MinION Sequencing of Fungi in Sub-Saharan African Air and a Novel LAMP Assay for Rapid Detection of the Tropical Phytopathogenic Genus Lasiodiplodia
Abstract
1. Introduction
2. Methods
2.1. Air Sample Collection and DNA Extraction
2.2. PCR Amplification of the ITS Region from Air Samples
2.3. MinION Amplicon Sequencing of Air Samples
2.4. Downstream Bioinformatics Analyses
2.5. Design, Validation, and Application of Lasiodiplodia LAMP Assay
3. Results
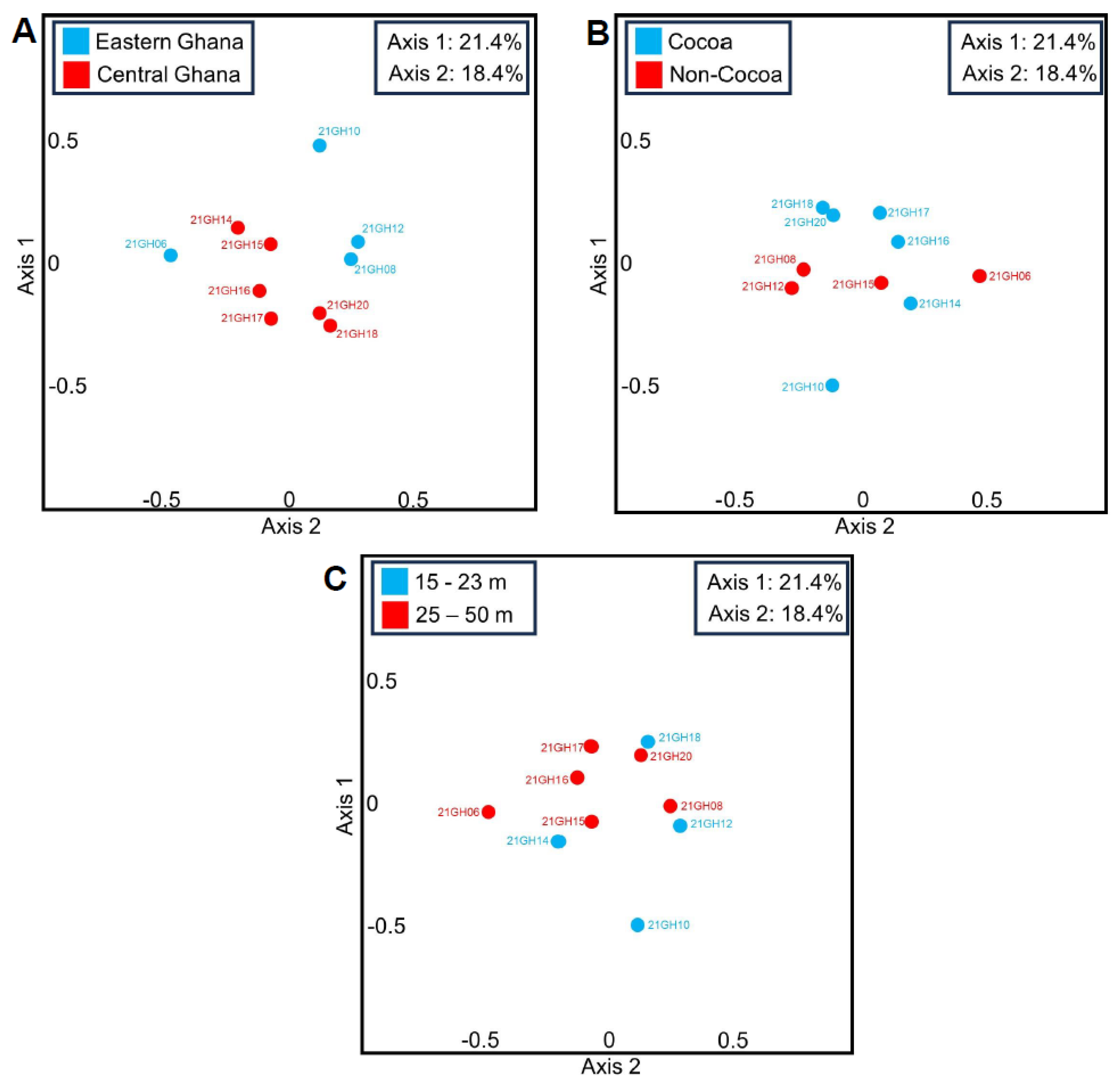
3.1. Principal Coordinate Analyses (PCoAs) and Beta Diversity of Ghanian Air Samples
3.2. Alpha Diversity in Ghanian Air Samples
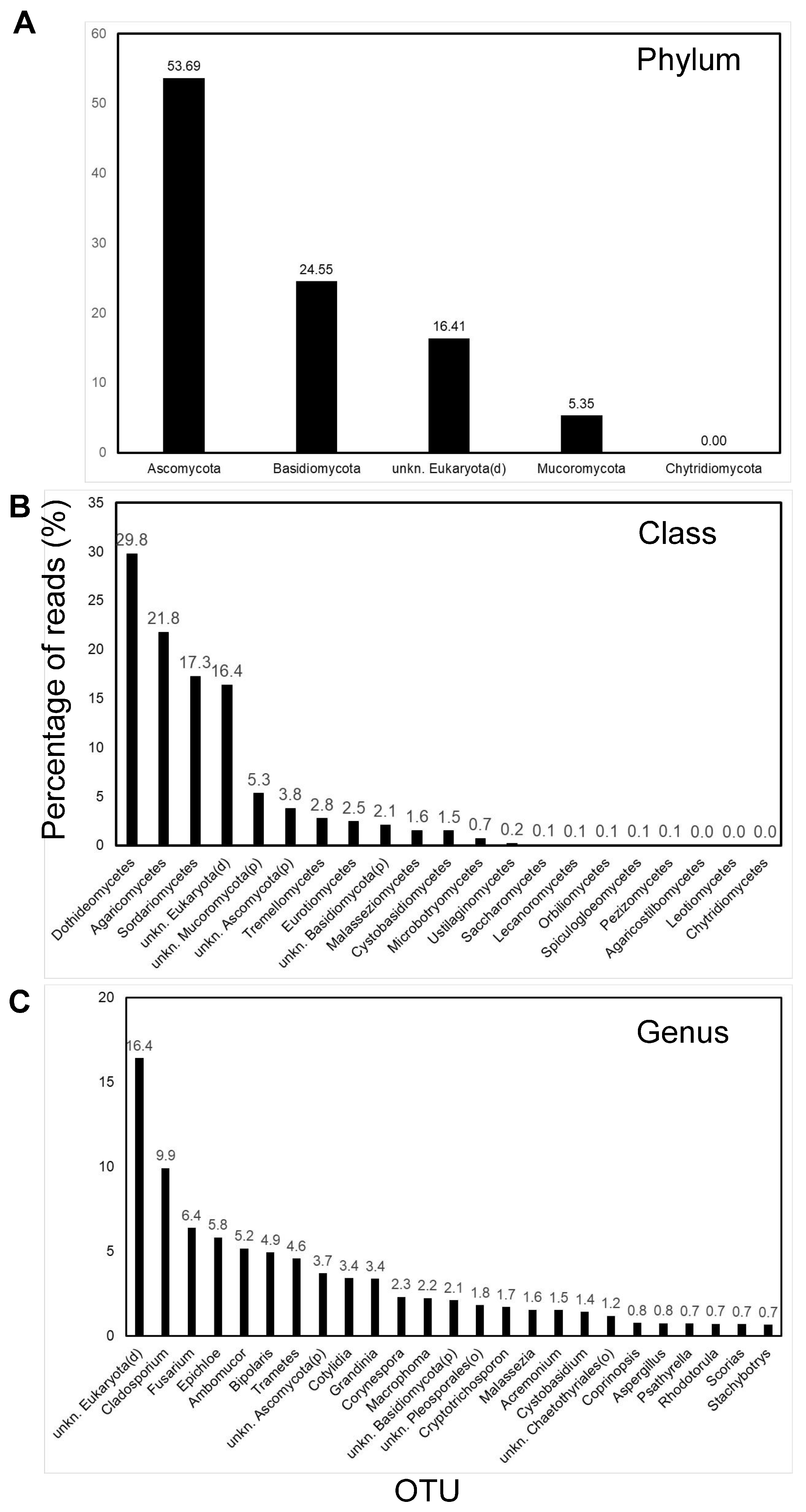
3.3. Predominant OTUs in Ghanaian Air
3.4. Design, Validation, and Application of Lasiodiplodia LAMP Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to global food security from emerging fungal and oomycete crop pathogens. Nat. Food 2020, 1, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Brachaczek, A.; Kaczmarek, J.; Jedryczka, M. Monitoring blackleg (Leptosphaeria spp.) ascospore release timing and quantity enables optimal fungicide application to improved oilseed rape yield and seed quality. Eur. J. Plant Pathol. 2016, 145, 643–657. [Google Scholar] [CrossRef][Green Version]
- Carisse, O.; Tremblay, D.M.; Lévesque, C.A.; Gindro, K.; Ward, P.; Houde, A. Development of a TaqMan real-time PCR assay for quantification of airborne conidia of Botrytis squamosa and management of botrytis leaf blight of onion. Phytopathology 2009, 99, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Carisse, O.; Bacon, R.; Lefebvre, A. Grape powdery mildew (Erysiphe necator) risk assessment based on airborne conidium concentration. Crop Prot. 2009, 28, 1036–1044. [Google Scholar] [CrossRef]
- Kennedy, R.; Wakeham, A.J. Development and use of detection systems for the sporangia of Peronospora destructor. Asp. Appl. Biol. 2008, 89, 55–56. [Google Scholar]
- Kunjeti, S.G.; Anchieta, A.; Martin, F.N.; Choi, Y.J.; Thines, M.; Michelmore, R.W.; Koike, S.T.; Tsuchida, C.; Mahaffee, W.; Subbarao, K.V.; et al. Detection and quantification of Bremia lactucae by spore trapping and quantitative PCR. Phytopathology 2016, 106, 426–1437. [Google Scholar] [CrossRef]
- Rogers, S.L.; Atkins, S.D.; West, J.S. Detection and quantification of airborne inoculum of Sclerotinia sclerotiorum using quantitative PCR. Plant Pathol. 2009, 58, 324–331. [Google Scholar] [CrossRef]
- Thiessen, L.D.; Keune, J.A.; Neill, T.M.; Turechek, W.W.; Grove, G.G.; Mahaffee, W.F. Development of a grower-conducted inoculum detection assay for management of grape powdery mildew. Plant Pathol. 2016, 65, 238–249. [Google Scholar] [CrossRef]
- Fraaije, B.A.; Cools, H.J.; Fountaine, J.; Lovell, D.J.; Motteram, J.; West, J.S.; Lucas, J.A. Role of ascospores in further spread of QoI-resistant Cytochrome b Alleles (G143A) in field populations of Mycosphaerella graminicola. Phytopathology 2005, 95, 933–941. [Google Scholar] [CrossRef]
- Vicentini, S.N.C.; Hawkins, N.J.; King, K.M.; Moreira, S.I.; de Paiva Custódio, A.A.; Leite Júnior, R.P.; Portalanza, D.; Garcés-Fiallos, F.R.; Krug, L.D.; West, J.S.; et al. Aerobiology of the wheat blast pathogen: Inoculum monitoring and detection of fungicide resistant alleles. Agronomy 2023, 13, 1238. [Google Scholar] [CrossRef]
- Fraaije, B.A.; Atkins, S.L.; Santos, R.F.; Hanley, S.J.; West, J.S.; Lucas, J.A. Epidemiological studies of pan-azole resistant Aspergillus fumigatus populations sampled during tulip cultivation show clonal expansion with acquisition of multi-fungicide resistance as potential driver. Microorganisms 2021, 9, 2379. [Google Scholar] [CrossRef]
- Jedryczka, M.; Sadyś, M.; Gilski, M.; Grinn-Gofron, A.; Kaczmarek, J.; Strzelczak, A.; Kennedy, R. Contribution of Leptosphaeria species ascospores to autumn asthma in areas of oilseed rape production. Ann. Allergy Asthma Immunol. 2016, 117, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Ezquerro, M.C.; Serrano-Silva, N.; Brunner-Mendoza, C. Metagenomic characterisation of bioaerosols during the dry season in Mexico City. Aerobiologia 2020, 36, 493–505. [Google Scholar] [CrossRef]
- Nicolaisen, M.; West, J.S.; Sapkota, R.; Canning, G.G.M.; Schoen, C.; Justesen, A.F. Fungal communities including plant pathogens in near surface air are similar across northwestern Europe. Front. Microbiol. 2017, 8, 1729. [Google Scholar] [CrossRef] [PubMed]
- West, J.S.; Kimber, R.B.E. Innovations in air sampling to detect plant pathogens. Ann. Appl. Biol. 2015, 166, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Kavhiza, N.J.; Zargar, M.; Prikhodko, S.I.; Pakina, E.N.; Murtazova, K.M.-S.; Nakhaev, M.R. Improving crop productivity and ensuring food security through the adoption of genetically modified crops in sub-saharan Africa. Agronomy 2022, 12, 439. [Google Scholar] [CrossRef]
- Baeza, M.; Barahona, S.; Alcaíno, J.; Cifuentes, V. Amplicon-metagenomic analysis of fungi from Antarctic terrestrial habitats. Front. Microbiol. 2017, 8, 2235. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Gat, D.; Paytan, A.; Rudich, Y. The response of airborne mycobiome to dust storms in the eastern Mediterranean. J. Fungi 2021, 7, 802. [Google Scholar] [CrossRef] [PubMed]
- Behzad, H.; Gojobori, T.; Mineta, K. Challenges and opportunities of airborne metagenomics. Genome Biol. Evol. 2015, 7, 1216–1226. [Google Scholar] [CrossRef]
- Nygaard, A.B.; Tunsjø, H.S.; Meisal, R.; Charnock, C. A preliminary study on the potential of Nanopore MinION and Illumina MiSeq 16S rRNA gene sequencing to characterize building-dust microbiomes. Sci. Rep. 2020, 10, 3209. [Google Scholar] [CrossRef]
- Kellogg, C.A.; Griffin, D.W.; Garrison, V.H.; Peak, K.K.; Royall, N.; Smith, R.R.; Shinn, E.A. Characterization of aerosolized bacteria and fungi from desert dust events in Mali, west Africa. Aerobiologia 2004, 20, 99–110. [Google Scholar] [CrossRef]
- RodrÍguez-Rajo, F.J.; Iglesias, I.; Jato, V. Variation assessment of airborne Alternaria and Cladosporium spores at different bioclimatical conditions. Mycol. Res. 2005, 109, 497–507. [Google Scholar] [CrossRef]
- Ismail, M.A.; Abdel-Hafez, S.I.I.; Moharram, A.M. Aeromycobiota of Western Desert of Egypt. Afr. J. Sci. Technol. 2002, 3, 1–9. [Google Scholar] [CrossRef]
- Yafetto, L.; Adatour, E.H. Fungal contaminations of indoor and outdoor air of buildings of the University of Cape Coast, Ghana. Stud. Fungi 2018, 3, 333–342. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zinger, L.; Nilsson, R.H.; Kennedy, P.G.; Yang, T.; Anslan, S.; Mikryukov, V. Best practices in metabarcoding of fungi: From experimental design to results. Mol. Ecol. 2022, 31, 2769–2795. [Google Scholar] [CrossRef] [PubMed]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef]
- Mafune, K.K.; Godfrey, B.J.; Vogt, D.J.; Vogt, K.A. A rapid approach to profiling diverse fungal communities using the MinION™ nanopore sequencer. BioTechniques 2020, 68, 72–78. [Google Scholar] [CrossRef]
- Paytuví, A.; Battista, E.; Scippacercola, F.; Cigliano, R.A.; Sanseverino, W. GAIA: An integrated metagenomics suite. BioRxiv 2019, 804690. [Google Scholar] [CrossRef]
- Postiglione, A.; Prigioniero, A.; Zuzolo, D.; Tartaglia, M.; Scarano, P.; Maisto, M.; Ranauda, M.A.; Sciarrillo, R.; Thijs, S.; Vangronsveld, J.; et al. Quercus ilex phyllosphere microbiome environmental-driven structure and composition shifts in a mediterranean contex. Plants 2022, 11, 3528. [Google Scholar] [CrossRef]
- Hirst, J.M.; Stedman, O.J.; Hogg, W.H. Long-distance Spore Transport: Methods of Measurement, Vertical Spore Profiles and the Detection of Immigrant Spores. J. Gen. Microbiol. 1967, 48, 329–355. [Google Scholar] [CrossRef][Green Version]
- Lacey, M.E.; West, J.S. The Air Spora. A Manual for Catching and Identifying Airborne Biological Particles; Springer: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Ovaskainen, O.; Abrego, N.; Somervuo, P.; Palorinne, I.; Hardwick, B.; Pitkänen, J.-M.; Andrew, N.R.; Niklaus, P.A.; Schmidt, N.M.; Seibold, S.; et al. Monitoring fungal communities with the global spore sampling project. Front. Ecol. Evol. 2020, 7, 511. [Google Scholar] [CrossRef]
- Becchimanzi, A.; Zimowska, B.; Nicoletti, R. Cryptic diversity in Cladosporium cladosporioides resulting from sequence-based species delimitation analyses. Pathogens 2021, 10, 1167. [Google Scholar] [CrossRef] [PubMed]
- Odebode, A.; Adekunle, A.; Stajich, J.; Adeonipekun, P. Airborne fungi spores distribution in various locations in Lagos, Nigeria. Environ. Monit. Assess. 2020, 192, 87. [Google Scholar] [CrossRef] [PubMed]
- Olaniyan, T.; Dalvie, M.A.; Röösli, M.; Naidoo, R.N.; Künzli, N.; de Hoogh, K.; Berman, D.; Parker, B.; Leaner, J.; Jeebhay, M.F. Short term seasonal effects of airborne fungal spores on lung function in a panel study of schoolchildren residing in informal settlements of the Western Cape of South Africa. Environ. Pollut. 2020, 260, 114023. [Google Scholar] [CrossRef]
- Voglmayr, H.; Jaklitsch, W.M. Corynespora, Exosporium and Helminthosporium revisited-new species and generic reclassification. Stud. Mycol. 2017, 87, 43–76. [Google Scholar] [CrossRef] [PubMed]
- Rondon, M.N.; Lawrence, K. The fungal pathogen Corynespora cassiicola: A review and insights for target spot management on cotton and soya bean. J. Phytopathol. 2021, 169, 329–338. [Google Scholar] [CrossRef]
- Lopez, D.; Ribeiro, S.; Label, P.; Fumanal, B.; Venisse, J.-S.; Kohler, A.; de Oliveira, R.R.; Labutti, K.; Lipzen, A.; Lail, K.; et al. Genome-wide analysis of Corynespora cassiicola leaf fall disease putative effectors. Front. Microbiol. 2018, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Wessel, M.; Quist-Wessel, P.M.F. Cocoa production in West Africa, a review and analysis of recent developments. NJAS Wagening J. Life Sci. 2015, 74, 1–7. [Google Scholar] [CrossRef]
- Adu-Acheampong, R.; Archer, S. Diversity of fungi associated with mirid (Hemiptera: Miridae) feeding lesions and dieback disease of cocoa in Ghana. Int. J. Agric. Res. 2011, 6, 660–672. [Google Scholar] [CrossRef]
- Anikwe, J.C.; Otuonye, H.A. Dieback of cocoa (Theobroma cacao L.) plant tissues caused by the brown cocoa mirid Sahlbergella singularis Haglund (Hemiptera: Miridae) and associated pathogenic fungi. Int. J. Trop. Insect Sci. 2015, 35, 193–200. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Mwangi, M.; Aigbe, S.O.; Leslie, J.F. Fusarium species from the cassava root rot complex in West Africa. Phytopathology 2006, 96, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Opoku-Asiama, Y.; Mbofung, G.A.; Amewowor, D.H.A.K. Incidence of cassava root rot in the central region of Ghana. J. Ghana Sci. Assoc. 1998, 1, 40–49. [Google Scholar] [CrossRef]
- Amoah, B.K.; Rezanoor, H.N.; Nicholson, P.; MacDonald, M.V. Variation in the Fusarium section Liseola: Pathogenicity and genetic studies of isolates of Fusarium moniliforme Sheldon from different hosts in Ghana. Plant Pathol. 1995, 44, 563–572. [Google Scholar] [CrossRef]
- Appiah-Kubi, Z.; Adomako, J.; Armooh, B.; Appaih-Kubi, D.; Oppong, A.; Quian, M.; Osekre, E.A.; Lamptey, J.N.; Kwoseh, C. Identification and characterization of Fusarium species associated with amaranth (Amaranthus species) wilt disease in the semi-deciduous and guinea savannah agro-ecological zones of Ghana. J. Agric. Sci. 2022, 14, 130–141. [Google Scholar] [CrossRef]
- Mbenoun, M.; Momo Zeutsa, E.H.; Samuels, G.; Nsouga Amougou, F.; Nyasse, S. Dieback due to Lasiodiplodia theobromae, a new constraint to cocoa production in Cameroon. Plant Pathol. 2008, 57, 381. [Google Scholar] [CrossRef]
- Ali, S.S.; Asman, A.; Shao, J.; Balidion, J.F.; Strem, M.D.; Puig, A.S.; Meinhardt, L.W.; Bailey, B.A. Genome and transcriptome analysis of the latent pathogen Lasiodiplodia theobromae, an emerging threat to the cacao industry. Genome 2020, 63, 37–52. [Google Scholar] [CrossRef]
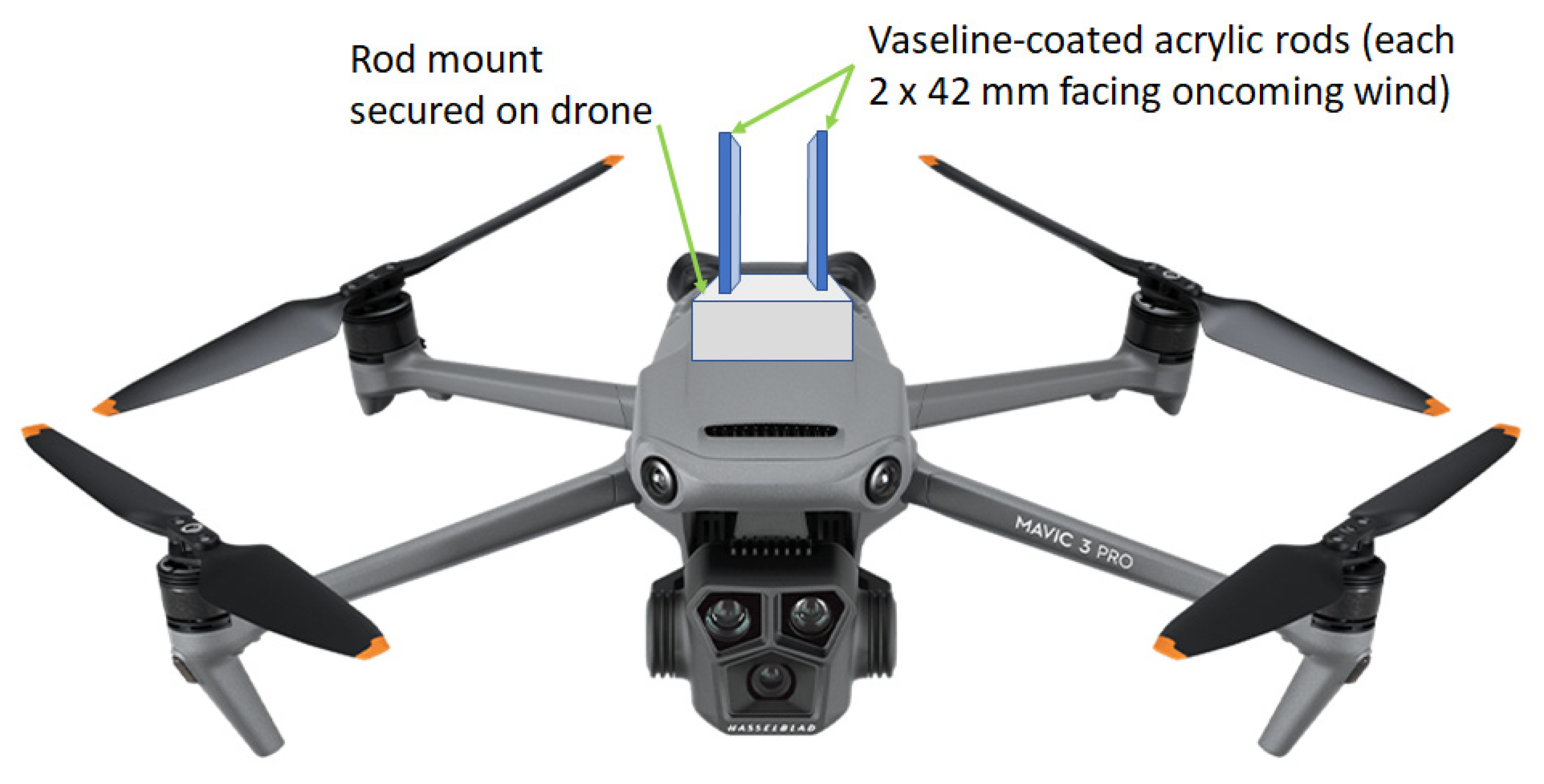
| Sample Code | Sample Date a | Origin (Ghana) | Region | Sampling Height above Ground (m) | Drone Flight Speed (Kph) | Air Sampling Duration (min) | Sampling Site Notes | FASTQ Reads Obtained |
|---|---|---|---|---|---|---|---|---|
| 21GH06 | 5 June 2021 | Aburi | East | 25 | 40 | 10 | Citrus plantation | 4000 |
| 21GH08 | 5 June 2021 | Aburi | East | 25 | 30 | 10 | Citrus plantation | 4000 |
| 21GH10 | 12 June 2021 | Koforidua | East | 20 | 40 | 15 | Cocoa farm | 4000 |
| 21GH12 | 12 June 2021 | Koforidua | East | 23 | 30 | 15 | General vegetation | 4000 |
| 21GH14 | 16 June 2021 | Assin Fosu | Central | 20 | 20 | 15 | Cocoa farm | 4000 |
| 21GH15 | 16 June 2021 | Assin Fosu | Central | 35 | 40 | 15 | Palm plantation | 4000 |
| 21GH16 | 16 June 2021 | Assin Fosu | Central | 30 | 20 | 15 | Replanted cocoa trees | 4000 |
| 21GH17 | 16 June 2021 | Assin Fosu | Central | 25 | 30 | 15 | Cocoa farm | 4000 |
| 21GH18 | 10 August 2021 | Assin Fosu | Central | 15 | 30 | 15 | Cocoa farm | 4000 |
| 21GH20 | 10 August 2021 | Assin Fosu | Central | 50 | ~40 | 15 | Extended cocoa farm | 4000 |
| Fungal Species | Isolate Code | Host | Source | Result 5 |
|---|---|---|---|---|
| Diplodia mutila | Bot-2017-DM21-apple | Apple | 1 | Negative |
| Di. mutila | Bot-11-walnut | Walnut | 1 | Negative |
| Di. mutila | Bot-15-walnut | Walnut | 1 | Negative |
| Di. mutila | Bot-16-hazelnut | Hazelnut | 1 | Negative |
| Di. sapinea | No data (FR) | No data | 2 | Negative |
| Di. seriata | Bot-2018-S3-apple | Apple | 1 | Negative |
| Di. seriata | Bot-09-grape | Grape | 1 | Negative |
| Dothiorella sarmentorum | Bot-12-walnut | Walnut | 1 | Negative |
| Lasiodiplodia pseudotheobromae | CBS116459 | Gamhar tree (Costa Rica) | 3 | Positive (~16 min) |
| Las. theobromae | Bot-2017-LT6-apple | Apple | 1 | Positive (~14.5 min) |
| Las. theobromae | CBS164.96 | Fruit (Papua New Guinea) | 3 | Positive (~16 min) |
| Neofusicoccum arbuti | Bot-2017-NA5-apple | Apple | 1 | Negative |
| Neo. parvum | Bot-10-grape | Grape | 1 | Negative |
| Neo. parvum | Bot-13-walnut | Walnut | 1 | Negative |
| Neo. parvum | Bot-14-blueberry | Blueberry | 1 | Negative |
| Plenodomus lingam | 22SURREY03 | Oilseed rape (UK) | 4 | Negative |
| Sclerotinia sclerotiorum | SS1 | Rapeseed mustard (UK) | 4 | Negative |
| Zymoseptoria tritci | 80.4 | Wheat (UK) | 4 | Negative |
| Air Sample ID | MinION Metabarcoding Result | LAMP Assay Result | |
|---|---|---|---|
| Proportion of Reads (Ladiodiplodia Genus) | Result | Overall Result | |
| 21GH06 | 0 | Negative | Negative |
| 21GH08 | 0.25% | Positive | Negative |
| 21GH10 | 0.13% | Positive | Negative |
| 21GH12 | 1.31% | Positive | Positive |
| 21GH14 | 0 | Negative | Negative |
| 21GH15 | 0.22% | Positive | Negative |
| 21GH16 | 0.75% | Positive | Negative |
| 21GH17 | 0 | Negative | Negative |
| 21GH18 | 0 | Negative | Negative |
| 21GH20 | 0 | Negative | Negative |
| Mean Value (Standard Error of Mean) | |||||
|---|---|---|---|---|---|
| OTUs | Chao1 | Shannon | Simpson | Fisher | |
| Ghana combined data (N = 10) | 90.1 (14.7) | 127.0 (20.2) | 2.5 (0.2) | 0.8 (0) | 17.5 (3.7) |
| Location: | |||||
| Eastern Ghana (N = 4) | 79 11.6) | 102.1 (14.5) | 2.4 (0.2) | 0.8 (0) | 14.1 (2.4) |
| Central Ghana (N = 6) | 97.5 (23.9) | 143.7 (31.7) | 2.6 (0.2) | 0.8 (0) | 19.8 (6.0) |
| t-test (p value) | 0.6 | 0.3 | 0.6 | 0.9 | 0.5 |
| Crop below: | |||||
| Cocoa (N = 6) | 76.8 (5.9) | 111.1 (9.9) | 2.4 (0.1) | 0.8 (0) | 14.2 (0.9) |
| Non-Cocoa (N = 4) | 110 (36.4) | 151 (50) | 2.7 (0.4) | 0.9 (0) | 22.4 (9.4) |
| t-test (p value) | 0.3 | 0.4 | 0.5 | 0.4 | 0.3 |
| Height above ground: | |||||
| 15–23 m (N = 4) | 79.3 (9.4) | 100.8 (10.6) | 2.3 (0.2) | 0.8 (0) | 15.1 (1.4) |
| 25–50 m (N = 6) | 97.3 (24.3) | 144.5 (32.0) | 2.7 (0.24) | 0.9 (0) | 19.1 (6.3) |
| t-test (p value) | 0.6 | 0.3 | 0.3 | 0.1 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, K.M.; Canning, G.G.M.; West, J.S. MinION Sequencing of Fungi in Sub-Saharan African Air and a Novel LAMP Assay for Rapid Detection of the Tropical Phytopathogenic Genus Lasiodiplodia. Pathogens 2024, 13, 330. https://doi.org/10.3390/pathogens13040330
King KM, Canning GGM, West JS. MinION Sequencing of Fungi in Sub-Saharan African Air and a Novel LAMP Assay for Rapid Detection of the Tropical Phytopathogenic Genus Lasiodiplodia. Pathogens. 2024; 13(4):330. https://doi.org/10.3390/pathogens13040330
Chicago/Turabian StyleKing, Kevin M., Gail G. M. Canning, and Jonathan S. West. 2024. "MinION Sequencing of Fungi in Sub-Saharan African Air and a Novel LAMP Assay for Rapid Detection of the Tropical Phytopathogenic Genus Lasiodiplodia" Pathogens 13, no. 4: 330. https://doi.org/10.3390/pathogens13040330
APA StyleKing, K. M., Canning, G. G. M., & West, J. S. (2024). MinION Sequencing of Fungi in Sub-Saharan African Air and a Novel LAMP Assay for Rapid Detection of the Tropical Phytopathogenic Genus Lasiodiplodia. Pathogens, 13(4), 330. https://doi.org/10.3390/pathogens13040330







