Trans-Cinnamaldehyde Primes More Robust Channel Catfish Immune Responses to Edwardsiella ictaluri Infection
Abstract
:1. Introduction
2. Material and Methods
2.1. Animal Ethical Oversight
2.2. Fish Management
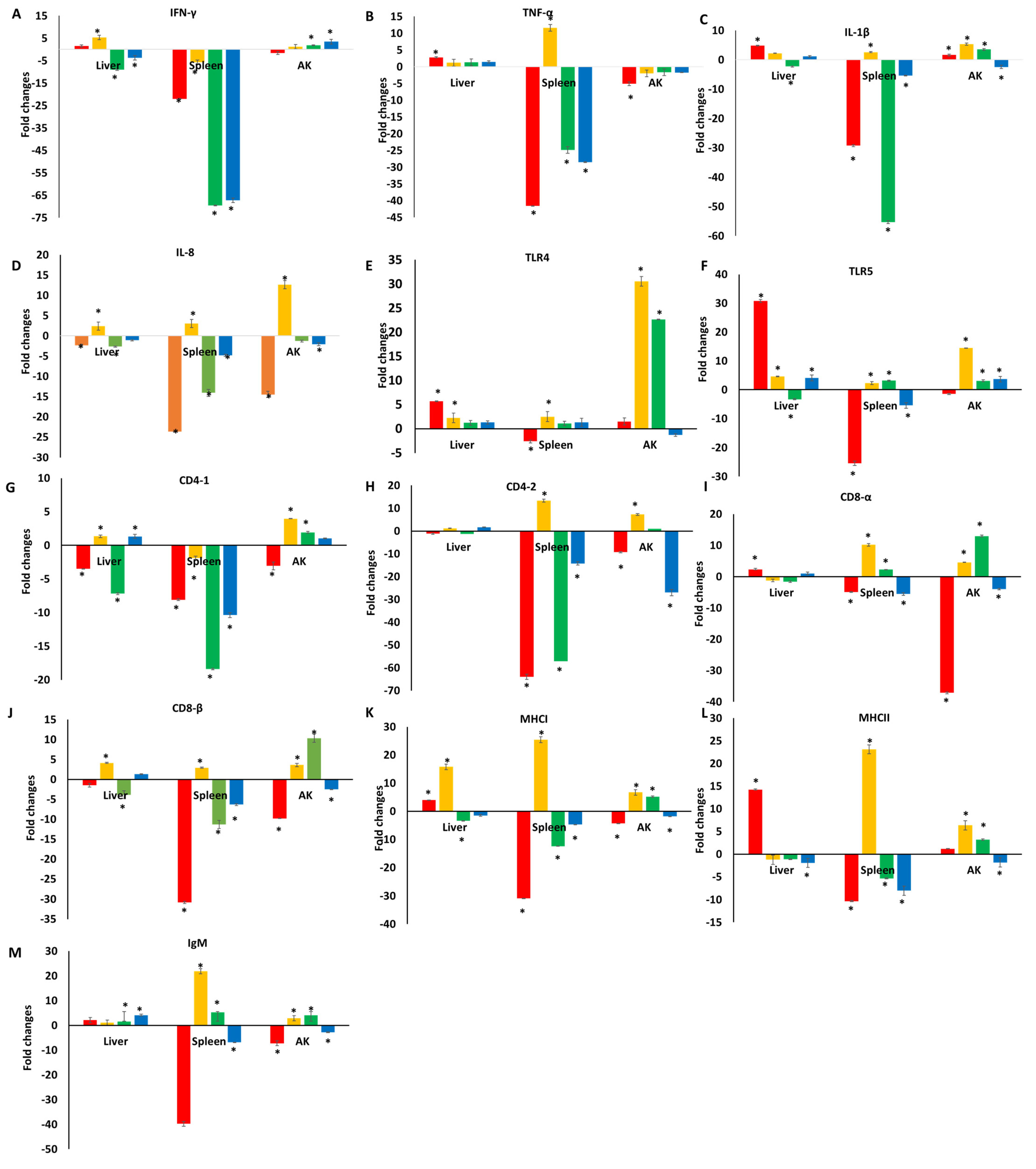
2.3. Analyses of Immune-Related Gene Expression in Catfish Fed Supplemental TC
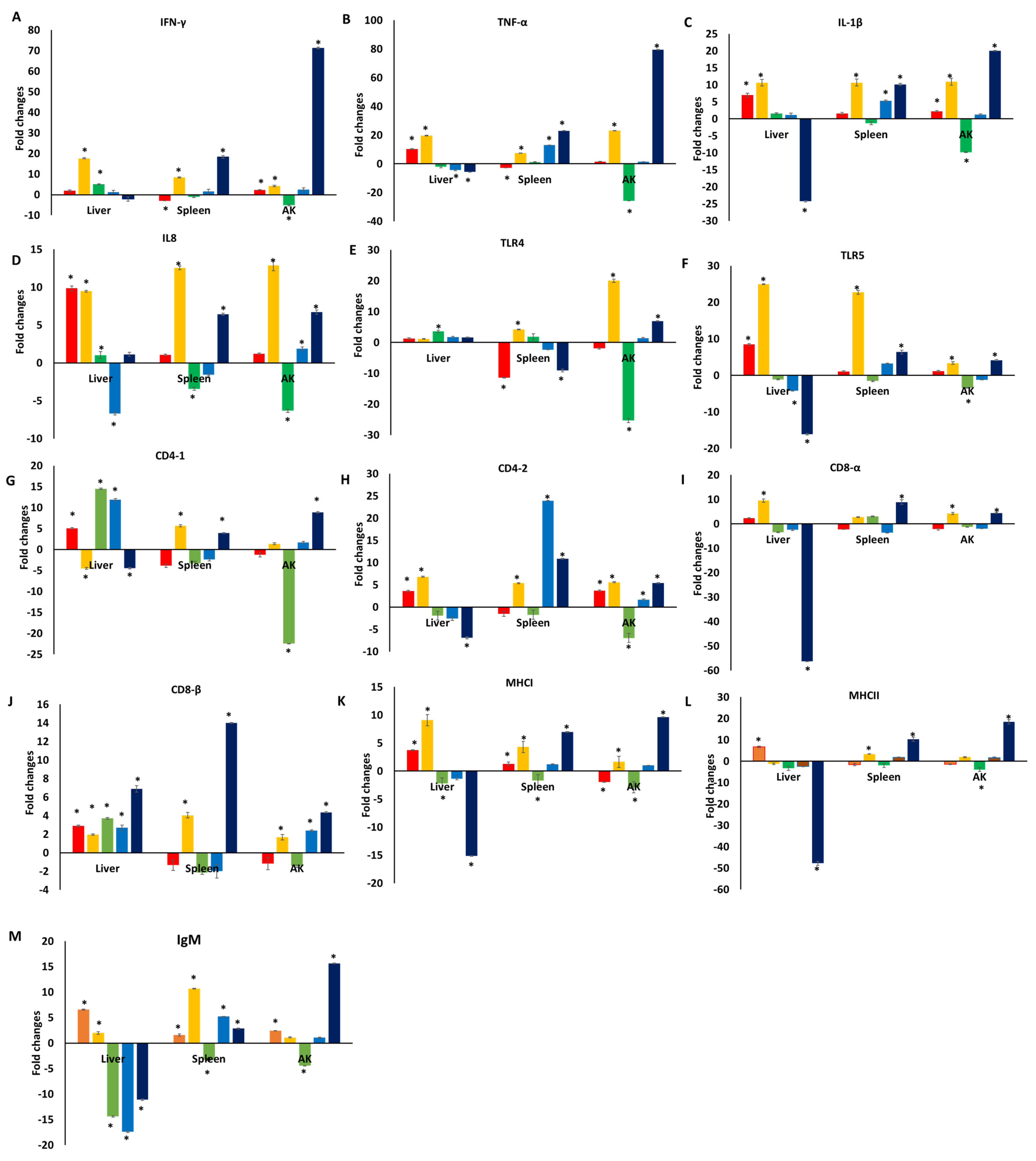
2.4. Analyses of Immune-Related Gene Expression in Catfish Fed TC following E. ictaluri Challenge
2.5. RNA Isolation and cDNA Synthesis
2.6. RT-qPCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Immune Responses of Catfish Fed a Diet Supplemented with TC
3.2. Immune Responses of Catfish Fed a Diet Supplemented with TC following E. ictaluri Challenge
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- USDA. Catfish Production, by the National Agricultural Statistics Service (NASS), Agricultural Statistics Board, United States Department of Agriculture (USDA). 2022. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/bg257f046/gh93jb451/rf560m06c/cfpd0223.pdf (accessed on 2 April 2024).
- Wise, A.L.; LaFrentz, B.R. A Review of Bacterial Co-Infections in Farmed Catfish: Components, Diagnostics, and Treatment Directions. Animals 2021, 11, 3240. [Google Scholar] [CrossRef]
- Hossain, M.J.; Sun, D.; McGarey, D.J.; Wrenn, S.; Alexander, L.M.; Martino, M.E.; Xing, Y.; Terhune, J.S.; Liles, M.R. An Asian origin of virulent Aeromonas hydrophila responsible for disease epidemics in United States-farmed catfish. mBio 2014, 5, e00848-14. [Google Scholar] [CrossRef]
- Hemstreet, B. An update on Aeromonas hydrophila from a fish health specialist for summer 2010. Catfish J. 2010, 24, 4. [Google Scholar]
- Wagner, B.A.; Wise, D.J.; Khoo, L.H.; Terhune, J.S. The Epidemiology of Bacterial Diseases in Food-Size Channel Catfish. J. Aquat. Anim. Health 2006, 18, 263–272. [Google Scholar] [CrossRef]
- Peterman, M.A.; Posadas, B.C. Direct economic impact of fish diseases on the East Mississippi catfish industry. N. Am. J. Aquac. 2019, 81, 222–229. [Google Scholar] [CrossRef]
- Wise, D.J.; Greenway, T.; Li, M.H.; Camus, A.C.; Robinson, E.H. Effects of variable periods of food deprivation on the development of enteric septicemia in channel catfish. J. Aquat. Anim. Health 2008, 20, 39–44. [Google Scholar] [CrossRef]
- Wise, D.J.; Greenway, T.E.; Byars, T.S.; Kumar, G.; Griffin, M.J.; Khoo, L.H.; Chesser, G.; Lowe, J. Validation of Edwardsiella ictaluri oral vaccination platform in experimental pond trials. J. World Aquac. Soc. 2020, 51, 346–363. [Google Scholar] [CrossRef]
- Kumar, G.; Gaunt, P. Medicated-Feed Intervention in Catfish Farming: An Economic Perspective. N. Am. J. Aquac. 2020, 82, 190–199. [Google Scholar] [CrossRef]
- Islam, S.; Riman, M.M.; Mannan, S.; Lawrence, M.L.; Abdelhamed, H. Characterisation and mobilisation of IncA/C plasmid-mediated antibiotic resistance in Edwardsiella ictaluri. J. Glob. Antimicrob. Resist. 2023, 33, 177–185. [Google Scholar] [CrossRef]
- Ali, I.A.A.; Matinlinna, J.P. Trans-Cinnamaldehyde Attenuates Enterococcus faecalis Virulence and Inhibits Biofilm Formation. Antibiotics. 2021, 10, 702. [Google Scholar] [CrossRef]
- Abdelhamed, H.; Ozdemir, O.; Ibrahim, I.; Lawrence, M.; Karsi, A. Antibacterial activities of trans-cinnamaldehyde, caprylic acid, and β-resorcylic acid against catfish pathogens. Aquaculture 2019, 504, 334–344. [Google Scholar] [CrossRef]
- Bisogno, F.; Mascoti, L.; Sanchez, C.; Garibotto, F.; Giannini, F.; Kurina-Sanz, M.; Enriz, R. Structure−Antifungal Activity Relationship of Cinnamic Acid Derivatives. J. Agric. Food Chem. 2007, 55, 10635–10640. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and Antimicrobial Activities of Cinnamic Acid Derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.R.; Costin, J.M.; Fink, R.C.; McMichael, M.; Fontaine, K.A.; Isern, S.; Michael, S.F. In vitro inhibition of dengue virus entry by p-sulfoxy-cinnamic acid and structurally related combinatorial chemistries. Antivir. Res. 2008, 80, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Yakhchali, M.; Taghipour, Z.; Mirabzadeh Ardakani, M.; Alizadeh Vaghasloo, M.; Vazirian, M.; Sadrai, S. Cinnamon and its possible impact on COVID-19: The viewpoint of traditional and conventional medicine. Biomed. Pharmacother. 2021, 143, 112221. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Upadhyaya, I.; Kollanoor-Johny, A.; Venkitanarayanan, K. Combating Pathogenic Microorganisms Using Plant-Derived Antimicrobials: A Minireview of the Mechanistic Basis. BioMed Res. Int. 2014, 2014, 761741. [Google Scholar] [CrossRef] [PubMed]
- Vijayaram, S.; Sun, Y.-Z.; Zuorro, A.; Ghafarifarsani, H.; Van Doan, H.; Hoseinifar, S.H. Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish Shellfish Immunol. 2022, 130, 294–308. [Google Scholar]
- Yılmaz, S.; Ergün, S. Trans-cinnamic acid application for rainbow trout (Oncorhynchus mykiss): I. Effects on haematological, serum biochemical, non-specific immune and head kidney gene expression responses. Fish Shellfish Immunol. 2018, 78, 140–157. [Google Scholar] [CrossRef]
- Kiron, V. Fish immune system and its nutritional modulation for preventive health care. Anim. Feed Sci. Technol. 2012, 173, 111–133. [Google Scholar] [CrossRef]
- Kordon, A.O.; Abdelhamed, H.; Karsi, A.; Pinchuk, L.M. Adaptive immune responses in channel catfish exposed to Edwardsiella ictaluri live attenuated vaccine and wild type strains through the specific gene expression profiles. Dev. Comp. Immunol. 2021, 116, 103950. [Google Scholar] [CrossRef]
- Flecknell, P. Chapter 6—Anaesthesia of common laboratory species: Special considerations. In Laboratory Animal Anaesthesia and Analgesia, 5th ed.; Flecknell, P., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 215–282. [Google Scholar]
- Conroy, C.J.; Papenfuss, T.; Parker, J.; Hahn, N.E. Use of tricaine methanesulfonate (MS222) for euthanasia of reptiles. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 28–32. [Google Scholar]
- Abdelhamed, H.; Lu, J.; Shaheen, A.; Abbass, A.; Lawrence, M.L.; Karsi, A. Construction and evaluation of an Edwardsiella ictaluri fhuC mutant. Vet Microbiol. 2013, 162, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Karsi, A.; Waldbieser, G.C.; Small, B.C.; Liu, Z.; Wolters, W.R. Molecular cloning of proopiomelanocortin cDNA and multi-tissue mRNA expression in channel catfish. Gen. Comp. Endocrinol. 2004, 137, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Kordon, A.O.; Abdelhamed, H.; Ahmed, H.; Baumgartner, W.; Karsi, A.; Pinchuk, L.M. Assessment of the Live Attenuated and Wild-Type Edwardsiella ictaluri-Induced Immune Gene Expression and Langerhans-Like Cell Profiles in the Immune-Related Organs of Catfish. Front. Immunol. 2019, 10, 432907. [Google Scholar] [CrossRef] [PubMed]
- Nik Mohamad Nek Rahimi, N.; Natrah, I.; Loh, J.-Y.; Ervin Ranzil, F.K.; Gina, M.; Lim, S.-H.E.; Lai, K.-S.; Chong, C.-M. Phytocompounds as an alternative antimicrobial approach in aquaculture. Antibiotics 2022, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Oulahal, N.; Degraeve, P. Phenolic-rich plant extracts with antimicrobial activity: An alternative to food preservatives and biocides? Front. Microbiol. 2022, 12, 753518. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Quiniou, S.M.; Boudinot, P.; Bengtén, E. Comprehensive survey and genomic characterization of Toll-like receptors (TLRs) in channel catfish, Ictalurus punctatus: Identification of novel fish TLRs. Immunogenetics 2013, 65, 511–530. [Google Scholar] [CrossRef]
- Vidya, M.K.; Kumar, V.G.; Sejian, V.; Bagath, M.; Krishnan, G.; Bhatta, R. Toll-like receptors: Significance, ligands, signaling pathways, and functions in mammals. Int. Rev. Immunol. 2018, 37, 20–36. [Google Scholar] [CrossRef]
- Martínez-López, A.; Tyrkalska, S.D.; Alcaraz-Pérez, F.; Cabas, I.; Candel, S.; Morcillo, F.J.M.; Sepulcre, M.P.; García-Moreno, D.; Cayuela, M.L.; Mulero, V. Evolution of LPS recognition and signaling: The bony fish perspective. Dev. Comp. Immunol. 2023, 145, 104710. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, P.; Wu, J.; Huang, A.; Bu, G.; Meng, F.; Kong, F.; Cao, X.; Han, X.; Yu, G. The potential sensing molecules and signal cascades for protecting teleost fishes against lipopolysaccharide. Fish Shellfish Immunol. 2020, 97, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, T.; Tsukada, H.; Nakao, M.; Oshiumi, H.; Matsumoto, M.; Tsukasa, S. Sensing bacterial flagellin by membrane and soluble orthologs of Toll-like receptor 5 in rainbow trout (Onchorhynchus mikiss). J. Biol. Chem. 2004, 279, 48588–48597. [Google Scholar] [CrossRef] [PubMed]
- Rebl, A.; Siegl, E.; Köllner, B.; Fischer, U.; Seyfert, H.-M. Characterization of twin toll-like receptors from rainbow trout (Oncorhynchus mykiss): Evolutionary relationship and induced expression by Aeromonas salmonicida salmonicida. Dev. Comp. Immunol. 2007, 31, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Z.; Xia, H.-Q.; Yang, H.-L.; Wang, Y.-L.; Zou, W.-C. TLR2 signaling may play a key role in the probiotic modulation of intestinal microbiota in grouper Epinephelus coioides. Aquaculture 2014, 430, 50–56. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, J.; Yan, Z.; Xiang, X.; Mu, R.; Zhu, P.; Yao, Y.; Zhu, F.; Chen, K.; Chi, S. Dietary Glycyrrhiza uralensis extracts supplementation elevated growth performance, immune responses and disease resistance against Flavobacterium columnare in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2020, 97, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Na, J.Y.; Lee, J.S. Anti-inflammatory effects of trans-cinnamaldehyde on lipopolysaccharide-stimulated macrophage activation via MAPKs pathway regulation. Immunopharmacol. Immunotoxicol. 2018, 40, 219–224. [Google Scholar] [CrossRef]
- Park, C.; Lee, H.; Hong, S.; Molagoda, I.M.N.; Jeong, J.-W.; Jin, C.-Y.; Kim, G.-Y.; Choi, S.H.; Hong, S.H.; Choi, Y.H. Inhibition of lipopolysaccharide-induced inflammatory and oxidative responses by trans-cinnamaldehyde in C2C12 myoblasts. Int. J. Med. Sci. 2021, 18, 2480. [Google Scholar] [CrossRef]
- Reda, R.M.; Mahmoud, R.; Selim, K.M.; El-Araby, I.E. Effects of dietary acidifiers on growth, hematology, immune response and disease resistance of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2016, 50, 255–262. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; He, S.; Shi, P.; Gao, X.; Yao, B.; Ringø, E. Effects of dietary potassium diformate (KDF) on growth performance, feed conversion and intestinal bacterial community of hybrid tilapia (Oreochromis niloticus ♀×O. aureus ♂). Aquaculture 2009, 291, 89–94. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J.; Long, S.; Miller, N.; Clem, L.; Chinchar, V. Molecular identification and expression analysis of tumor necrosis factor in channel catfish (Ictalurus punctatus). Dev. Comp. Immunol. 2003, 27, 845–858. [Google Scholar] [CrossRef]
- Wang, E.; Wang, J.; Long, B.; Wang, K.; He, Y.; Yang, Q.; Chen, D.; Geng, Y.; Huang, X.; Ouyang, P. Molecular cloning, expression and the adjuvant effects of interleukin-8 of channel catfish (Ictalurus Punctatus) against Streptococcus iniae. Sci. Rep. 2016, 6, 29310. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Peng, Y.; Zhou, X.-Q. Cinnamaldehyde improved intestine immune function and alleviated inflammation associated with NF-κB pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Aquac. Rep. 2021, 21, 100837. [Google Scholar] [CrossRef]
- Dee, C.T.; Nagaraju, R.T. CD4-Transgenic Zebrafish Reveal Tissue-Resident Th2- and Regulatory T Cell-like Populations and Diverse Mononuclear Phagocytes. J. Immunol. 2016, 197, 3520–3530. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Dickerson, H.W.; Findly, R.C. Vertebrate adaptive immunity—Comparative insights from a Teleost Model. Front. Immunol. 2017, 8, 1379. [Google Scholar] [CrossRef]
- Nhu, T.Q.; Hang, B.T.B.; Vinikas, A.; Hue, B.T.B.; Quetin-Leclercq, J.; Scippo, M.-L.; Phuong, N.T.; Kestemont, P. Screening of immuno-modulatory potential of different herbal plant extracts using striped catfish (Pangasianodon hypophthalmus) leukocyte-based in vitro tests. Fish Shellfish Immunol. 2019, 93, 296–307. [Google Scholar] [CrossRef]
- Kondera, E.; Bojarski, B.; Ługowska, K.; Kot, B.; Witeska, M. Hematological and hematopoietic effects of bactericidal doses of trans-cinnamaldehyde and thyme oil on Cyprinus carpio juveniles. Front. Physiol. 2021, 12, 771243. [Google Scholar] [CrossRef]
- Findly, R.C.; Zhao, X.; Noe, J.; Camus, A.C.; Dickerson, H.W. B cell memory following infection and challenge of channel catfish with Ichthyophthirius multifiliis. Dev. Comp. Immunol. 2013, 39, 302–311. [Google Scholar] [CrossRef]
- Bengtén, E.; Clem, L.W.; Miller, N.W.; Warr, G.W.; Wilson, M. Channel catfish immunoglobulins: Repertoire and expression. Dev. Comp. Immunol. 2006, 30, 77–92. [Google Scholar] [CrossRef]
- Yu, X.B.; Liu, G.L.; Zhu, B.; Hao, K.; Ling, F.; Wang, G.X. In vitro immunocompetence of two compounds isolated from Polygala tenuifolia and development of resistance against grass carp reovirus (GCRV) and Dactylogyrus intermedius in respective host. Fish Shellfish Immunol. 2014, 41, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, A.; Viswanath, K.; Satoh, S. Real-time quantification of the immune gene expression in rainbow trout fed different forms of probiotic bacteria Lactobacillus rhamnosus. Aquac. Res. 2011, 42, 906–917. [Google Scholar] [CrossRef]
| Diet Composition | Percent in Control Feed | Ingredients |
|---|---|---|
| Crude protein | 32.0% | Soybean meal |
| Crude fat | 2.5% | Rapeseed meal |
| Crude fiber | 7.0% | Corn gluten meal |
| Phosphorus | 0.4% | Fish meal |
| Wheat shorts | ||
| Rapeseed oil | ||
| Mineral premix | ||
| Sodium chloride | ||
| Vitamin premix |
| Genes | Accession NO. | Primers | References |
|---|---|---|---|
| 18S ribosomal RNA | AF021880 | F-GAGAAACGGCTACCACATCC R-GATACGCTCATTCCGATTACAG | [26] |
| CD4-1 | DQ435305 | F-GATGTCATCATTGTAGATCTCG R-GAGGTAGCTGGCATTTCACTCC | [27] |
| CD4-2 | DQ435304 | F-CTGTATGTTGTATCAGCCTCTG R-CAGTCACCTCCTTACTTTGGCTA | [27] |
| CD8-α | HQ446239 | F-CTACGCGGAGAGACAGTCCCAA R-CTCACAACCCAAAAGCACATC | [27] |
| CD8-β | HQ446240 | F-CCATCAGGCCTGGAGAAAGCA R-TCACCACCAGGAGTAGGACA | [27] |
| IL-1β | DQ157743 | F-TGATCCTTTGGCCATGAGCGGC R-AGACATTGAAAAGCTCCTGGTC | [27] |
| TLR-4 | x79482 | F-ACCTGACTACCACACCCATA R-TCCTAGACGAGTGGAGGTTATT | This study |
| TLR-5 | x79482 | F-GGAAGCGCTACAAATCCTACT R-GTATGCCAGATCAAGTCGTATCA | This study |
| INFγ | NC_030434 | F-TTGGGCAAAGTAGAGGACACC R-TGTTTCCACACTGCCTGTTCG | [27] |
| MHC class II | AF103002 | F-GACACCAGGACATGGGAGGTG R-CGAGGAAGAAAGTTCCGGTAG | [27] |
| MHC class I I | AF103 | F-GACCGAGAACTCGACTACTACA R-GAGTGCCTTTCTCCCAGTAATC | This study |
| TNFα | AJ417565 | F-GCACAACAAACCAGACGAGA R-TCGTTGTCCTCCAGTTTCAA | [27] |
| IgM | x79482 | F-AAGAAGCGAGTTATGCACCAG R-ATGCTTCATGTTCCACCTCAC | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramachandran, R.; Ford, E.; Gomaa, B.; Abdelhamed, H. Trans-Cinnamaldehyde Primes More Robust Channel Catfish Immune Responses to Edwardsiella ictaluri Infection. Pathogens 2024, 13, 310. https://doi.org/10.3390/pathogens13040310
Ramachandran R, Ford E, Gomaa B, Abdelhamed H. Trans-Cinnamaldehyde Primes More Robust Channel Catfish Immune Responses to Edwardsiella ictaluri Infection. Pathogens. 2024; 13(4):310. https://doi.org/10.3390/pathogens13040310
Chicago/Turabian StyleRamachandran, Reshma, Emerald Ford, Basant Gomaa, and Hossam Abdelhamed. 2024. "Trans-Cinnamaldehyde Primes More Robust Channel Catfish Immune Responses to Edwardsiella ictaluri Infection" Pathogens 13, no. 4: 310. https://doi.org/10.3390/pathogens13040310
APA StyleRamachandran, R., Ford, E., Gomaa, B., & Abdelhamed, H. (2024). Trans-Cinnamaldehyde Primes More Robust Channel Catfish Immune Responses to Edwardsiella ictaluri Infection. Pathogens, 13(4), 310. https://doi.org/10.3390/pathogens13040310






