Molecular Detection and Genetic Characterization of Feline Immunodeficiency Virus (FIV) in Seropositive Cats in Northern Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria, Sampling and Groups
2.2. Detection of FIV Proviral DNA
2.3. Sequence Analysis
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Detection of FIV Proviral DNA
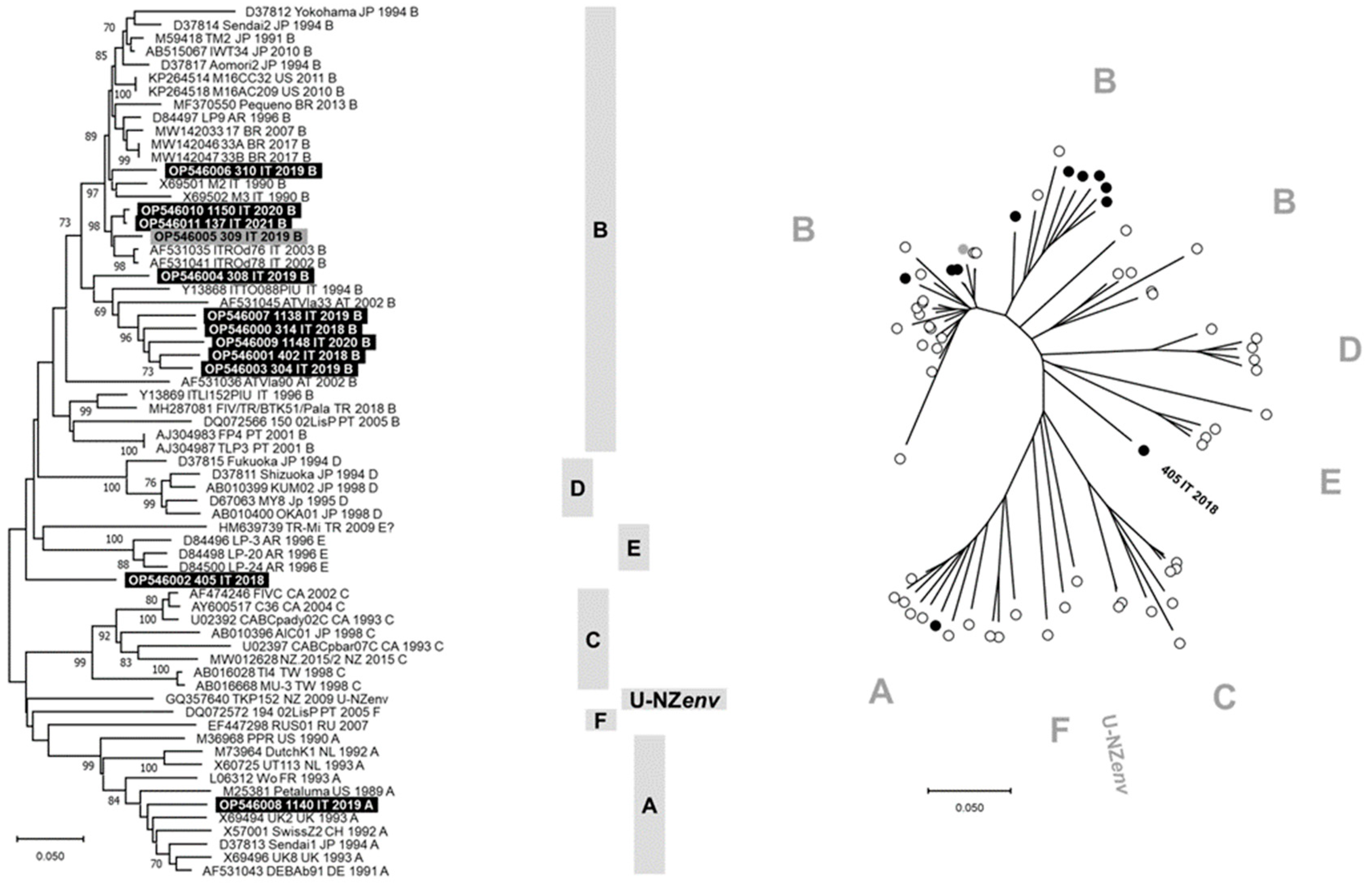
3.3. Sequence Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartmann, K. Clinical aspects of feline immunodeficiency and feline leukemia virus infection. Vet. Immunol. Immunopathol. 2011, 143, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Giunti, M.; Balboni, A. Feline Immunodeficiency Virus Infection. In Feline Emergency and Critical Care Medicine; Byers, C.R., Giunti, M., Eds.; Edra S.p.A.: Milano, Italy, 2021; pp. 231–235. [Google Scholar]
- de Mello, L.S.; Ribeiro, P.R.; de Almeida, B.A.; Bandinelli, M.B.; Sonne, L.; Driemeier, D.; Pavarini, S.P. Diseases associated with feline leukemia virus and feline immunodeficiency virus infection: A retrospective study of 1470 necropsied cats (2010–2020). Comp. Immunol. Microbiol. Infect. Dis. 2023, 95, 101963. [Google Scholar] [CrossRef] [PubMed]
- Hosie, M.J.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Lloret, A.; Lutz, H.; et al. Feline immunodeficiency. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Hayward, J.J.; Taylor, J.; Rodrigo, A.G. Phylogenetic analysis of feline immunodeficiency virus in feral and companion domestic cats of New Zealand. J. Virol. 2007, 81, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.A.; Sykes, J.E. Feline immunodeficiency virus infection. In Greene’s Infectious Diseases of the Dog and Cat, 5th ed.; Sykes, J.E., Ed.; Elsevier: London, UK, 2023; pp. 414–428. [Google Scholar]
- Reggeti, F.; Bienzle, D. Feline immunodeficiency virus subtypes A, B and C and intersubtype recombinants in Ontario, Canada. J. Gen. Virol. 2004, 85, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Pistello, M.; Cammarota, G.; Nicoletti, E.; Matteucci, D.; Curcio, M.; Del Mauro, D.; Bendinelli, M. Analysis of the genetic diversity and phylogenetic relationship of Italian isolates of feline immunodeficiency virus indicates a high prevalence and heterogeneity of subtype B. J. Gen. Virol. 1997, 78, 2247–2257. [Google Scholar] [CrossRef]
- Crawford, P.C.; Levy, J.K. New challenges for the diagnosis of feline immunodeficiency virus infection. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Griessmayr, P.; Schulz, B.; Greene, C.E.; Vidyashankar, A.N.; Jarrett, O.; Egberink, H.F. Quality of different in-clinic test systems for feline immunodeficiency virus and feline leukaemia virus infection. J. Feline Med. Surg. 2007, 9, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K. Clinical aspects of feline retroviruses: A review. Viruses 2012, 4, 2684–2710. [Google Scholar] [CrossRef]
- Kann, R.K.; Kyaw-Tanner, M.T.; Seddon, J.M.; Lehrbach, P.R.; Zwijnenberg, R.J.; Meers, J. Molecular subtyping of feline immunodeficiency virus from domestic cats in Australia. Aust. Vet. J. 2006, 84, 112–116. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gaschen, B.; Blay, W.; Foley, B.; Haigwood, N.; Kuiken, C.; Korber, B. Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology 2004, 14, 1229–1246. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Levy, J.; Hartmann, K.; Hofmann-Lehmann, R.; Hosie, M.; Olah, G.; Denis, K.S. 2020 AAFP Feline retrovirus testing and management guidelines. J. Feline Med. Surg. 2020, 22, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Ammersbach, M.; Little, S.; Bienzle, D. Preliminary evaluation of a quantitative polymerase chain reaction assay for diagnosis of feline immunodeficiency virus infection. J. Feline Med. Surg. 2013, 15, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Weng, H.Y.; Litster, A.; Leutenegger, C.; Guptill, L. Commercially available enzyme-linked immunosorbent assay and polymerase chain reaction tests for detection of feline immunodeficiency virus infection. J. Vet. Intern. Med. 2017, 31, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Westman, M.E.; Coggins, S.J.; van Dorsselaer, M.; Norris, J.M.; Squires, R.A.; Thompson, M.; Malik, R. Feline immunodeficiency virus (FIV) infection in domestic pet cats in Australia and New Zealand: Guidelines for diagnosis, prevention and management. Aust. Vet. J. 2022, 100, 345–359. [Google Scholar] [CrossRef]
- Steinrigl, A.; Klein, D. Phylogenetic analysis of feline immunodeficiency virus in Central Europe: A prerequisite for vaccination and molecular diagnostics. J. Gen. Virol. 2003, 84, 1301–1307. [Google Scholar] [CrossRef]
- Studer, N.; Lutz, H.; Saegerman, C.; Gönczi, E.; Meli, M.L.; Boo, G.; Hartmann, K.; Hosie, M.J.; Moestl, K.; Tasker, S.; et al. Pan-European Study on the Prevalence of the Feline Leukaemia Virus Infection—Reported by the European Advisory Board on Cat Diseases (ABCD Europe). Viruses 2019, 11, 993. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Marati, L.; Pugliese, A.; Levante, M.; Ferrara, G.; de Carlo, E.; Amoroso, M.G.; Montagnaro, S. Prevalence of feline leukemia virus and feline immunodeficiency virus in cats from southern Italy: A 10-year cross-sectional study. Front. Vet. Sci. 2023, 10, 1260081. [Google Scholar] [CrossRef] [PubMed]
- Koç, B.T.; Oğuzoğlu, T.Ç. A phylogenetic study of Feline Immunodeficiency Virus (FIV) among domestic cats in Turkey. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101544. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, J.; Meili, T.; Meli, M.L.; Riond, B.; Helfer-Hungerbuehler, A.K.; Bönzli, E.; Pineroli, B.; Hofmann-Lehmann, R. Decreased Sensitivity of the Serological Detection of Feline Immunodeficiency Virus Infection Potentially Due to Imported Genetic Variants. Viruses 2019, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.H.; Mathiason-Dubard, C.; Learn, G.H.; Rodrigo, A.G.; Sodora, D.L.; Mazzetti, P.; Hoover, E.A.; Mullins, J.I. Genetic diversity of feline immunodeficiency virus: Dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J. Virol. 1977, 71, 4241–4253. [Google Scholar] [CrossRef]
- Bęczkowski, P.M.; Hughes, J.; Biek, R.; Litster, A.; Willett, B.J.; Hosie, M.J. Feline immunodeficiency virus (FIV) env recombinants are common in natural infections. Retrovirology 2014, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Spada, E.; Proverbio, D.; della Pepa, A.; Perego, R.; Baggiani, L.; DeGiorgi, G.B.; Domenichini, G.; Ferro, E.; Cremonesi, F. Seroprevalence of feline immunodeficiency virus, feline leukaemia virus and Toxoplasma gondii in stray cat colonies in northern Italy and correlation with clinical and laboratory data. J. Feline Med. Surg. 2012, 14, 369–377. [Google Scholar] [CrossRef] [PubMed]
- da Silva Serpa, P.B.; Messick, J.B. A case of acute monocytic leukemia (AMoL or AML-M5) in an adult FeLV/FIV-positive cat. Vet. Clin. Pathol. 2021, 50, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Battilani, M.; Kaehler, E.; Tirolo, A.; Balboni, A.; Dondi, F. Clinicopathological findings in cats tested for feline immunodeficiency virus (FIV) and feline leukaemia virus (FeLV). Acta Vet Beogr. 2022, 72, 419–432. [Google Scholar] [CrossRef]
- Wang, W.; Nie, J.; Prochnow, C.; Truong, C.; Jia, Z.; Wang, S.; Chen, X.S.; Wang, Y. A systematic study of the N-glycosylation sites of HIV-1 envelope protein on infectivity and antibody-mediated neutralization. Retrovirology 2013, 10, 14. [Google Scholar] [CrossRef]
- Bęczkowski, P.M.; Harris, M.; Techakriengkrai, N.; Beatty, J.A.; Willett, B.J.; Hosie, M.J. Neutralising antibody response in domestic cats immunised with a commercial feline immunodeficiency virus (FIV) vaccine. Vaccine 2015, 33, 977–984. [Google Scholar] [CrossRef] [PubMed]
| PCR and Primers | Nucleotide Sequence (5′-3′) | Genome Position a | Fragment Size (Base Pairs) a |
|---|---|---|---|
| LTR region PCR | |||
| FIV_LTR_F | AGCTGCYTAACCGCRAAACCACATC | 116–140 | 259 |
| FIV_LTR_R | TCCCTGTTCGGGCGCCAACTG | 354–374 | |
| env gene A (V3-V4) PCR | |||
| FIV_env_SU3 | ATWCCAAAATGTGGATGGTGG | 7316–7336 | 493 |
| FIV_env_SU4 | AATAAGGTCATCTACCTTCAT | 7787–7807 | |
| env gene B (V3-V4) PCR | |||
| FIV_env_SU3 | ATWCCAAAATGTGGATGGTGG | 7316–7336 | 493 |
| FIV_env_SU4_modB | AATAAGGTCCTCTATTTTCAT | 7787–7807 | |
| env gene C (V4-V5) PCR | |||
| FIV_env_SU5 | AATCCTGTAGATTGTACCATG | 7721–7741 | 283 |
| FIV_env_SU6 | TCCTGCYACTGGRTTATACCA | 7982–8002 | |
| env gene D (V4-V5) PCR | |||
| FIV_env_SU5_modB | AACCCGGTAGATTGTACTATG | 7721–7741 | 283 |
| FIV_env_SU6 | TCCTGCYACTGGRTTATACCA | 7982–8002 | |
| env gene E (V3-V5) PCR | |||
| FIV_env_SU3 | ATWCCAAAATGTGGATGGTGG | 7316–7336 | 687 |
| FIV_env_SU6 | TCCTGCYACTGGRTTATACCA | 7982–8002 |
| Cat | Year of Sampling | Breed | Sex | Age | Group | Clinical and Clinicopathological Findings Referable to FIV Infection | Concomitant Diseases | FeLV Antigens Test |
|---|---|---|---|---|---|---|---|---|
| 313/2018 | 2018 | DSH | MC | 5y 11m | SC | Anemia, sarcoma of the right anterior limb | Neg | |
| 314/2018 | 2018 | DSH | MC | 17y 8m | SC | Anemia, azotemia | Hypovolemic shock | Neg |
| 396/2018 | 2018 | DSH | F | 16y 11m | SC | Anemia, stomatitis, fever | Neg | |
| 397/2018 | 2018 | DSH | MC | 9y 6m | SC | Anemia, azotemia | Neg | |
| 398/2018 | 2018 | DSH | M | 6m | SC | Stomatitis, gastroenteritis | Neg | |
| 402/2018 | 2018 | DSH | FS | 10y 5m | SC | Dysorexia, weight loss, dermatitis | Neg | |
| 403/2018 | 2018 | DSH | M | 5y 3m | SC | Anorexia, lymphadenomegaly | Neg | |
| 405/2018 | 2018 | DSH | MC | 8y 6m | SC | Lymphoma | Neg | |
| 406/2018 | 2018 | DSH | MC | 8y 11m | AC | Congestive heart failure | Neg | |
| 407/2018 | 2018 | DSH | F | 8y 1m | SC | Blindness, azotemia, neurological signs | Neg | |
| 408/2018 | 2018 | DSH | MC | 10y 2m | SC | Rhinitis | Constipation | Neg |
| 409/2018 | 2018 | DSH | FS | 15y 10m | SC | Anorexia, depression, fever | hepatic lipidosis | Neg |
| 303/2019 | 2019 | DSH | FS | 15y 7m | AC | Neg | ||
| 304/2019 | 2019 | DSH | MC | 13y 11m | SC | Nasal adenocarcinoma | Neg | |
| 305/2019 | 2019 | DSH | MC | 16y 4m | SC | Anemia | Dysuria | Neg |
| 306/2019 | 2019 | DSH | M | 4y 4m | AC | Trauma | Neg | |
| 307/2019 | 2019 | DSH | FS | 17y 11m | SC | Itching, dermatitis | Neg | |
| 308/2019 | 2019 | DSH | MC | 9y 2m | SC | Anorexia, stomatitis, rhinitis | Pos | |
| 309/2019 | 2019 | DSH | M | 3y 7m | AC | Trauma | Neg | |
| 310/2019 | 2019 | DSH | MC | 11y 10m | SC | Anorexia, vomiting, fever | Neg | |
| 311/2019 | 2019 | DSH | MC | 12y | SC | Stomatitis | Neg | |
| 312/2019 | 2019 | DSH | M | 6y | AC | Trauma | Neg | |
| 318/2019 | 2019 | DSH | MC | 5y 3m | AC | Urinary tract obstruction | Neg | |
| 323/2019 | 2019 | DSH | M | 3y 7m | AC | Trauma | Pos | |
| 1127/2019 | 2019 | DSH | M | 15y 11m | AC | Trauma, septic shock | Pos | |
| 1136/2019 | 2019 | DSH | M | 10y 5m | SC | Stomatitis with ulcerations, AKI/CKD | Neg | |
| 1137/2019 | 2019 | DSH | MC | 20y 1m | SC | Dysorexia, stomatitis | Hydronephrosis, ureteral obstruction | Neg |
| 1138/2019 | 2019 | DSH | FS | 11y 9m | SC | Fever, depression, stomatitis, sublingual neoformation | Neg | |
| 1140/2019 | 2019 | DSH | MC | 8y 10m | SC | Anemia, azotemia | Neg | |
| 1142/2019 | 2019 | DSH | MC | 3y 4m | AC | Hypertrophic cardiomyopathy | Neg | |
| 1143/2019 | 2019 | DSH | MC | 13y 11m | SC | Fever, oral ulcerations, depression | Hypertrophic cardiomyopathy | Neg |
| 399/2020 | 2020 | DSH | MC | 7y 10m | AC | Neg | ||
| 400/2020 | 2020 | DSH | MC | 16y 6m | SC | Dysorexia, weight loss, mast cell tumor | Neg | |
| 401/2020 | 2020 | DSH | M | 10y 11m | SC | Fibrosarcoma | Neg | |
| 404/2020 | 2020 | DSH | MC | 2y 11m | AC | Neg | ||
| 1144/2020 | 2020 | DSH | MC | 10y 1m | AC | Trauma | Neg | |
| 1145/2020 | 2020 | DSH | MC | 14y 1m | AC | Trauma | Neg | |
| 1146/2020 | 2020 | DSH | FS | 6y 4m | SC | Anemia, depression, anorexia | Neg | |
| 1147/2020 | 2020 | DSH | M | 4y | AC | Trauma | Neg | |
| 1148/2020 | 2020 | DSH | MC | 6y 5m | SC | Anemia, stomatitis | Neg | |
| 1149/2020 | 2020 | DSH | FS | 3y 6m | SC | Lymphadenomegaly, severe dermatopathy | Cardiogenic pulmonary edema | Neg |
| 1150/2020 | 2020 | DSH | MC | 3y 1m | SC | Stomatitis with oral ulcerations | Neg | |
| 1151/2020 | 2020 | DSH | MC | 5y 4m | SC | Dermatopathy, lymphadenomegaly | Neg | |
| 1152/2020 | 2020 | DSH | MC | 10y 8m | SC | Stomatitis, azotemia, proteinuria | Constipation | Neg |
| 1197/2020 | 2020 | DSH | MC | 7y 4m | AC | Urinary tract obstruction | Neg | |
| 1204/2020 | 2020 | DSH | MC | 5y 10m | AC | Bladder lithiasis | Neg | |
| 1210/2020 | 2020 | Persian | FS | 5y 11m | SC | Thrombocytopenia | Neg | |
| 1211/2020 | 2020 | DSH | MC | 7y 7m | SC | AKI/CKD | Neg | |
| 137/2021 | 2021 | DSH | M | 2y 1m | SC | Anemia, fever | Neg | |
| 395/2021 | 2021 | DSH | MC | 10y | AC | Trauma | Neg |
| Variables | Total | SC | AC | p Value |
|---|---|---|---|---|
| Number of cats | 50 | 33 | 17 | |
| Year of sampling | ||||
| 2018 | 12 (24%) | 11 (33.3%) | 1 (5.9%) | 0.1953 |
| 2019 | 19 (38%) | 11 (33.3%) | 8 (47.1%) | |
| 2020 | 17 (34%) | 10 (30.3%) | 7 (41.2%) | |
| 2021 | 2 (4%) | 1 (3%) | 1 (5.9%) | |
| Sex | ||||
| Male | 40 (80%) | 24 (72.7%) | 16 (94.1%) | 0.1562 |
| Female | 10 (20%) | 9 (27.3%) | 1 (5.9%) | |
| Age a | 8y 10m [6m–20y 1m] | 10y 2m [6m–20y 1m] | 6y [2y 11m–15y 11m] | <0.0001 |
| Breed | ||||
| DSH | 49 (98%) | 32 (97%) | 17 (100%) | 0.7330 |
| Persian | 1 (2%) | 1 (3%) | 0 (0%) | |
| FeLV antigen test | ||||
| Positive | 3 (6%) | 1 (3%) | 2 (11.8%) | 0.5462 |
| Negative | 47 (94%) | 32 (97%) | 15 (88.2%) |
| Total (N 50) | ||||
|---|---|---|---|---|
| Positive | Negative | |||
| LTR region PCR | 46 (92%) | 4 (8%) | ||
| env gene PCR assays | 43 (86%) | 7 (86%) | ||
| env gene A (V3-V4) PCR | 1 (2%) | 49 (98%) | ||
| env gene B (V3-V4) PCR | 35 (70%) | 15 (30%) | ||
| env gene C (V4-V5) PCR | 2 (4%) | 48 (96%) | ||
| env gene D (V4-V5) PCR | 15 (30%) | 35 (70%) | ||
| env gene E (V3-V5) PCR | 16 (32%) | 34 (68%) | ||
| LTR region PCR | ||||
| Positive | Negative | Total | ||
| env gene PCR assays | Positive | 39 (78%) | 4 (8%) | 43 (86%) |
| Negative | 7 (14%) | 0 (0%) | 7 (14%) | |
| Total | 46 (92%) | 4 (8%) | 50 (100%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balboni, A.; Facile, V.; Gallina, L.; Sabetti, M.C.; Dondi, F.; Battilani, M. Molecular Detection and Genetic Characterization of Feline Immunodeficiency Virus (FIV) in Seropositive Cats in Northern Italy. Pathogens 2024, 13, 463. https://doi.org/10.3390/pathogens13060463
Balboni A, Facile V, Gallina L, Sabetti MC, Dondi F, Battilani M. Molecular Detection and Genetic Characterization of Feline Immunodeficiency Virus (FIV) in Seropositive Cats in Northern Italy. Pathogens. 2024; 13(6):463. https://doi.org/10.3390/pathogens13060463
Chicago/Turabian StyleBalboni, Andrea, Veronica Facile, Laura Gallina, Maria Chiara Sabetti, Francesco Dondi, and Mara Battilani. 2024. "Molecular Detection and Genetic Characterization of Feline Immunodeficiency Virus (FIV) in Seropositive Cats in Northern Italy" Pathogens 13, no. 6: 463. https://doi.org/10.3390/pathogens13060463






