in Peru, epidemiology of taeniosis and cysticercosis in a Peruvian village. Am. J. Epidemiol. 1992, 135, 875–882. [Google Scholar] [CrossRef][Green Version]
- Shey-Njila, O.; Zoli, P.A.; Awah-Ndukum, J.; Nguekam; Assana, E.; Myambas, P.; Dorny, O.; Brandt, J.; Geerts, S. Porcine cysticercosis in village pigs of North-West Cameroon. JHL 2003, 77, 351–354. [Google Scholar] [CrossRef]
- Ngowi, H.A.; Kassuku, A.A.; Maeda, G.E.; Boa, M.E.; Carabin, H.; Willingham, A.L., III. Risk factors for the prevalence of porcine cysticercosis in Mbulu District, Tanzania. Vet. Parasitol. 2004, 120, 275–283. [Google Scholar] [CrossRef]
- Krecek, R.C.; Mohammed, H.; Michael, L.M.; Schantz, P.M.; Ntanjana, L.; Morey, L.; Rakem Werre, S.; Willingham, A.L., III. Risk factors of porcine cysticercosis in the Eastern Cape Province, South Africa. PLoS ONE 2012, 7, e37718. [Google Scholar] [CrossRef]
- Eshitera, E.E.; Githigia, S.M.; Kitala, P.; Thomas, L.F.; Fèvre, E.M.; Harrison, L.J.; Mwihia, E.W.; Otieno, R.O.; Ojiambo, F.; Maingi, N. Prevalence of porcine cysticercosis and associated risk factors in Homa Bay District, Kenya. BMC Vet. Res. 2012, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Boa, M.E.; Mahundi, E.A.; Kassuku, A.A.; Willingham, A.L., 3rd; Kyvsgaard, N.C. Epidemiological survey of swine cysticercosis using ante-mortem and post-mortem examination tests in the southern highlands of Tanzania. Vet. Parasitol. 2006, 139, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Sikasunge, C.S.; Phiri, I.K.; Phiri, A.M.; Dorny, P.; Siziya, S.; Willingham, A.L., 3rd. Risk factors associated with porcine cysticercosis in selected districts of Eastern and Southern provinces of Zambia. Vet. Parasitol. 2007, 143, 59–66. [Google Scholar] [CrossRef]
- Carabin, H.; Millogo, A.; Cissé, A.; Gabriël, S.; Sahlu, I.; Dorny, P.; Bauer, C.; Tarnagda, Z.; Covan, L.D.; Ganaba, R. Prevalence of and factors associated with human cysticercosis in 60 villages in three provinces of Burkina Faso. PLoS Negl. Trop. Dis. 2015, 9, e0004248. [Google Scholar] [CrossRef]
| Name | Sequence | Size (bp) | PCR Conditions |
|---|---|---|---|
| T. asiatica | 5′-ACGGTTGGATTAGATGTTAAGACTA-3 | 269 | 35 cycles: 94 °C 30 s. 60 °C 30 s. 72 °C 90 s. |
| T. solium American/African genotype | 5′-GGTAGATTTTTTAATGTTTTCTTTA-3′, | 720 | |
| T. solium Asian genotype | 5′-TTGTTATAAATTTTTGATTACTAAC-3 | 984 | |
| Common reverse primer | Rev, 5′-GACATAACATAATGAAAATG-3 |
| Community Number | Sublingual Inspection | Ag-ELISA | ||||||
|---|---|---|---|---|---|---|---|---|
| Pigs (n) | Positive Pigs | Frequency (%) | 95% CI | Pigs (n) | Positive Pigs | Frequency (%) | 95% CI | |
| 1 | 9 | 2 | 22.2 | (2.8–60) | 10 | 1 | 10 | (0.2–44.5) |
| 3 | 6 | 1 | 16.6 | (0.4–64.12) | 26 | 2 | 7.7 | (0.9–25.1) |
| 4 | 5 | 2 | 40 | (5.3–85.3) | 6 | 1 | 16.7 | (0.4–64.1) |
| 7 | 3 | 1 | 33.3 | (0.8–90.6) | 16 | 1 | 6.2 | (0.2–30.2) |
| 8 | 7 | 2 | 28.6 | (3.7–7.9) | 34 | 3 | 8.8 | (1.8–23.7) |
| 9 | 9 | 3 | 33.3 | (4.5–70.1) | 38 | 6 | 15.8 | (6.0–31.2) |
| 15 | 8 | 2 | 25 | (3.2–65.1) | 18 | 3 | 16.7 | (3.6–41.4) |
| 16 | 11 | 5 | 45.5 | (16.7–76.6) | 24 | 13 | 54.2 | (32.8–74.4) |
| 18 | 6 | 1 | 16.7 | (0.4–64.1) | 10 | 2 | 20 | (2.5–55.7) |
| 19 | 8 | 1 | 12.5 | (0.3–52.6) | 20 | 4 | 20 | (5.7–43.7) |
| 23 | 7 | 3 | 42.9 | (9.9–81.6) | 18 | 7 | 38.9 | (17.3–64.2) |
| 27 | 3 | 1 | 33.3 | (0.8–90.6) | 8 | 2 | 25 | (3.2–65.1) |
| 29 | 5 | 1 | 20 | (0.5–71.6) | 18 | 1 | 5.6 | (0.1–27.3) |
| Negative 1 | 0 | 0 | 0 | 226 | 0 | 0 | ||
| Total | 87 | 25 | 28.7 | (19.4–39.4) | 472 | 46 | 9.7 | (7.2–12.8) |
| Factor | Level | n | Positive Case | Negative Case | Odd Ratio | p Value |
|---|---|---|---|---|---|---|
| Pigs raised in a free-range environment | Yes | 259 | 33 | 226 | 2.315 (1.128–4.754) | 0.01 |
| No | 213 | 13 | 200 | |||
| Consumption of pork with visible cysticerci | Yes | 51 | 11 | 40 | 2.696 (1.202–6.045) | 0.01 |
| No | 421 | 35 | 386 | |||
| Commercialized the pigs at home | Yes | 242 | 31 | 211 | 3.113 (1.485–6.526) | 0.00 |
| No | 230 | 15 | 215 | |||
| Sale of pigs with cysticercosis | Yes | 39 | 8 | 31 | 3.234 (1.373–7.616) | 0.00 |
| No | 433 | 38 | 395 | |||
| Pigs had access to consume human feces | Yes | 305 | 40 | 265 | 3.367 (1.383–8.194) | 0.00 |
| No | 167 | 6 | 161 | |||
| No washing of hands before and after handling food | Yes | 118 | 21 | 97 | 3.408 (1.763–6.590) | 0.00 |
| No | 354 | 25 | 329 | |||
| Did not know the consequences of eating pork with cysticerci | Yes | 172 | 29 | 143 | 3.638 (1.844–7.178) | 0.00 |
| No | 300 | 17 | 283 | |||
| Lack of knowledge of transmission of Taenia solium | Yes | 339 | 42 | 297 | 3.832 (1.336–10.986) | 0.00 |
| No | 133 | 4 | 129 | |||
| Slaughtered pigs at home | Yes | 262 | 34 | 228 | 4.179 (1.809–9.653) | 0.00 |
| No | 210 | 12 | 198 | |||
| No | 451 | 34 | 417 | |||
| Lack of knowledge of transmission of porcine cysticercosis | Yes | 16 | 5 | 11 | 5.468 (1.798–16.623) | 0.00 |
| No | 456 | 41 | 415 | |||
| No | 466 | 38 | 428 | |||
| The pigs that die are destined for consumption by other animals | Yes | 271 | 41 | 230 | 5.814 (2.235–15.123) | 0.00 |
| No | 201 | 5 | 196 | |||
| No habit of washing hands after defecation | Yes | 112 | 24 | 88 | 5.864 (2.987–11.511) | 0.00 |
| No | 360 | 22 | 338 | |||
| Use of antiparasitic treatments in pigs | Yes | 204 | 9 | 195 | 10.732 (3.259–35.334) | 0.00 |
| No | 268 | 37 | 231 | |||
| Illiterate | Yes | 198 | 41 | 157 | 15.000 (5.243–42.916) | 0.00 |
| No | 274 | 5 | 269 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arango-Londoño, M.M.; López-Osorio, S.; Rojas-Bermudéz, F.; Chaparro-Gutiérrez, J.J. The Frequency of Porcine Cysticercosis and Factors Associated with Taenia solium Infection in the Municipality of Tuchín-Córdoba, Colombia. Pathogens 2024, 13, 311. https://doi.org/10.3390/pathogens13040311
Arango-Londoño MM, López-Osorio S, Rojas-Bermudéz F, Chaparro-Gutiérrez JJ. The Frequency of Porcine Cysticercosis and Factors Associated with Taenia solium Infection in the Municipality of Tuchín-Córdoba, Colombia. Pathogens. 2024; 13(4):311. https://doi.org/10.3390/pathogens13040311
Chicago/Turabian StyleArango-Londoño, Margarita M., Sara López-Osorio, Fernando Rojas-Bermudéz, and Jenny J. Chaparro-Gutiérrez. 2024. "The Frequency of Porcine Cysticercosis and Factors Associated with Taenia solium Infection in the Municipality of Tuchín-Córdoba, Colombia" Pathogens 13, no. 4: 311. https://doi.org/10.3390/pathogens13040311
APA StyleArango-Londoño, M. M., López-Osorio, S., Rojas-Bermudéz, F., & Chaparro-Gutiérrez, J. J. (2024). The Frequency of Porcine Cysticercosis and Factors Associated with Taenia solium Infection in the Municipality of Tuchín-Córdoba, Colombia. Pathogens, 13(4), 311. https://doi.org/10.3390/pathogens13040311